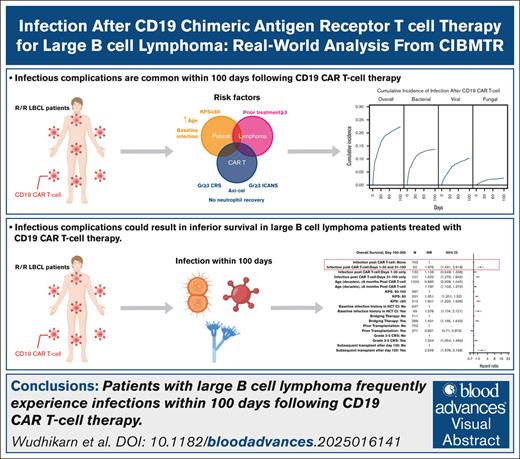
Key Points
Patients with LBCL frequently experience infections within 100 days after CD19 CAR T-cell therapy.
Infectious complications may potentially compromise survival in patients with LBCL receiving CD19 CAR T-cell therapy.
Visual Abstract
Infection is increasingly recognized as a significant cause of morbidity and mortality in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) receiving CD19 chimeric antigen receptor (CAR) T-cell therapy. The current study analyzed the natural history, risk factors, and outcomes of infection in 3350 patients with R/R LBCL receiving commercial CD19 CAR T-cell therapy (n = 2804 axicabtagene ciloleucel [axi-cel], n = 546 tisagenlecleucel) from December 2017 to June 2022. Infection developed in 834 patients (24.9%) within 100 days after infusion, resulting in an infection density of 0.43 per 100 patient days and a 100-day cumulative incidence of 22%. Bacterial, viral, and fungal infections were recorded in 527 (15.7%), 374 (11.2%), and 108 patients (3.2%), respectively, with corresponding infection densities of 0.23, 0.15, and 0.04 per 100 patient days. After a 24-month median follow-up, 1482 patients (44%) had died, with infection as the primary cause in 173 cases (12%). The 100-day infection-related mortality (IRM) was 1.6% (95% confidence interval, 1.2-2.0). Patients with a Karnofsky performance score of ≤80, infection history before CAR T-cell therapy, axi-cel therapy, severe cytokine release syndrome (grade ≥3), and severe immune effector cell–associated neurotoxicity syndrome (grade ≥3) had increased infection risk. Infections within 100 days were an independent risk factor for inferior overall survival beyond day 100 after CD19 CAR T-cell therapy. In conclusion, study results show a significant incidence of infection and IRM in patients with R/R LBCL treated with CD19 CAR T-cell therapy. Furthermore, results identify patients at a heightened risk of infection, offering insights to guide potential interventions aimed at mitigating infection and improving patient outcomes after CAR T-cell therapy.
Introduction
Recently, CD19 chimeric antigen receptor (CAR) T-cell therapy has become the standard treatment of patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL), eliciting an outstanding response and providing a potential long-term survival.1-6 Despite its efficacy, CAR T-cell therapy associates with significant side effects including immune-mediated cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), organ toxicities, cytopenia, hypogammaglobulinemia, and infection.7 Management of CRS and ICANS has become more standardized, resulting in lower incidence of severe events. In contrast, cytopenia and infection have become increasingly recognized complications that can result in significant morbidity and mortality and can be associated with inferior outcomes after CAR T-cell therapy.8-10 Evidence from both pivotal registration trials and largely single institutional studies has highlighted the common occurrence of infectious complications, with reported incidences ranging from 20% to 60%.11-15 Although predisposing factors of infection have been identified, inconsistencies exist among studies attributable to the heterogeneity of patients and the limited study size. Therefore, the current study aimed to define the incidence, patterns, risk factors, and outcomes of clinically significant infections within the initial 100 days after CD19 CAR T-cell therapy in patients with R/R LBCL after CD19 CAR T-cell therapy reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
Study design and data source
This retrospective, noninterventional study included adult patients with R/R LBCL who received commercial CD19-directed CAR T-cell therapy, specifically axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel), between December 2017 and June 2022 at cellular therapy centers in the United States and internationally. Eligible patients were those diagnosed as having R/R diffuse LBCL not otherwise specified, high-grade B-cell lymphoma, and transformed indolent lymphoma who underwent axi-cel or tisa-cel as their first CAR T-cell infusion. The CIBMTR is a research collaborative organization between the Medical College of Wisconsin and NMDP (formerly known as the National Marrow Donor Program) and maintains the Stem Cell Therapeutic Outcomes Database to which 195 US centers voluntarily report data on CAR T-cell therapy recipients (supplemental Material).16 Systematic operational and internal reviews are regularly conducted at each participating center to verify compliance, accuracy, and quality of submitted data. All patients participating in CIBMTR observational research provide consent to research that has received approval from the NMDP Institutional Review Board.
Data collection
Baseline clinical characteristics, CAR T-cell–associated information, relevant post-CAR T-cell adverse events, and infection-related data were collected through CIBMTR transplant essential and comprehensive report forms. Baseline comorbidities, as defined by the hematopoietic cell transplantation comorbidity index (HCT-CI), and relevant preexisting conditions, particularly baseline infections, were documented. The baseline infection history included a composite record of documented infection, fever of unknown origin, and infection requiring continued antimicrobial therapy beyond day 0, as outlined by the HCT-CI and recorded in the CIBMTR case report form.17 Diagnosis and assessment of CRS and ICANS were defined using the 2019 standard consensus criteria established by the American Society of Transplant and Cellular Therapy.18 Neutrophil recovery, defined as the time at which the absolute neutrophil count reached ≥500/mm3 (or ≥0.5 × 109/L) for 3 consecutive laboratory measurements obtained on separate days, was recorded. Data for infectious complications included the types and severity of infection observed within the initial 100 days after CAR T-cell infusion. Infection episodes including organism, timeframe, and site were defined and reported in accordance with the CIBMTR instruction manual. Infections were stratified based on causative microorganisms, including bacterial, viral, and fungal agents. Each pathogen-specific infection was further categorized by organism grouping (ie, gram-negative, gram-positive, or anaerobic bacteria for “bacterial infections”; cytomegalovirus [CMV], non-CMV herpes virus, severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], BK polyomavirus, or common respiratory viruses for “viral infections”; and yeast, mold, or pneumocystis for “fungal infections”). Only clinically significant infections were recorded, defined as infections requiring treatments according to the CIBMTR instruction manual.19 Prophylaxis protocols, diagnostic methodologies, and treatment strategies of infections were not standardized across each center. Viral load and specific information on preemptive protocols for pathogen surveillance were also not collected.
End point and definition
The primary end point of this study was infection density of overall infectious complications and those specific to organism type (bacterial, viral, or fungal) within the initial 100 days after infusion. Analysis of infectious complications was limited to the first 100 days after CAR T-cell therapy, given that data completeness was fully ensured during this period. Infection density was estimated as the number of infections per person per day at risk. Infection densities were reported for overall infections and further categorized by organism type, stratified into 2 distinct time intervals after CAR T-cell infusion: days 0 to 30 and days 31 to 100.
