We conducted this systematic review to evaluate outcomes of thrombolysis followed by anticoagulation vs anticoagulation alone in pediatric patients with venous thromboembolism (VTE). This systematic review addresses mortality, VTE resolution, recurrence, bleeding, and organ-specific outcomes in 5 PICO (population, intervention, comparison, outcomes) questions on thrombolysis across pulmonary embolism (PE), extremity deep vein thrombosis (DVT), right atrial thrombosis (RAT), cerebral sinus venous thrombosis (CSVT), and renal vein thrombosis (RVT). Meta-analysis reported risk ratios or differences (95% confidence intervals [CIs]), and absolute effects per 1000 patients. Certainty of evidence was assessed using GRADE (Grading of recommendation, assessment, Development, and Evaluation) guidelines. Thirteen nonrandomized studies were included and no randomized clinical trials addressed these questions. Thrombolysis might be associated with a higher risk of major bleeding, clinically relevant nonmajor bleeding, or unspecified bleeding with risk differences of 0.09 (95% CI, −0.06 to 0.23), 0.06 (95% CI, −0.11 to 0.22), and 0.09 (95% CI, −0.04 to 0.23), respectively. In PE with hemodynamic compromise, thrombolysis might be associated with a lower risk of mortality but conclusions on PE progression were uncertain in submassive PE. In DVT, thrombolysis may have little to no effect on mortality or thrombus resolution but might be associated with lower risk of postthrombotic syndrome. In RAT, thrombolysis might have little to no effect on thrombus resolution but a higher risk of major bleeding and mortality. For CSVT and RVT, the evidence was very limited. These findings were based on very-low-certainty evidence because of confounding and imprecision from small sample sizes. This systematic review highlights key challenges in developing recommendations for thrombolysis in children with VTE.
Introduction
The incidence of venous thromboembolism (VTE) in pediatric patients has significantly increased over the last decades, especially in hospitalized children.1-6 VTE in the pediatric population can lead to significant morbidity and mortality, particularly in cases of occlusive VTE and pulmonary embolism (PE) with hemodynamic compromise.7-10
Anticoagulation (AC) is the standard of care for VTE treatment. However, thrombolytic therapy may be necessary to rapidly restore venous patency in cases of life-, organ-, or limb-threatening VTEs.11 Thrombolytic therapy can be delivered via IV systemic administration or a catheter-directed approach. Randomized controlled trials investigating the efficacy and safety of thrombolysis compared with AC for the management of VTE in children are lacking, despite its continued use in the pediatric population. This may be partly attributed to an increase in the availability of pediatric interventional radiologists with expertise in this therapy, as well as to the perceived benefits associated with thrombolysis use, including the rapid resolution of acute VTE symptoms (ie, swelling and pain) and the potential to decrease the frequency of the postthrombotic syndrome (PTS).12 Although for the most part thrombolytic therapy in pediatric VTE can be delivered safely, it can be associated with serious adverse outcomes including significant bleeding complications or rarely mortality. Despite the risks, there is limited high-quality clinical evidence to guide its use.
In 2024, the American Society of Hematology (ASH), and the International Society on Thrombosis and Haemostasis (ISTH) assembled a panel of experts to define the scope and priority areas for updated guidelines on the treatment of VTE in pediatric patients.13,14 Thrombolytic therapy emerged as a priority area because of the lack of clinical data and conflicting evidence regarding its long-term benefits (such as decreasing the incidence of PTS). In this article, we describe the methods and report the findings of a systematic review conducted to inform recommendations regarding the role of thrombolytic therapy in the treatment of VTE in the pediatric population relevant to the ASH-ISTH updated guidelines on the management of VTE.
Methods
We followed the Cochrane Handbook for Systematic Reviews of Interventions when conducting this review.15 We reported this study according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.16 A protocol was registered with PROSPERO (CRD 42024527382).
This systematic review addresses the following question: should thrombolytic therapy followed by AC vs AC alone be used in pediatric patients with the following:
submassive PE (PE with echocardiographic or biochemical evidence of right ventricular dysfunction but without hemodynamic compromise) and PE with hemodynamic compromise (massive PE/high-risk PE);
extremity deep vein thrombosis (DVT);
right atrial thrombosis (RAT);
cerebral sinus venous thrombosis (CSVT); or
renal vein thrombosis (RVT).
Search strategy
We systematically searched the published literature in PubMed, Embase, and The Cochrane Central Register of Controlled Trials from 2018 until February 2024 for relevant studies. An experienced librarian modified and updated the original search strategy used for the 2018 guidelines to align with the updated guideline questions with input from the methods team and the panel members. Modifications included the addition of direct oral anticoagulant-related terms, and removal of terms related to questions that were no longer addressed, such as protein C and S deficiency. The full search strategy pertinent to this review is outlined in the supplemental Table 1. In addition, the reference lists of the included studies were manually reviewed for additional relevant reports, and we reached out to the panel members asking for any relevant studies not retrieved by the search.
Inclusion and exclusion criteria
Studies were eligible for inclusion if the following criteria were met: (1) study design: randomized controlled trials, nonrandomized comparative studies, and noncomparative studies (eg, case series) addressing the use of thrombolysis in at least one of the VTE types of interest; (2) population: children (aged 0 to <21 years); (3) intervention: at least 1 arm receiving a thrombolytic therapy delivered systemic or via catheter-directed thrombolysis (CDT) for the management of the following VTE types: RAT, RVT (unilateral or bilateral, with or without into the inferior vena cava [IVC]), submassive PE (defined as a PE with echocardiographic or biochemical evidence of right ventricular dysfunction but without hemodynamic compromise), PE with hemodynamic compromise, extremity DVT (including upper and lower limb DVT, May-Thurner syndrome, Paget-Schroetter syndrome, and IVC atresia), and CSVT; and (4) outcomes: reporting on at least 1 outcome of interest including VTE resolution, VTE progression or recurrence, major bleeding (MB) or clinically relevant nonmajor bleeding (CRNMB) or unspecified bleeding, mortality (all-cause), and the long-term outcomes of chronic thromboembolic pulmonary hypertension (CTEPH) and PTS.
We excluded protocols, reviews (narrative and systematic), case reports; studies addressing thrombolytic therapy for arterial thrombosis; studies that reported on thrombectomy without the use of thrombolysis, studies reporting only on patients with VTE types distal DVT and cardiac thrombosis in other cardiac cavities (ventricles or left atrium); and non-English studies. We also excluded conference abstracts published before 2021.
Study selection process and data extraction
Teams of 2 reviewers independently and in duplicate screened the title and abstract followed by the full text of all the identified citations using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia, https://www.covidence.org) to assess their eligibility. Disagreements between the reviewers were resolved by a third reviewer (H.K., M.A., or R.A.M.) or by panel consensus. For each included study, 2 reviewers (H.K. and M.A.) independently extracted information about study characteristics (study design, year of publication, and countries), population (sample size, age, type of VTE, and VTE risk factors), intervention, and outcomes with follow-up duration.
Data synthesis
For outcomes with comparative data, we excluded single-arm studies, except in the case of RVT, for which evidence was particularly very limited. We conducted meta-analysis for outcomes with ≥2 studies using Review Manager web. For bleeding outcomes, we presented pooled estimates across all VTE types as well as separately for each VTE subtype. In contrast, efficacy outcomes were analyzed only by individual VTE subtype. For dichotomous outcomes without 0 events in both arms of any included study, we calculated risk ratios (RRs) with corresponding 95% confidence intervals (CIs). If at least 1 study had 0 events in both arms, we calculated risk differences (RDs; 95% CI) instead. Given that the number of studies per outcome was ≤5, we used a fixed-effects model throughout. For RR estimates, we applied the inverse variance method, whereas for RD estimates, we used the Mantel-Haenszel method because of the low number of events in both arms of included studies. RR and RD estimates were subsequently converted to absolute effects per 1000 patients (with 95% CIs) using the GRADEpro (Grading of Recommendation, Assessment, Development, and Evaluation) Guideline Development Tool (https://gradepro.org). A formal assessment of publication bias was not feasible because of the small number of studies (<10 studies) included per outcome.
Risk of bias and quality of evidence assessment
All studies were assessed for risk of bias by 2 reviewers (H.K. and M.A.) using the Risk of Bias in Non-Randomized Studies of Interventions (https://methods.cochrane.org/bias/) tool for nonrandomized comparative studies.17 Similarly, 2 reviewers (H.K. and M.A.) assessed the quality of evidence of all included studies using the GRADEpro Guideline Development Tool (https://gradepro.org). The reviewers considered study design, risk of bias, inconsistency, indirectness, imprecision, publication bias, presence of large effects, dose-response gradient, and residual confounding for the assessment of the quality of evidence for each outcome.18 The panel members assessed whether each outcome was critical or important for decision-making.
Results
Search results
A total of 17 326 records were identified through the initial electronic search. No additional records were identified through manual review of reference lists of the included studies. After removing duplicates and completing title and abstract screening, a total of 2440 full-text articles were reviewed, of these, 13 were determined to meet all criteria for inclusion (Figure 1).