Additional end points included the cumulative incidence of infection, infection-related mortality (IRM), and overall survival (OS). Cumulative incidence of infection was determined as the initial occurrence of infection throughout the observed period, with relapse/progression, noninfection-related death, and hematopoietic cell transplantation (HCT) after CD19 CAR T-cell therapy considered as competing events. IRM was defined as the cumulative incidence of death directly attributable to infection as either the primary cause or a contributing cause, with relapse and death from noninfectious causes as competing events. The CIBMTR collects cause-of-death data using Case Report Form 2900, adhering to the definitions established by the Centers for Disease Control and Prevention and the National Center for Health Statistics. The primary cause of death is defined as the underlying disease or injury that initiated the sequence of events directly leading to death. All other conditions that may have contributed to the patient’s death are documented and reported as contributing causes.20,21 OS was defined as the time from CD19 CAR T-cell infusion to death from any cause. OS was reported as median OS and OS at 1 year after infusion was reported.
Statistical analysis
Baseline characteristics and infection data were reported using descriptive statistics. Continuous variables were presented as median and range or interquartile range (IQR), whereas categorical variables were depicted by the actual number and proportion of events. Patient-, disease-, and CAR T-cell infusion–related factors were subjected to comparison between groups using Pearson χ2 test for categorical variables and Kruskal-Wallis or Wilcoxon tests for continuous variables. Cumulative incidences of infection and IRM were evaluated at day 100 using the Aalen-Johansen estimator. OS was described using Kaplan-Meier estimates, with the variance estimated via Greenwood’s formula. Point estimates and 95% confidence intervals (CIs) were provided.
Multivariable Cox proportional hazards models were used to identify factors associated with infectious complications and IRM within 100 days after CAR T-cell infusion. A landmark analysis was conducted to evaluate the impact of infections on survival after CAR T-cell therapy using Cox models with a day 100 landmark, where patients were categorized into 4 groups based on their infection history after CAR T-cell therapy: no infections during days 0 to 100, had infection during days 0 to 30 only, had infection during days 31 to 100 only, and had infections during both days 0 to 30 and 31 to 100. Prespecified risk factors considered for the multivariable analysis models included age, Karnofsky performance score (KPS), baseline infection history, previous lines of antilymphoma treatment (1-2 vs >2), pre-CAR T bridging therapy, pre-CAR T transplant, CAR T-cell type (axi-cel vs tisa-cel), neutrophil recovery, onset and grade of CRS, and onset and grade of ICANS. CRS, ICANS, and neutrophil recovery were treated as time-dependent covariates in the Cox models. A stepwise selection procedure was used to identify variables to be selected for final models, with a significance level of 0.05 set as the inclusion criterion. The existence of center effects was tested using the score test of Commenges and Andersen22; if the score test found significance of center effects, a marginal Cox model was used to account for these effects.23 Statistical significance was determined as having a 2-sided P value of < .05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 4.3.0.
Results
Baseline clinical characteristics
A total of 3350 patients with R/R LBCL received CD19 CAR T-cell therapy, with 2804 patients (83.7%) receiving axi-cel and 546 patients (16.3%) receiving tisa-cel across 121 centers during the study period (supplemental Figure 1). The median age of patients at the initiation of CAR T-cell therapy was 62.5 years (IQR, 53.8-69.4), with 1958 patients (58.4%) aged 60 years or older. The predominant indication for CAR T-cell therapy was diffuse LBCL, noted in 3241 patients (96.7%). Among all patients, 640 (19.1%) had a KPS of <80 and 1050 (31.3%) had an HCT-CI score of 3 or more. The median number of previous lines of treatment before CAR T-cell therapy was 3 (IQR, 2-4). A total of 865 patients (25.8%) underwent HCT before receiving CD19 CAR T-cell therapy, including 811 patients who underwent autologous HCT, 30 who underwent allogeneic HCT, and 4 who underwent both autologous and allogeneic HCT. Among 34 patients who previously underwent allogeneic HCT, 26 received reduced intensity or nonmyeloablative conditioning regimens. The median duration from HCT to CD19 CAR T-cell infusion was 13.4 months (IQR, 7.2-28.2 months), with 431 patients (12.9%) receiving CAR T-cell therapy within 12 months after HCT. Baseline characteristics of the entire patient cohort are presented in Table 1.
Baseline characteristics of 3350 patients with R/R LBCL treated with CD19 CAR T-cell therapy in this cohort
| Parameters . | N = 3350 (%) . |
|---|---|
| Median age at the time of CAR T-cell therapy, y (IQR) | 62.5 (53.8-69.4) |
| Age ≥60 y at the time of CAR T-cell therapy, n (%) | 1958 (58.4) |
| Male sex, n (%) | 2104 (62.9) |
| Race, n (%) | |
| Caucasian | 2646 (79.0) |
| African American | 183 (5.5) |
| Asian | 161 (4.8) |
| Native American and Pacific Islander | 24 (0.7) |
| Mixed | 15 (0.4) |
| HCT-CI, n (%) | |
| 1-2 | 1157 (34.5) |
| 3-4 | 757 (22.6) |
| 5 or more | 293 (8.7) |
| Missing | 171 (5.1) |
| Diagnostic indication of CD19 CAR T-cell therapy, n (%) | |
| Diffuse large B-cell lymphoma | 3241 (96.7) |
| High-grade B-cell lymphoma | 80 (2.4) |
| Other | 29 (0.9) |
| Transformed lymphoma, n (%) | |
| Transformed | 925 (27.6) |
| De novo | 2340 (69.9) |
| Missing/Unknown | 85 (2.5) |
| Previous lines of treatment before CAR T-cell therapy, n (%) | |
| 1-3 | 2030 (60.6) |
| 4 or more lines | 1222 (36.5) |
| Missing | 98 (2.9) |
| Previous HCT before CAR T-cell therapy, n (%) | 865 (25.8) |