Patient and outcome characteristics
The included studies reported outcomes on a total of 233 patients with VTE, 101 receiving thrombolysis followed by AC therapy, and 132 receiving AC therapy alone. Among the 13 included studies,19-31 12 were retrospective, and only 1 was prospective.22 All were nonrandomized observational studies. Two studies addressed neonates (aged <28 days), and both were in patients with RVT; 1 study only included infants (aged <6 months), whereas the rest addressed all pediatric age groups. Regarding the type of thrombolytic therapy, in 7 studies, systemic thrombolysis was used, whereas CDT was used in 2 studies, and 4 studies used either systemic thrombolysis or CDT. There was variability in the doses and protocol of thrombolysis used across the studies. The characteristics of patients and the outcomes reported in the included studies are shown in Table 1.
Characteristics of the included studies for all locations
| Author (year) . | Study design . | Age group . | Population . | Thrombolytic therapy followed by AC . | AC . | Outcomes . | Additional information on thrombolysis . | Follow-up time . |
|---|---|---|---|---|---|---|---|---|
| Ross et al20 (2020) | Retrospective cohort | 0-18 years | Submassive PE | Systemic thrombolysis or CDT (n = 14) | AC (n = 9) | Mortality, bleeding, and progression | Systemic thrombolysis or CDT | N/A |
| Ross et al20 (2020) | Retrospective cohort | 0-18 years | PE with hemodynamic compromise | Systemic thrombolysis or CDT (n = 7) | AC (n = 1) | Mortality, bleeding | Systemic thrombolysis or CDT | N/A |
| Belsky et al21 (2020) | Case series | 0-18 years | Submassive PE | CDT (n = 5) | AC (n = 3) | Thrombus resolution (complete or partial), PTS, MB, and CRNMB | Up-front thrombolytic therapy consists of suction thrombectomy or mechanical clot disruption. After clot reduction, catheter-directed lysis. Alteplase dose used for CDT was 0.03 mg/kg per hour (maximum dose 2 mg/h). | 6 months |
| Pelland-Marcotte et al19 (2019) | Retrospective cohort | 0-18 years | PE with hemodynamic compromise | Systemic thrombolysis or CDT (n = 7) | AC (n = 15) | All-cause mortality, thrombus recurrence | Systemic thrombolysis or CDT | Median, 2.4 years |
| Warad et al25 (2020) | Retrospective cohort | 0-18 years | May-Thurner | Systemic thrombolysis or CDT (n = 6) | AC (n = 3) | Mortality, thrombus resolution (complete or partial) thrombus recurrence, and PTS | Systemic and CDT (catheter-directed, 5; systemic, 1) Catheter-directed alteplase (0.5 and 1 mg/h) Systemic t-PA (0.03 and 0.06 mg/kg per hour) | Median, 1.2 years |
| van Ommen et al22 (2023) | Prospective observational | <6 months | DVT (CVAD) | Systemic thrombolysis (n = 1) | AC (n = 36) | Mortality, thrombus resolution (complete or partial) MB, CRNMB, and thrombus recurrence | Systemic r-tPA The starting and maximum dosages of r-tPA varied between 0.1 and 0.3 mg/kg per hour and between 0.3 and 0.5 mg/kg per hour, respectively. The duration of thrombolysis varied between 6 and 24 hours. | Mean, 40 days |
| Tarango et al24 (2018) | Retrospective cohort | 0-18 years | Lower extremity DVT with IVC atresia | CDT (n = 12) | AC (n = 6) | Mortality, thrombus recurrence, and PTS | Percutaneous endovascular thrombolysis in addition to AC therapy, n = 12 | Range, 6-53 months |
| Kumar et al23 (2019) | Retrospective cohort | 0-18 years | Paget-Schroetter syndrome | Systemic thrombolysis or CDT (n = 10) | AC (n = 12) | Mortality, PTS, MB, CRNMB thrombus resolution (complete or partial), and thrombus recurrence | 10 patients underwent up-front thrombolytic therapy (9 patients had CDT and 3 patients had pharmacomechanical thrombolysis [AngioJet]). CDT was performed through an infusion catheter placed in the interventional radiology suite. r-tPA (dose 0.03 mg/kg per hour [maximum dose 2 mg/h]) was infused directly into the thrombus. Median (IQR) duration of CDT in all patients was 48 hours (24-72). | Median, ∼4 years |
| Goldenberg et al26 (2007) | Retrospective cohort | 6 months to 21 years | Lower extremity DVT, different risk factors | Systemic or CDT, thrombolysis (n = 9) | AC (n = 13) | Thrombus resolution (complete or partial), PTS, and MB | Systemic and CDT (n = 7 systemic, n = 2 CDT) tPA, infusion was begun at 0.03 mg/kg per hour. Maximum duration of systemic tPA infusion was 96 hours. | 24 months |
| van Ommen et al22 (2023) | Prospective observational | <6 months | RAT CVAD | Systemic thrombolysis (n = 6) | AC (n = 14) | MB, CRNMB, extension, thrombus resolution (complete or partial), and recurrence of thrombus | Six infants were treated with r-tPA. The starting and maximum dosages of r-tPA varied between 0.1 and 0.3 .5 mg/kg per hour and between 0.3 and 0.5 mg/kg per hour, respectively. The duration of thrombolysis varied between 6 and 24 hours. | Mean, 56 days |
| Odaman Al et al27 (2022) | Case series | 0-18 years | RAT | Systemic thrombolysis (n = 4) | AC (n = 9) | Mortality, thrombus resolution (complete or partial), and bleeding | Systemic r-tPA: 0.2-0.5 mg/kg per hour (6-hour infusion); 0.01-0.06 mg/kg per hour (24-hour infusion) | 6.5 months |
| Kara et al28 (2021) | Retrospective observational | 0-18 years | RAT | Systemic thrombolysis (n = 7) | AC (n = 4) | Mortality, thrombus resolution (complete or partial) | Systemic thrombolysis. In 3 patients, r-tPA was started with low dose (0.01 mg/kg per hour) and increased gradually to 0.06 mg/kg per hour. In 3 patients, r-tPA was started with standard dose (0.5 mg/kg per hour). In 1 patient, r-tPA was started with low dose (0.01 mg/kg per hour) and increased to standard dose. | N/A |
| Rong et al29 (2020) | Case series | 3-10 years | CSVT (nephrotic syndrome) | Systemic thrombolysis (n = 6) | AC (n = 4) | Mortality, thrombus resolution (complete or partial), and thrombus recurrence | Urokinase was used as a thrombolytic therapy as well as xueshuantong, which is a Chinese patent medicine containing extractive ingredients of herb for AC (n = 6/10). | N/A |
| Likoho et al31 (2023) | Retrospective observational | Neonates (<28 days) | Bilateral RVT | Systemic thrombolysis (n = 4) | AC (n = 3) | Mortality, bleeding, thrombus resolution (complete or partial), thrombus recurrence, proteinuria, CKD, HBP, and long-term kidney feature | Received fibrinolysis with tPA | Median, 5.7 years |
| Niada et al30 (2018) | Case series | Neonates (<28 days) | Unilateral RVT | Systemic thrombolysis (n = 3) | N/A | Thrombus resolution (complete or partial), progression, atrophic kidney function, normal HBP, and CKD | Patients 3, 4, and 5 received antithrombotic treatment with r-tPA. Thrombolysis was attempted as follows: r-tPA 0.1 mg/kg as bolus, followed by 0.3 mg/kg over 3 hours (patients 4 and 5) and 0.9 mg/kg over 3 hours in patient 3. If repermeabilization was deemed unsatisfactory, repeat doses were administered, with 0.3 mg/kg over 3 hours after a time interval of 12-24 hours after the previous perfusion. | 6 months |
| Author (year) . | Study design . | Age group . | Population . | Thrombolytic therapy followed by AC . | AC . | Outcomes . | Additional information on thrombolysis . | Follow-up time . |
|---|---|---|---|---|---|---|---|---|
| Ross et al20 (2020) | Retrospective cohort | 0-18 years | Submassive PE | Systemic thrombolysis or CDT (n = 14) | AC (n = 9) | Mortality, bleeding, and progression | Systemic thrombolysis or CDT | N/A |
| Ross et al20 (2020) | Retrospective cohort | 0-18 years | PE with hemodynamic compromise | Systemic thrombolysis or CDT (n = 7) | AC (n = 1) | Mortality, bleeding | Systemic thrombolysis or CDT | N/A |
| Belsky et al21 (2020) | Case series | 0-18 years | Submassive PE | CDT (n = 5) | AC (n = 3) | Thrombus resolution (complete or partial), PTS, MB, and CRNMB | Up-front thrombolytic therapy consists of suction thrombectomy or mechanical clot disruption. After clot reduction, catheter-directed lysis. Alteplase dose used for CDT was 0.03 mg/kg per hour (maximum dose 2 mg/h). | 6 months |
| Pelland-Marcotte et al19 (2019) | Retrospective cohort | 0-18 years | PE with hemodynamic compromise | Systemic thrombolysis or CDT (n = 7) | AC (n = 15) | All-cause mortality, thrombus recurrence | Systemic thrombolysis or CDT | Median, 2.4 years |
| Warad et al25 (2020) | Retrospective cohort | 0-18 years | May-Thurner | Systemic thrombolysis or CDT (n = 6) | AC (n = 3) | Mortality, thrombus resolution (complete or partial) thrombus recurrence, and PTS | Systemic and CDT (catheter-directed, 5; systemic, 1) Catheter-directed alteplase (0.5 and 1 mg/h) Systemic t-PA (0.03 and 0.06 mg/kg per hour) | Median, 1.2 years |