| Median time from HCT to CAR T-cell therapy (IQR), mo | 13.4 (7.2-28.2) |
| Disease status at the time of CAR T-cell therapy, n (%) | |
| Chemosensitive relapse, complete remission | 140 (4.2) |
| Chemosensitive relapse, partial remission | 688 (20.5) |
| Chemoresistant relapse | 2175 (64.9) |
| Relapse, untreated | 197 (5.9) |
| Unknown | 150 (4.5) |
| KPS, n (%) | |
| 90-100 | 1361 (40.6) |
| 80 | 991 (29.6) |
| <80 | 640 (19.1) |
| Missing | 358 (10.7) |
| Receipt of bridging therapy, n (%) | |
| No | 2451 (73.2) |
| Yes | 801 (23.9) |
| Missing | 98 (2.9) |
| History of infection before CAR T-cell therapy, n (%) | 133 (4.0) |
| Parameters . | N = 3350 (%) . |
|---|---|
| Median age at the time of CAR T-cell therapy, y (IQR) | 62.5 (53.8-69.4) |
| Age ≥60 y at the time of CAR T-cell therapy, n (%) | 1958 (58.4) |
| Male sex, n (%) | 2104 (62.9) |
| Race, n (%) | |
| Caucasian | 2646 (79.0) |
| African American | 183 (5.5) |
| Asian | 161 (4.8) |
| Native American and Pacific Islander | 24 (0.7) |
| Mixed | 15 (0.4) |
| HCT-CI, n (%) | |
| 1-2 | 1157 (34.5) |
| 3-4 | 757 (22.6) |
| 5 or more | 293 (8.7) |
| Missing | 171 (5.1) |
| Diagnostic indication of CD19 CAR T-cell therapy, n (%) | |
| Diffuse large B-cell lymphoma | 3241 (96.7) |
| High-grade B-cell lymphoma | 80 (2.4) |
| Other | 29 (0.9) |
| Transformed lymphoma, n (%) | |
| Transformed | 925 (27.6) |
| De novo | 2340 (69.9) |
| Missing/Unknown | 85 (2.5) |
| Previous lines of treatment before CAR T-cell therapy, n (%) | |
| 1-3 | 2030 (60.6) |
| 4 or more lines | 1222 (36.5) |
| Missing | 98 (2.9) |
| Previous HCT before CAR T-cell therapy, n (%) | 865 (25.8) |
| Median time from HCT to CAR T-cell therapy (IQR), mo | 13.4 (7.2-28.2) |
| Disease status at the time of CAR T-cell therapy, n (%) | |
| Chemosensitive relapse, complete remission | 140 (4.2) |
| Chemosensitive relapse, partial remission | 688 (20.5) |
| Chemoresistant relapse | 2175 (64.9) |
| Relapse, untreated | 197 (5.9) |
| Unknown | 150 (4.5) |
| KPS, n (%) | |
| 90-100 | 1361 (40.6) |
| 80 | 991 (29.6) |
| <80 | 640 (19.1) |
| Missing | 358 (10.7) |
| Receipt of bridging therapy, n (%) | |
| No | 2451 (73.2) |
| Yes | 801 (23.9) |
| Missing | 98 (2.9) |
| History of infection before CAR T-cell therapy, n (%) | 133 (4.0) |
CAR T-cell therapy–associated characteristics
Bridging therapy was administered before CAR T-cell infusion in 801 patients (23.9%). At the time of CAR T-cell therapy, 140 patients (4.2%) were in complete remission. The most frequently used lymphodepletion chemotherapy regimen was fludarabine and cyclophosphamide, given in 3185 patients (95.1%), followed by single-agent bendamustine in 101 patients (3.0%). The median total CAR T-cell dose was 2 × 108 cells (IQR, 1.4 × 108 to 7 × 108 cells). CRS was observed in 2573 patients (76.8%), with 268 (8.0%) experiencing grade 3 or higher CRS. The median duration from CAR T-cell infusion to the onset of CRS was 4 days (IQR, 2-6 days). ICANS (any grade) was noted in 1143 patients (34.1%) with a median onset of 7 days (IQR, 5-9 days) after CAR T-cell infusion. Grade 3 or more severe ICANS was observed in 581 patients (17.3%). Tocilizumab was administered to 1816 patients (54.2%), and systemic corticosteroids were administered to 1450 patients (43.3%). Thirty-five patients (1.0%) required anakinra, and 34 patients (1.0%) received siltuximab. The median time for neutrophil recovery after CAR T-cell infusion was 10 days (IQR, 6-13 days). A total of 1850 patients (55.2%) developed hypogammaglobulinemia (<600 mg/dL), and 866 patients (25.8%) received intravenous immunoglobulin replacement therapy. The details of CAR T-cell therapy are presented in Table 2.
Detailed information on CD19 CAR T-cell therapy and related events
| Parameters . | N = 3350 (%) . |
|---|---|
| LD chemotherapy, n (%) | |
| Fludarabine-based regimens∗ | 3224 (96.2) |
| Bendamustine-based regimens† | 103 (3.1) |
| Single-agent cyclophosphamide | 8 (0.2) |
| Others or not specified | 15 (0.4) |
| Median time from LD chemotherapy to CAR T-cell infusion (IQR), d | 5 (5-5) |
| CAR T-cell product | |
| Axi-cel | 2804 (83.7) |
| Tisa-cel | 546 (16.3) |
| Median total CAR T-cell dose (IQR), ×108 cells | 2 (1.4-7) |
| Median time from CAR T-cell infusion to CRS onset (range), d | 4 (2-6) |
| Severity of CRS,‡ n (%) | |
| Grade 0 | 777 (23.2) |
| Grade 1-2 | 2305 (68.8) |
| Grade ≥3 | 268 (8.0) |
| Median time from CAR T-cell infusion to ICANS onset (range), d | 7 (5-9) |
| Severity of ICANS,‡ n (%) | |
| Grade 0 | 2207 (65.9) |
| Grade 1-2 | 562 (16.8) |
| Grade ≥3 | 581 (17.3) |
| Receipt of tocilizumab, n (%) | |
| Yes | 1816 (54.2) |
| No | 1489 (44.4) |
| Missing | 45 (1.3) |
| Receipt of systemic corticosteroid, n (%) | |
| Yes | 1450 (43.3) |
| No | 1831 (54.7) |
| Missing | 69 (2.1) |
| Median time to neutrophil recovery (range), d | 10 (6-13) |
| Delayed neutrophil recovery,§ n (%) | 388 (11.6) |
| Parameters . | N = 3350 (%) . |
|---|---|
| LD chemotherapy, n (%) | |
| Fludarabine-based regimens∗ | 3224 (96.2) |
| Bendamustine-based regimens† | 103 (3.1) |
| Single-agent cyclophosphamide | 8 (0.2) |
| Others or not specified | 15 (0.4) |
| Median time from LD chemotherapy to CAR T-cell infusion (IQR), d | 5 (5-5) |
| CAR T-cell product | |
| Axi-cel | 2804 (83.7) |
| Tisa-cel | 546 (16.3) |
| Median total CAR T-cell dose (IQR), ×108 cells | 2 (1.4-7) |
| Median time from CAR T-cell infusion to CRS onset (range), d | 4 (2-6) |
| Severity of CRS,‡ n (%) | |
| Grade 0 | 777 (23.2) |
| Grade 1-2 | 2305 (68.8) |
| Grade ≥3 | 268 (8.0) |
| Median time from CAR T-cell infusion to ICANS onset (range), d | 7 (5-9) |
| Severity of ICANS,‡ n (%) | |
| Grade 0 | 2207 (65.9) |
| Grade 1-2 | 562 (16.8) |
| Grade ≥3 | 581 (17.3) |
| Receipt of tocilizumab, n (%) | |
| Yes | 1816 (54.2) |
| No | 1489 (44.4) |
| Missing | 45 (1.3) |
| Receipt of systemic corticosteroid, n (%) | |
| Yes | 1450 (43.3) |
| No | 1831 (54.7) |
| Missing | 69 (2.1) |
| Median time to neutrophil recovery (range), d | 10 (6-13) |
| Delayed neutrophil recovery,§ n (%) | 388 (11.6) |
LD, lymphodepletion.
Fludarabine-based regimens: fludarabine + cyclophosphamide, fludarabine + carboplatin, fludarabine + cytarabine, fludarabine + cytarabine + cyclophosphamide, fludarabine + cyclophosphamide + others, single-agent fludarabine.
Bendamustine-based regimens: bendamustine, bendamustine + cytarabine, bendamustine + cyclophosphamide.
Severity grading according to the American Society of Transplant and Cellular Therapy Consensus Criteria.
No neutrophil recovery after 30 days of CAR T-cell infusion.
Infections
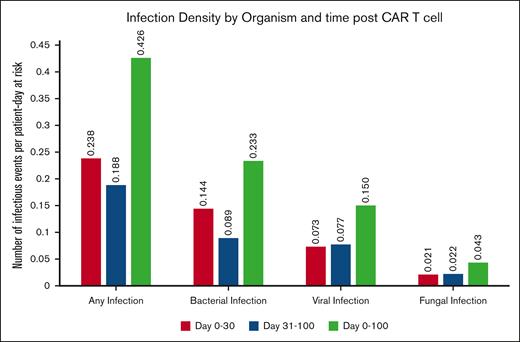
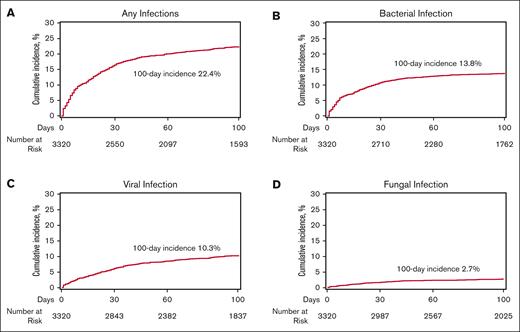
Within the initial 100 days, 834 patients (24.9%) experienced at least 1 episode of infection, resulting in an infection density of 0.43 infections per 100 patient days at risk and a 100-day cumulative incidence of infection of 22.4% (95% CI, 21-23.8). The infection densities during days 0 to 30 and days 30 to 100 were 0.24 and 0.19 per 100 patient days at risk, respectively, as shown in Figure 1. For organism types, 527 (15.7%), 374 (11.2%), and 108 (3.2%) were bacterial, viral, and fungal infections, respectively. The corresponding 100-day infection densities for bacterial, viral, and fungal infections were 0.23, 0.15, and 0.04 per patient per 100 days at risk, respectively (Figure 1). The 100-day cumulative incidences of bacterial, viral, and fungal infection were 13.8% (95% CI, 12.6-15), 10.3% (95% CI, 9.2-11.3), and 2.7% (95% CI, 2.1-3.2), respectively (Figure 2).
The 100-day incidence of infection after CAR T-cell therapy. (A) Any infection, (B) bacterial infection, (C) viral infection, and (D) fungal infection.
The 100-day incidence of infection after CAR T-cell therapy. (A) Any infection, (B) bacterial infection, (C) viral infection, and (D) fungal infection.
Among patients with bacterial infection, the 3 most common pathogens were Staphylococcus spp (n = 151, 28.7%), followed by gram-negative bacilli from the bacterial order Enterobacterales (n = 147, 27.9%) and Clostridioides difficile (n = 117, 22.2%) (supplemental Tables 1 and 2). Bloodstream infection was the most common infection documented in 222 patients (42.1%). Of all 374 patients who developed viral infection, community respiratory viruses were the most common (n = 188, 50.3%), followed by CMV (n = 118, 31.6%) and non-CMV herpes viruses (n = 61, 16.3%). SARS-CoV-2 was observed in 60 patients (16%). Besides SARS-CoV-2, rhinovirus was the most common respiratory viral infection. Of all viral infections, the respiratory tract was the most common site in 187 patients (50%), followed by viremia in 157 patients (42%). Details of viral infection by pathogen and site of infection are presented in supplemental Tables 3 and 4. Fungal infections included yeast infection in 78 patients (2.3%), mold infection in 28 patients (0.8%), and Pneumocystis jirovecii infection in 7 patients (0.2%). Fungemia and respiratory tract fungal infection were the 2 most common fungal infections observed in 40 patients (1.2%) and 38 patients (1.1%), respectively. Among 38 patients who had fungal infection of the respiratory system, 12 (31.5%) and 26 (68.4%) were reported to involve upper and lower respiratory tracts, respectively. Among upper respiratory tract fungal infections, the identified pathogens included Candida albicans (n = 7), non-albicans Candida (n = 1), an unspecified yeast (n = 1), Aspergillus fumigatus (n = 1), Fusarium spp (n = 1), and mucormycetes (n = 1). Lower respiratory tract fungal infections were attributed to non-albicans Candida (n = 9), Coccidioides spp (n = 1), unspecified Aspergillus spp (n = 9), Aspergillus fumigatus (n = 3), Aspergillus niger (n = 1), and mucormycetes (n = 3). Additional details of specific fungal pathogen subtypes are presented in the Supplementary Material (supplemental Tables 5 and 6).
Survival outcomes
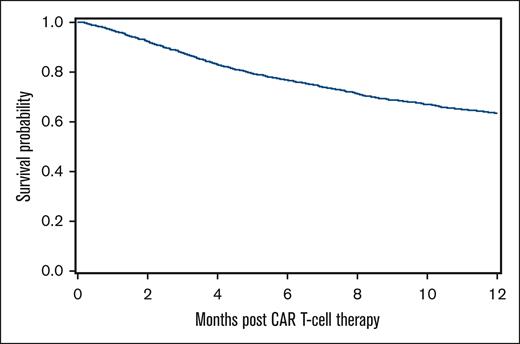
At a median follow-up of 24 months, 1482 patients (44.2%) had died. The most common cause of death was primary disease in 1037 patients (70.0%). Infection was the primary cause of death in 173 patients (11.7%). Among 1209 patients whose primary cause of death was noninfectious etiologies, infection was the contributing cause of death in 107 patients (8.8%). The 100-day IRM was 1.6% (95% CI, 1.2-2). The 100-day and 1-year OS were 86.3% (95% CI, 85.1-87.4) and 63.4% (95% CI, 61.7-65.2), respectively (Figure 3).