| van Ommen et al22 (2023) | Prospective observational | <6 months | DVT (CVAD) | Systemic thrombolysis (n = 1) | AC (n = 36) | Mortality, thrombus resolution (complete or partial) MB, CRNMB, and thrombus recurrence | Systemic r-tPA The starting and maximum dosages of r-tPA varied between 0.1 and 0.3 mg/kg per hour and between 0.3 and 0.5 mg/kg per hour, respectively. The duration of thrombolysis varied between 6 and 24 hours. | Mean, 40 days |
| Tarango et al24 (2018) | Retrospective cohort | 0-18 years | Lower extremity DVT with IVC atresia | CDT (n = 12) | AC (n = 6) | Mortality, thrombus recurrence, and PTS | Percutaneous endovascular thrombolysis in addition to AC therapy, n = 12 | Range, 6-53 months |
| Kumar et al23 (2019) | Retrospective cohort | 0-18 years | Paget-Schroetter syndrome | Systemic thrombolysis or CDT (n = 10) | AC (n = 12) | Mortality, PTS, MB, CRNMB thrombus resolution (complete or partial), and thrombus recurrence | 10 patients underwent up-front thrombolytic therapy (9 patients had CDT and 3 patients had pharmacomechanical thrombolysis [AngioJet]). CDT was performed through an infusion catheter placed in the interventional radiology suite. r-tPA (dose 0.03 mg/kg per hour [maximum dose 2 mg/h]) was infused directly into the thrombus. Median (IQR) duration of CDT in all patients was 48 hours (24-72). | Median, ∼4 years |
| Goldenberg et al26 (2007) | Retrospective cohort | 6 months to 21 years | Lower extremity DVT, different risk factors | Systemic or CDT, thrombolysis (n = 9) | AC (n = 13) | Thrombus resolution (complete or partial), PTS, and MB | Systemic and CDT (n = 7 systemic, n = 2 CDT) tPA, infusion was begun at 0.03 mg/kg per hour. Maximum duration of systemic tPA infusion was 96 hours. | 24 months |
| van Ommen et al22 (2023) | Prospective observational | <6 months | RAT CVAD | Systemic thrombolysis (n = 6) | AC (n = 14) | MB, CRNMB, extension, thrombus resolution (complete or partial), and recurrence of thrombus | Six infants were treated with r-tPA. The starting and maximum dosages of r-tPA varied between 0.1 and 0.3 .5 mg/kg per hour and between 0.3 and 0.5 mg/kg per hour, respectively. The duration of thrombolysis varied between 6 and 24 hours. | Mean, 56 days |
| Odaman Al et al27 (2022) | Case series | 0-18 years | RAT | Systemic thrombolysis (n = 4) | AC (n = 9) | Mortality, thrombus resolution (complete or partial), and bleeding | Systemic r-tPA: 0.2-0.5 mg/kg per hour (6-hour infusion); 0.01-0.06 mg/kg per hour (24-hour infusion) | 6.5 months |
| Kara et al28 (2021) | Retrospective observational | 0-18 years | RAT | Systemic thrombolysis (n = 7) | AC (n = 4) | Mortality, thrombus resolution (complete or partial) | Systemic thrombolysis. In 3 patients, r-tPA was started with low dose (0.01 mg/kg per hour) and increased gradually to 0.06 mg/kg per hour. In 3 patients, r-tPA was started with standard dose (0.5 mg/kg per hour). In 1 patient, r-tPA was started with low dose (0.01 mg/kg per hour) and increased to standard dose. | N/A |
| Rong et al29 (2020) | Case series | 3-10 years | CSVT (nephrotic syndrome) | Systemic thrombolysis (n = 6) | AC (n = 4) | Mortality, thrombus resolution (complete or partial), and thrombus recurrence | Urokinase was used as a thrombolytic therapy as well as xueshuantong, which is a Chinese patent medicine containing extractive ingredients of herb for AC (n = 6/10). | N/A |
| Likoho et al31 (2023) | Retrospective observational | Neonates (<28 days) | Bilateral RVT | Systemic thrombolysis (n = 4) | AC (n = 3) | Mortality, bleeding, thrombus resolution (complete or partial), thrombus recurrence, proteinuria, CKD, HBP, and long-term kidney feature | Received fibrinolysis with tPA | Median, 5.7 years |
| Niada et al30 (2018) | Case series | Neonates (<28 days) | Unilateral RVT | Systemic thrombolysis (n = 3) | N/A | Thrombus resolution (complete or partial), progression, atrophic kidney function, normal HBP, and CKD | Patients 3, 4, and 5 received antithrombotic treatment with r-tPA. Thrombolysis was attempted as follows: r-tPA 0.1 mg/kg as bolus, followed by 0.3 mg/kg over 3 hours (patients 4 and 5) and 0.9 mg/kg over 3 hours in patient 3. If repermeabilization was deemed unsatisfactory, repeat doses were administered, with 0.3 mg/kg over 3 hours after a time interval of 12-24 hours after the previous perfusion. | 6 months |
CKD, chronic kidney disease; CVAD, central venous access device; HBP, high blood pressure; IQR, interquartile range; N/A, not available; r-tPA, recombinant tissue plasminogen activator; tPA, tissue plasminogen activator.