Factors associated with infections and outcomes
Multivariable regression analyses were used to identify factors associated with overall and organism-specific infections within the initial 100 days after CAR T-cell therapy. Severe (grade ≥3) CRS and ICANS were independent factors for overall and any organism-specific (bacterial, viral, and fungal) infection (Table 3). In addition to severe CRS and ICANS, factors contributing to an increased risk of overall infection included older age (hazard ratio [HR] per decade increase, 1.07; 95% CI, 1.01-1.13), baseline infection history before CAR T-cell therapy (HR, 1.71; 95% CI, 1.25-2.34), lower KPS (HR for <80 vs 90-100, 2.09; 95% CI, 1.72-2.54), absence of neutrophil recovery (HR, 1.35; 95% CI, 1.08-1.69), and the receipt of axi-cel (HR, 1.48; 95% CI, 1.18-1.87). Upon stratification by organism, in addition to severe CRS and ICANS, risk of bacterial infection was associated with older age (HR, 1.12; 95% CI, 1.04-1.20), lower KPS (HR for <80 vs 90-100, 2.34; 95% CI, 1.84-2.98), history of infection before CAR T-cell therapy (HR, 1.83; 95% CI, 1.27-2.65), and axi-cel treatment (HR, 1.80; 95% CI, 1.32-2.45). In addition to severe CRS and ICANS, lower KPS (HR for <80 vs 90-100, 2.19; 95% CI, 1.63-2.96), a higher number of previous treatments before CAR T-cell (HR for ≥3 vs 1-2 lines, 1.42; 95% CI, 1.10-1.83), and absence of neutrophil recovery (HR, 1.47; 95% CI, 1.04-2.09) were associated with viral infection. Finally, poor KPS (HR for <80 vs 90-100, 2.35; 95% CI, 1.34-4.10), baseline infection history before CAR T-cell therapy (HR, 3.31; 95% CI, 1.68-6.52), and absence of neutrophil recovery (HR, 2.32; 95% CI, 1.36-3.95) were associated with fungal infection within 100 days after CAR T-cell therapy. Risk factors of infections and IRM during the first 30 days after CAR T-cell therapy were analyzed and are presented in supplemental Table 7.
Multivariable analysis of risk factors associated with infection-related complication during day 0-100 after CAR T-cell therapy
| Variable . | Overall infection . | Bacterial infection . | Viral infection . | Fungal infection . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | |
| Age (decades)∗ | 3296 | 695 | 1.067 (1.006-1.131) | .030 | 3304 | 439 | 1.117 (1.037-1.203) | .003 | NA | NA | NA | NA | NA | NA | NA | NA |
| KPS | <.001 (3 df) | <.001 | <.0001 (3 df) | .014 (3 df) | ||||||||||||
| 90-100 | 1344 | 207 | Ref | Ref | 1345 | 125 | Ref | Ref | 1349 | 89 | Ref | Ref | 1349 | 20 | Ref | Ref |
| 80 | 976 | 237 | 1.644 (1.366-1.979) | <.001 | 980 | 134 | 1.466 (1.152-1.864) | .002 | 979 | 114 | 1.867 (1.416-2.461) | <.001 | 981 | 25 | 1.708 (0.968-3.014) | .065 |
| <80 | 627 | 196 | 2.089 (1.716-2.544) | <.001 | 629 | 138 | 2.343 (1.840-2.984) | <.001 | 633 | 89 | 2.194 (1.627-2.960) | <.001 | 635 | 28 | 2.347 (1.344-4.099) | .003 |
| Missing | 349 | 75 | 1.472 (1.129-1.920) | .004 | 350 | 42 | 1.320 (0.937-1.861) | .113 | 352 | 39 | 1.746 (1.196-2.548) | .004 | 353 | 13 | 2.467 (1.219-4.994) | .012 |
| Baseline infection history | .004 (2 df) | .006 (2 df) | .002 (2 df) | |||||||||||||
| No | 2997 | 636 | Ref | Ref | 3004 | 388 | Ref | Ref | NA | NA | NA | NA | 3017 | 74 | Ref | Ref |
| Yes | 130 | 43 | 1.707 (1.246-2.338) | .001 | 131 | 30 | 1.831 (1.266-2.650) | .001 | NA | NA | NA | NA | 132 | 10 | 3.305 (1.675-6.520) | .001 |
| Missing | 169 | 36 | 0.972 (0.694-1.363) | .870 | 169 | 21 | 0.956 (0.621-1.473) | .840 | NA | NA | NA | NA | 169 | 2 | 0.656 (0.207-2.077) | .474 |
| No. of lines of previous therapy | .017 (2 df) | .006 (2 df) | ||||||||||||||
| 1-2 | 977 | 177 | Ref | Ref | NA | NA | NA | NA | 981 | 76 | Ref | Ref | NA | NA | NA | NA |
| ≥3 | 2234 | 521 | 1.270 (1.073-1.503) | .005 | NA | NA | NA | NA | 2247 | 251 | 1.417 (1.095-1.834) | .008 | NA | NA | NA | NA |
| Missing | 85 | 17 | 1.021 (0.616-1.695) | .934 | NA | NA | NA | NA | 85 | 4 | 0.536 (0.194-1.477) | .228 | NA | NA | NA | NA |
| CAR T-cell product | ||||||||||||||||
| Tisa-cel | 538 | 82 | Ref | Ref | 539 | 44 | Ref | Ref | NA | NA | NA | NA | NA | NA | NA | NA |
| Axi-cel | 2758 | 633 | 1.483 (1.177-1.867) | .001 | 2765 | 395 | 1.800 (1.321-2.453) | <.001 | NA | NA | NA | NA | NA | NA | NA | NA |
| Grade 3-5 CRS† | ||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| Yes | 1.762 (1.385-2.241) | <.001 | 1.898 (1.422-2.534) | <.001 | 1.546 (1.076-2.222) | .018 | 2.555 (1.418-4.603) | .002 | ||||||||
| Grade 3-5 ICANS† | ||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| Yes | 1.707 (1.408-2.070) | <.001 | 1.733 (1.356-2.214) | <.001 | 1.488 (1.125-1.968) | .005 | 2.629 (1.580-4.375) | <.001 | ||||||||
| Neutrophil recovery | ||||||||||||||||
| Yes | Ref | Ref | NA | NA | Ref | Ref | Ref | Ref | ||||||||
| No | 1.350 (1.075-1.694) | .01 | NA | NA | 1.471 (1.037-2.085) | .030 | 2.318 (1.362-3.945) | .001 | ||||||||
| Variable . | Overall infection . | Bacterial infection . | Viral infection . | Fungal infection . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | n . | Event . | HR (95% CI) . | P value . | |
| Age (decades)∗ | 3296 | 695 | 1.067 (1.006-1.131) | .030 | 3304 | 439 | 1.117 (1.037-1.203) | .003 | NA | NA | NA | NA | NA | NA | NA | NA |
| KPS | <.001 (3 df) | <.001 | <.0001 (3 df) | .014 (3 df) | ||||||||||||
| 90-100 | 1344 | 207 | Ref | Ref | 1345 | 125 | Ref | Ref | 1349 | 89 | Ref | Ref | 1349 | 20 | Ref | Ref |
| 80 | 976 | 237 | 1.644 (1.366-1.979) | <.001 | 980 | 134 | 1.466 (1.152-1.864) | .002 | 979 | 114 | 1.867 (1.416-2.461) | <.001 | 981 | 25 | 1.708 (0.968-3.014) | .065 |
| <80 | 627 | 196 | 2.089 (1.716-2.544) | <.001 | 629 | 138 | 2.343 (1.840-2.984) | <.001 | 633 | 89 | 2.194 (1.627-2.960) | <.001 | 635 | 28 | 2.347 (1.344-4.099) | .003 |
| Missing | 349 | 75 | 1.472 (1.129-1.920) | .004 | 350 | 42 | 1.320 (0.937-1.861) | .113 | 352 | 39 | 1.746 (1.196-2.548) | .004 | 353 | 13 | 2.467 (1.219-4.994) | .012 |
| Baseline infection history | .004 (2 df) | .006 (2 df) | .002 (2 df) | |||||||||||||
| No | 2997 | 636 | Ref | Ref | 3004 | 388 | Ref | Ref | NA | NA | NA | NA | 3017 | 74 | Ref | Ref |
| Yes | 130 | 43 | 1.707 (1.246-2.338) | .001 | 131 | 30 | 1.831 (1.266-2.650) | .001 | NA | NA | NA | NA | 132 | 10 | 3.305 (1.675-6.520) | .001 |
| Missing | 169 | 36 | 0.972 (0.694-1.363) | .870 | 169 | 21 | 0.956 (0.621-1.473) | .840 | NA | NA | NA | NA | 169 | 2 | 0.656 (0.207-2.077) | .474 |
| No. of lines of previous therapy | .017 (2 df) | .006 (2 df) | ||||||||||||||
| 1-2 | 977 | 177 | Ref | Ref | NA | NA | NA | NA | 981 | 76 | Ref | Ref | NA | NA | NA | NA |
| ≥3 | 2234 | 521 | 1.270 (1.073-1.503) | .005 | NA | NA | NA | NA | 2247 | 251 | 1.417 (1.095-1.834) | .008 | NA | NA | NA | NA |
| Missing | 85 | 17 | 1.021 (0.616-1.695) | .934 | NA | NA | NA | NA | 85 | 4 | 0.536 (0.194-1.477) | .228 | NA | NA | NA | NA |
| CAR T-cell product | ||||||||||||||||
| Tisa-cel | 538 | 82 | Ref | Ref | 539 | 44 | Ref | Ref | NA | NA | NA | NA | NA | NA | NA | NA |
| Axi-cel | 2758 | 633 | 1.483 (1.177-1.867) | .001 | 2765 | 395 | 1.800 (1.321-2.453) | <.001 | NA | NA | NA | NA | NA | NA | NA | NA |
| Grade 3-5 CRS† | ||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| Yes | 1.762 (1.385-2.241) | <.001 | 1.898 (1.422-2.534) | <.001 | 1.546 (1.076-2.222) | .018 | 2.555 (1.418-4.603) | .002 | ||||||||
| Grade 3-5 ICANS† | ||||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| Yes | 1.707 (1.408-2.070) | <.001 | 1.733 (1.356-2.214) | <.001 | 1.488 (1.125-1.968) | .005 | 2.629 (1.580-4.375) | <.001 | ||||||||
| Neutrophil recovery | ||||||||||||||||
| Yes | Ref | Ref | NA | NA | Ref | Ref | Ref | Ref | ||||||||
| No | 1.350 (1.075-1.694) | .01 | NA | NA | 1.471 (1.037-2.085) | .030 | 2.318 (1.362-3.945) | .001 | ||||||||
Center effects were found to be significant by score test (P < .001) for all outcomes. Therefore, marginal Cox regression models were fitted to account for these effects.
df, degrees of freedom; NA, not applicable; Ref, reference.
Age was treated as a continuous variable.
CRS, ICANS onset, and neutrophil recovery were treated as time-dependent covariates.
Patients with advanced age (HR per decade increase, 1.63; 95% CI, 1.19-2.23), lower KPS (HR for <80 vs 90-100, 14.31; 95% CI, 4.24-48.27), baseline infection history before CAR T-cell therapy (HR, 3.92; 95% CI, 1.60-9.62), and severe CRS (HR, 3.73; 95% CI, 2.00-6.99) had a significantly increased risk of IRM within the first 100 days (Figure 4). In addition, patients who developed infections between day 31 and 100 after CAR T-cell infusion, had poor KPS (HR for <80 vs 90-100, 1.60; 95% CI, 1.32-1.94), received bridging therapy (HR, 1.40; 95% CI, 1.20-1.64), and baseline infection history before CAR T-cell therapy (HR, 1.58; 95% CI, 1.17-2.12) had lower OS (Figure 4). Moreover, older patients exhibited poorer OS than their younger counterparts during long-term follow-up (exceeding 246 days) after CAR T-cell infusion (HR per decade increase, 1.19; 95% CI, 1.11-1.28). Severe ICANS (HR, 1.24; 95% CI, 1.05-1.47) and subsequent HCT after day 100 after CAR T-cell therapy (HR, 2.25; 95% CI, 1.58-3.20) were associated with increased mortality.
Forest plots of multivariable analysis of factors associated with IRM and OS.
Discussion
The current study presents the largest real-world data set of infections after CD19 CAR T-cell therapy in patients with R/R LBCL. In addition to characterizing infection burden in patients receiving CAR T-cell therapy, the study identified a subgroup of patients exhibiting a significantly elevated risk of infections after CD19 CAR T-cell therapy, suggesting the importance of both patient selection and preventive measures to mitigate infection within this particularly vulnerable subgroup.24-26
The incidence of infection and rate of IRM in the current study were similar or lower than contemporary literature.12,14,27-31 This difference could be attributed to several factors, including differences in the definition of infection across all studies, variations in patient demographics, shifts in the use of antimicrobial prophylaxis, and the evolution of treatment for immune-mediated toxicities over time. Furthermore, owing to the retrospective nature of the registry, incomplete reporting of follow-up data remains a plausible limitation. Finally, the current study included only clinically significant infection complications, which may have resulted in a lower observed incidence of infections than previous reports.