Safety of thrombolysis in patients with VTE
Seven studies (N = 162) compared bleeding rates between thrombolysis followed by AC (n = 62) and AC alone (n = 100). When assessed across different VTE locations, thrombolysis might be associated with a higher risk of MB (5 studies, n = 118), CRNMB (4 studies, n = 96), and unspecified bleeding (5 studies, n = 83), with absolute effect risk (of 90 more per 1000 (95% CI, 60 fewer to 230 more), 60 more per 1000 (95% CI, 110 fewer to 220 more), and 90 more per 1000 (95% CI, 40 fewer to 230 more), respectively. Forest plots showing the individual study estimates and the pooled estimates for each outcome are displayed in Figure 2. These estimates were based on very low certainty in the evidence due to concerns related to risk of bias (selection bias and confounding) and imprecision.
Forest plots showing individual study and pooled estimates for bleeding outcomes comparing thrombolysis followed by AC vs AC alone in patients with VTE (different types). (A) Major bleeding, (B) clinically relevant nonmajor bleeding, and (C) bleeding (unspecified). M-H, Mantel-Haenszel.
Forest plots showing individual study and pooled estimates for bleeding outcomes comparing thrombolysis followed by AC vs AC alone in patients with VTE (different types). (A) Major bleeding, (B) clinically relevant nonmajor bleeding, and (C) bleeding (unspecified). M-H, Mantel-Haenszel.
Efficacy and safety of thrombolysis across different VTE types
Submassive PE
Two nonrandomized observational studies compared the use of thrombolytic therapy followed by AC (n = 19) with AC alone (n = 12) in pediatric patients with submassive PE.20,21 The outcomes assessed in these studies included mortality, thrombus resolution, progression to massive PE, bleeding, and CTEPH. Compared with AC alone, thrombolytic therapy followed by AC might be associated with a lower risk of progression from submassive to massive PE with an absolute risk of 40 fewer per 1000 (95% CI, 106 fewer to 892 more) but might have no effect on the rates of thrombus resolution with an absolute risk of 0 fewer per 1000 (95% CI, 360 fewer to 560 more). There were no events of CTEPH (defined as pulmonary hypertension on echocardiogram at least 6 months after PE without other known cause) or bleeding in either arm. For the study reporting mortality outcome, mortality was reported in 1 patient (1/9) in the AC-alone arm compared with 0 (0/14) in the thrombolysis arm.20 The certainty of evidence for all the outcomes is very low because of concerns about risk of bias and imprecision. Table 2 summarizes the findings for thrombolysis followed by AC vs AC alone in pediatric patients with submassive PE.
GRADE summary of evidence for thrombolysis followed by AC vs AC alone in patients with submassive PE
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 120 | Nonrandomized studies | 0/14 (0.0) | 1/9 (11.1) | Not estimable | Not estimable | ⊕○○○ Very low | Critical |
| Thrombus resolution (follow-up: 6 months; assessed with: complete or partial resolution) | |||||||
| 121 | Nonrandomized studies | 5/5 (100.0) | 3/3 (100.0) | RR, 1.00 (0.64-1.56) | 0 fewer per 1000 (360 fewer to 560 more) | ⊕○○○ Very low∗,† | Critical |
| Progression (submassive to massive) | |||||||
| 120 | Nonrandomized studies | 1/14 (7.1) | 1/9 (11.1) | RR, 0.64 (0.05-9.03) | 40 fewer per 1000 (106 fewer to 892 more) | ⊕○○○ Very low∗,† | Critical |
| Chronic thromboembolic pulmonary hypertension (follow-up: 6 months) | |||||||
| 121 | Nonrandomized studies | 0/5 (0.0) | 0/2 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified) | |||||||
| 220,21 | Nonrandomized studies | 0/19 (0.0) | 0/9 (0.0) | Not pooled | Not estimable | ⊕○○○ Very low∗,† | Critical |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 120 | Nonrandomized studies | 0/14 (0.0) | 1/9 (11.1) | Not estimable | Not estimable | ⊕○○○ Very low | Critical |
| Thrombus resolution (follow-up: 6 months; assessed with: complete or partial resolution) | |||||||
| 121 | Nonrandomized studies | 5/5 (100.0) | 3/3 (100.0) | RR, 1.00 (0.64-1.56) | 0 fewer per 1000 (360 fewer to 560 more) | ⊕○○○ Very low∗,† | Critical |
| Progression (submassive to massive) | |||||||
| 120 | Nonrandomized studies | 1/14 (7.1) | 1/9 (11.1) | RR, 0.64 (0.05-9.03) | 40 fewer per 1000 (106 fewer to 892 more) | ⊕○○○ Very low∗,† | Critical |
| Chronic thromboembolic pulmonary hypertension (follow-up: 6 months) | |||||||
| 121 | Nonrandomized studies | 0/5 (0.0) | 0/2 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified) | |||||||
| 220,21 | Nonrandomized studies | 0/19 (0.0) | 0/9 (0.0) | Not pooled | Not estimable | ⊕○○○ Very low∗,† | Critical |
ROBINS-I, Risk Of Bias in Nonrandomized Studies-of Interventions.