Consistent with findings from prospective clinical trials and previous real-world evidence, the current study shows that infection risk was most pronounced during the initial 30 days after CAR T-cell therapy, with bacterial infections being the predominant pathogens during this period.11-13,32 Notably, patients in this cohort had a higher median age at infusion and a greater proportion of poor performance status, representing a more vulnerable population at an increased risk of complications than those treated in clinical trials. Severe CRS/ICANS, low KPS, and the administration of axi-cel were associated with infection within 30 days after CAR T-cell therapy. After 30 days, the incidence of infection exhibited a gradual decline,32 with viral infections becoming increasingly predominant. Factors contributing to the risk of infection after day 30 included advanced age, a history of extensive treatments, and failure to achieve neutrophil recovery. The predominance of viral infections in later stages could be attributed to impaired pathogen-specific humoral and cellular-mediated immunity.11,14,33
Consistent with previous studies, community respiratory viruses constituted the predominant viral pathogen after CD19 CAR T-cell therapy.10,13,32,34,35 Only 3.5% of patients in this cohort had CMV infection. Although the observed incidence of CMV infection may be underestimated owing to the absence of a standardized CMV monitoring protocol in patients undergoing CAR T-cell therapy, this finding may also reflect a low incidence of clinically significant CMV infection truly requiring antiviral therapy. Data from pivotal studies and initial retrospective studies suggested a low incidence of CMV infection in patients receiving CD19 CAR T-cell therapy.1,12,13 However, subsequent studies with more defined testing protocols reported a higher incidence, with approximately 40% of patients experiencing CMV reactivation and 10% developing clinically significant CMV infection.36-41 Consequently, the real incidence of CMV reactivation and the burden of clinically significant CMV infection after CAR T-cell therapy require larger studies. Finally, among invasive fungal infections, Candida infections—particularly candidemia—were the most prevalent in the present study. Interestingly, Pneumocystis infection was observed in 7 patients, which may reflect prolonged T-cell depletion. It is important to acknowledge that, given the retrospective nature of the CIBMTR database, accurate identification of causative pathogens particularly in fungal infections may be limited. For instance, respiratory tract infections caused by Candida spp are uncommon. Therefore, reported organisms in certain sites may represent colonization rather than true infection. Taken together, antiviral and antifungal prophylaxis for patients undergoing CD19 CAR T-cell therapy should be considered and guided by institutional epidemiology and assessment of patient infection risk.
The current study identified a high-risk patient group particularly susceptible to infections after CAR T-cell therapy. In particular, patients experiencing severe CRS and ICANS had higher overall infection rates, organism-specific infectious complications, and IRM.12-14,42-45 The observed association with severity of CRS/ICANS and infection risk, especially early infection within 30 days after CAR T-cell therapy, may reflect severe cytopenia or prolonged exposure to immunosuppression such as systemic corticosteroids for managing severe CRS and ICANS.46 Older patients with impaired performance status, particularly those who experienced severe CRS and ICANS, represent a subgroup at a significantly higher risk of infection. Such an elevated infection risk was most pronounced during the first 30 days after CAR T-cell therapy but could persist up to 100 days after treatment depending on the degree of host comorbidities and CAR T-cell–associated complications. Baseline infection history before CAR T-cell infusion especially those requiring ongoing antimicrobial therapy beyond day 0 was also a significant independent risk factor for bacterial and fungal infections, consistent with observations from previous studies.13,43,47,48 This finding underscores the critical need for infection control before delivering CAR T-cell therapy and highlights baseline active infection as a potential contraindication or need to delay the administration of CAR T cells. However, owing to the large-scale real-world nature of this study, the availability of detailed baseline infection histories was limited. As such, broader application of general infection history rather than specific pathogen-related histories may not be suitable for determining risk factors for infection complications across diverse clinical settings given that the nature and risk factors of infections can vary significantly between pathogen subtypes and diagnostic entities. Finally, receipt of axi-cel was found to be an independent risk factor for overall infection, especially bacterial infection. Axi-cel therapy may lead to a higher incidence of CAR T-cell toxicities,49,50 thereby inducing more pronounced immune dysregulation and necessitating more frequent immunosuppressive therapy.
The current study underscores that infection after CAR T-cell therapy is an independent prognostic factor for overall mortality, particularly infections occurring later (days 30-100) after CAR T-cell therapy. The substantial impact of delayed- vs early-onset infection on mortality after CAR T-cell therapy mirrors findings observed in allogeneic HCT patients,51,52 suggesting prolonged immune dysregulation (persistent cytopenia, CD4+ lymphopenia, or hypogammaglobulinemia) after CAR T-cell therapy. Despite 55.2% of patients having hypogammaglobulinemia, intravenous immunoglobulin replacement was administered in only 25.8% of cases, probably reflecting variability in immunoglobulin G monitoring and intravenous immunoglobulin replacement practices.25 Future investigation is needed to explore the potential benefits of intravenous immunoglobulin replacement in preventing infection, as well as a validated threshold for its administration in the post-CAR T-cell therapy setting.
As the largest multi-institutional study to date in patients with R/R LBCL receiving CD19 CAR T-cell therapy within real-world clinical practice settings, the current study offers a detailed characterization of infections and conducts a comprehensive analysis of risk factors for infection within the initial 100 days after CAR T-cell therapy. In doing so, the study establishes a framework for future clinical trials incorporating clinical practice guidelines for antimicrobial and vaccination prophylaxis in CAR T-cell therapy patients. However, the current study is not without limitations. Owing to the retrospective design of the study and the limitations of the CIBMTR case report forms, relevant data are missing such as detailed cytopenia, infection, and immunosuppression (dose and duration) for the management of CRS and ICANS. These factors are important in assessing infection risk in patients undergoing CAR T-cell therapy. Institutional variability in antimicrobial prophylaxis protocols, growth factor administration, intravenous immunoglobulin replacement strategies, and infection surveillance programs is also not captured to evaluate their impact on infection. Finally, only data from patients treated with axi-cel and tisa-cel were analyzed, so findings cannot be extended to other CD19 CAR T-cell products such as lisocabtagene maraleucel.
In conclusion, infection has emerged as a longitudinal complication that can adversely affect the outcomes of patients with R/R LBCL treated with CD19 CAR T-cell therapy. Our study identifies an adult patient population at a high risk of infection after CAR T-cell therapy for R/R LBCL. Future investigation is needed to determine best practices for infection surveillance and prevention in this patient population.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Additional federal support is provided by U01AI184132 from the NIAID and UG1HL174426 from the NHLBI. Support is also provided by the Medical College of Wisconsin, NMDP, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium, and the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune LLC; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc; Amgen, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; Autolus Limited; BeiGene; BioLineRx; Blue Spark Technologies; bluebird bio, Inc; Blueprint Medicines; Bristol Myers Squibb; CareDx, Inc; Caribou Biosciences, Inc; CytoSen Therapeutics, Inc; DKMS; Editas Medicine; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; HistoGenetics; IN8Bio, Inc; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite Pharma, a Gilead company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Merck; Millennium, the Takeda Oncology company; Miller Pharmacal Group, Inc; Miltenyi Biomedicine; Miltenyi Biotec, Inc; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea; Stemcell Technologies; Stemline Technologies; Stemsoft; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the National Institutes of Health, the Department of Health and Human Services, the Department of the Navy, the Department of Defense, the HRSA, or any other agency of the US government.
Authorship
Contribution: K.W. proposed the study concept, reviewed the literature, designed the study, drafted the study protocol, interpreted the analysis, wrote the first draft, and edited, reviewed, and approved the final version of the manuscript; J.H.B., L.G., and G.F. coproposed the study and approved the final draft of the manuscript; M.C. and M.J.M. cleaned and analyzed the data and provided the study reports; H.G.R. proposed the study, provided the feedback, and reviewed the manuscript; M.B.A., M.A.K.-D., K.M.W., S.G., J.-A.H.Y., A.S., T.J., C.G.K., D.M., N.S.G., B.S., M.V.B., P.V., D.E.Y., A.M.B., M.M.H., A.H.K., T.N., J.H.C., U.G., M.S., and Z.E.B. provided the feedback and reviewed the manuscript; C.E.D., J.A.H., H.S.M., and R.F.C. provided feedback, reviewed the manuscript, and edited the manuscript; M.-A.P. coproposed the study and reviewed and edited the manuscript; M.R., A.R.H., and J.J.A. supervised the study, reviewed the proposal, and reviewed and edited the manuscript; and all coauthors approved the final draft of the manuscript.
Conflict-of-interest disclosure: J.H.B. reports honorarium from Kite Pharma, a Gilead company and research funding from Kite Pharma (a Gilead company), Genentech-Roche, Regeneron Pharmaceuticals, Janssen Pharmaceuticals, and Cargo Therapeutics. L.G. received honorarium from Bristol Myers Squibb (BMS). H.G.R. reports consultancy fees from Medexus and Vertex Therapeutics and medical monitoring for CD33CAR T for NMDP (honorary). M.A.K.-D. receives research grant support from BMS, Novartis, and Pharmacyclics, and honorarium from Kite Pharma. S.G. serves on advisory boards for BMS, Kite, Pfizer, and Sanofi. J.-A.H.Y. receives clinical trial reimbursement (through the institution) from AiCuris, AlloVir, Ansun, Basilea, F2G, Gilead, GlaxoSmithKline, Mundipharma (Cidara), Pfizer, Pulmocide, Scynexis, Takeda, and Vedanta. A.S. provides consulting services to Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, Editas Medicine, and BioLineRx; receives research funding from Crispr Therapeutics; serves as a clinical trial site principal investigator (PI) for Crispr Therapeutics, Vertex Pharmaceuticals, Novartis Pharmaceuticals, Magenta Therapeutics, and Beam Therapeutics; receives honoraria from Vindico Medical Education and Blackwood CME; serves as medical monitor for RCI BMT for NMDP; and serves as a data safety monitoring board (DSMB) member for a trial of Children’s Hospital of Philadelphia. G.F. reports advisory board/consultancy for Johnson & Johnson, Knight Pharma, Sanofi, and Astellas; speakers' bureau membership for Novartis, Johnson & Johnson, Takeda, Astellas, Knight, Libbs, Alexion/AstraZeneca, and Sanofi; manuscript writing for Johnson & Johnson, Novartis, and Astellas; research grant from Libbs; and support for meetings and travels from Celgene, Merck Sharp & Dohme (MSD), AstraZeneca, Astellas, and Kite Pharma (a Gilead company). T.J. receives research grant support from CTI Biopharma, Kartos Therapeutics, Incyte, BMS, and TScan Therapeutics, and participated on the advisory boards for BMS, Incyte, AbbVie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios Pharma, Protagonist Therapeutics, Galapagos, TScan Therapeutics, Karyopharm, MorphoSys, and IN8Bio. C.G.K. is an employee of the National Institutes of Health (NIH) and expressed the authors’ own opinions, which do not reflect the views of the NIH, the Department of Health and Human Services (DHHS), or the US government. D.M. reports consulting/advisory role for ADC Therapeutics, Genmab, Genentech (spouse), Daiichi Sankyo (spouse), and AstraZeneca (spouse); research funding for investigator initiated trials from Genentech, Genmab, Karyopharm, and AstraZeneca (spouse); and expert testimony for AstraZeneca. N.S.G. reports research grants from BMS, Cabaletta, and Affimed; serves on data safety monitoring board for Novartis; serves on advisory board for Genentech and Regeneron; and receives research funding from Regeneron and Poseida. M.V.B. received research grants paid to his institution from the NIH/National Cancer Institute (RO1), CNPq (National Council for Scientific and Technological Development), and FAPESP (São Paulo Research Foundation); and honoraria/consulting fees from Takeda, Merck, AbbVie, Janssen/Johnson & Johnson, and Knight. P.V. receives research grant support from Cidara, Mundipharma, Scynexis, Ansun, and F2G via the institution; provides consulting service for Scynexis; and receives honoraria from the MSD Manuals. D.E.Y. is an employee of the NIH and the DHHS and expressed the author’s own opinions, which do not reflect the views of the NIH, the DHHS, or the US government. A.M.B. reports serving as a clinical trial site PI for Crispr Therapeutics, Kite, Autolus, TESSA, and Lyell, and provided consulting services for Kite Pharma (a Gilead company), Autolus, and Legend. T.N. receives clinical trial support to the institution by Novartis and by Karyopharm (drug only supply), and reports consultancy for Alexion. U.G. reports serving as a site PI for Janssen, Takeda, Allogene, Cargo Therapeutics, and BMS; is a speaker's bureau member for Kite; and is an advisory board member for Kyverna and Autolus. M.S. provides consulting service for CVS Caremark and serves on the advisory boards of BMS and A28 Therapeutics. C.E.D. reports honorarium from Omeros, Alexion Pharmaceuticals, and from Alexion for a presentation at the European Society for Blood and Marrow Transplantation. H.S.M. reports advisory board/consultancy for Crispr Therapeutics, BMS, Jazz, Incyte, Sobi, Autolus, and Senti Bioscience, and serves as medical monitor for NMDP/BMT CTN. A.R.H. receives honorarium from Elsevier for the Clinical Overview Chapter. M.-A.P. reports honoraria from Adicet, Allogene, Caribou Biosciences, Celgene, BMS, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite Pharma (a Gilead company), MSD, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, Takeda, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences; has ownership interests in Omeros and OrcaBio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite Pharma (a Gilead company), Miltenyi Biotec, Nektar Therapeutics, and Novartis. R.F.C. serves as a consultant, speaker, or scientific adviser for ADMA Biologics, MSD, Takeda, Shionogi, AiCuris, Astellas, Adagio Therapeutics, Tether, Oxford Immunotec, Pfizer, Moderna, Karius, IntegerBio, Assembly Bio, and Ansun Pharmaceuticals, and received research support (paid to the institution) from MSD, Karius, AiCuris, Ansun Pharmaceuticals, Takeda, Roche/Genentech, Oxford Immunotec, Freestyle, and Eurofins Viracor. J.A.H. reports consultancy for Moderna, AlloVir, Gilead, Takeda, CSL Behring, Karius, Symbio, GeoVax, and Sanofi; reports research funding from Gilead, Takeda, MSD, GeoVax, and Sanofi; and reports significant payments (scientific advisory board) from Karius. M.R. reports being an employee of Iqvia Biotech, a clinical research organization. J.J.A. serves on the advisory committee for AscellaHealth. The remaining authors declare no competing financial interests.
Correspondence: Kitsada Wudhikarn, Division of Hematology and Center of Excellence in Translational Hematology, Faculty of Medicine, Chulalongkorn University, 1873 Rama 4 Rd, Bangkok 10330, Thailand; email: kitsada.w@chula.ac.th.
References
Author notes
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.