Risk of bias, assessed using ROBINS-I, was judged to be serious because of selection bias without adjustment for potential confounders.
Imprecision because of the small number of included patients and patients with events in the included studies.
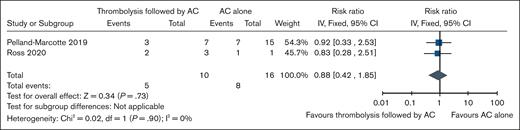
PE with hemodynamic compromise
Two nonrandomized observational studies compared the outcomes of thrombolytic therapy followed by AC (n = 14) with AC alone (n = 16) in patients with PE with hemodynamic compromise.19,20 Compared with AC alone, thrombolysis might be associated with lower mortality rates in patients with massive PE with an absolute risk of 60 fewer per 1000 (95% CI, 290 fewer to 425 more) but might be associated with higher risk of thrombosis recurrence with absolute risk of 228 more per 1000 (95% CI, 86 fewer to 1000 more). Bleeding was reported in only 1 study, and absolute effect was not estimable, with 1 event among 7 patients in the thrombolysis arm vs no bleeding in 1 patient in the AC-alone arm. The certainty of evidence is very low for all the outcomes because of concerns about risk of bias and imprecision. Table 3 summarizes the findings for thrombolysis followed by AC vs AC alone in pediatric patients with PE with hemodynamic compromise. Forest plots showing individual study data and pooled estimates for these outcomes are presented in Figure 3.
GRADE summary of evidence for thrombolysis followed by AC vs AC alone in patients with massive PE
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 219,20 | Nonrandomized studies | 6/10 (50.0) | 8/16 (50.0) | RR, 0.88 (0.42-1.85) | 60 fewer per 1000 (290 fewer to 425 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 119 | Nonrandomized studies | 3/7 (42.9) | 3/15 (20.0) | RR, 2.14 (0.57-8.09) | 228 more per 1000 (86 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified bleed [intracranial/extracranial]) | |||||||
| 120 | Nonrandomized studies | 1/7 (14.3) | 0/1 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 219,20 | Nonrandomized studies | 6/10 (50.0) | 8/16 (50.0) | RR, 0.88 (0.42-1.85) | 60 fewer per 1000 (290 fewer to 425 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 119 | Nonrandomized studies | 3/7 (42.9) | 3/15 (20.0) | RR, 2.14 (0.57-8.09) | 228 more per 1000 (86 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified bleed [intracranial/extracranial]) | |||||||
| 120 | Nonrandomized studies | 1/7 (14.3) | 0/1 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
Risk of bias, assessed using ROBINS-I, was judged to be serious because of selection bias without adjustment for potential confounders.
Imprecision because of the small number of included patients and patients with events in the included studies.
Forest plot showing individual study and pooled estimates for mortality (all-cause mortality) comparing thrombolysis followed by AC vs AC alone in patients with massive PE.
Forest plot showing individual study and pooled estimates for mortality (all-cause mortality) comparing thrombolysis followed by AC vs AC alone in patients with massive PE.
Extremity DVT
Five nonrandomized comparative studies addressing the outcomes of thrombolysis followed by AC vs AC alone in patients with DVT were included.22-26 The total number of patients receiving thrombolysis followed by AC was 38, whereas 70 received AC alone. Risk factors for extremity DVT varied across studies. One study focused on patients with IVC atresia,24 another on those with May-Thurner syndrome,25 a third on central venous catheter–related thrombosis,22 and another on Paget-Schroetter syndrome.23 Additionally, 1 study included patients with multiple risk factors.26
When compared with AC alone, thrombolysis followed by AC may be associated with little to no effect on mortality, and thrombus resolution (complete or partial) with an absolute risk of 10 fewer per 1000 (95% CI, 140 fewer to 120 more), and 0 fewer per 1000 (95% CI, 186 fewer to 248 more), respectively. Thrombolysis followed by AC might be associated with a higher rate of thrombus recurrence when compared with AC alone, with an absolute risk of 50 more per 1000 (95% CI, 160 fewer to 260 more) but may be associated with a lower rate of PTS, with an absolute risk of 31 fewer per 1000 (95% CI, 199 fewer to 291 more). For bleeding, thrombolysis followed by AC may be associated with a higher rate of MB with 110 more per 1000 (95% CI, 60 fewer to 270 more) but might have little to no effect on CRNMB with absolute risk of 0 fewer per 1000 (95% CI, 210 fewer to 210 more). These results were based on very low certainty in the evidence because of concerns related to imprecision and risk of bias in the included studies because they are nonrandomized studies without any adjustment for confounders and imprecision. Table 4 summarizes the findings for thrombolysis followed by AC vs AC alone in pediatric patients with DVT. Forest plots showing individual study data and pooled estimates for these outcomes are presented in the Figure 4.
GRADE summary of findings for thrombolysis followed by AC vs AC alone in patients with extremity DVT
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 522-26 | Nonrandomized studies | 0/38 (0.0) | 4/59 (6.8) | RD, −0.01 (−0.14 to 0.12) | 10 fewer per 1000 (140 fewer to 120 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (assessed with: complete or partial resolution) | |||||||
| 422,23,25,26 | Nonrandomized studies | 21/26 (80.8) | 41/53 (77.4) | RR, 1.00 (0.76-1.32) | 0 fewer per 1000 (186 fewer to 248 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 422-25 | Nonrandomized studies | 6/29 (20.7) | 3/46 (6.5) | RD, 0.05 (−0.16 to 0.26) | 50 more per 1000 (160 fewer to 260 more) | ⊕○○○ Very low∗,† | Critical |
| Postthrombotic syndrome | |||||||
| 423-26 | Nonrandomized studies | 13/36 (36.1) | 13/34 (38.2) | RR, 0.92 (0.48-1.76) | 31 fewer per 1000 (199 fewer to 291 more) | ⊕○○○ Very low∗,† | Critical |
| Major bleeding | |||||||
| 422,23,25,26 | Nonrandomized studies | 2/26 (7.7) | 3/64 (4.7) | RD, 0.11 (−0.06 to 0.27) | 110 more per 1000 (60 fewer to 270 more) | ⊕○○○ Very low∗,† | Critical |
| Clinically relevant non major bleeding | |||||||
| 422,23,25 | Nonrandomized studies | 0/17 (0.0) | 1/51 (2.0) | RD, 0.00 (−0.21 to 0.21) | 0 fewer per 1000 (210 fewer to 210 more) | ⊕○○○ Very low∗,† | Critical |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 522-26 | Nonrandomized studies | 0/38 (0.0) | 4/59 (6.8) | RD, −0.01 (−0.14 to 0.12) | 10 fewer per 1000 (140 fewer to 120 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (assessed with: complete or partial resolution) | |||||||
| 422,23,25,26 | Nonrandomized studies | 21/26 (80.8) | 41/53 (77.4) | RR, 1.00 (0.76-1.32) | 0 fewer per 1000 (186 fewer to 248 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 422-25 | Nonrandomized studies | 6/29 (20.7) | 3/46 (6.5) | RD, 0.05 (−0.16 to 0.26) | 50 more per 1000 (160 fewer to 260 more) | ⊕○○○ Very low∗,† | Critical |
| Postthrombotic syndrome | |||||||
| 423-26 | Nonrandomized studies | 13/36 (36.1) | 13/34 (38.2) | RR, 0.92 (0.48-1.76) | 31 fewer per 1000 (199 fewer to 291 more) | ⊕○○○ Very low∗,† | Critical |
| Major bleeding | |||||||
| 422,23,25,26 | Nonrandomized studies | 2/26 (7.7) | 3/64 (4.7) | RD, 0.11 (−0.06 to 0.27) | 110 more per 1000 (60 fewer to 270 more) | ⊕○○○ Very low∗,† | Critical |
| Clinically relevant non major bleeding | |||||||
| 422,23,25 | Nonrandomized studies | 0/17 (0.0) | 1/51 (2.0) | RD, 0.00 (−0.21 to 0.21) | 0 fewer per 1000 (210 fewer to 210 more) | ⊕○○○ Very low∗,† | Critical |
Risk of bias, assessed using ROBINS-I, was judged to be serious because of selection bias without adjustment for potential confounders.
Imprecision because of the small number of included patients and patients with events in the included studies.
Forest plots showing individual study and pooled estimates for outcomes comparing thrombolysis followed by AC vs AC alone in patients with extremity DVT. (A) Resolution of thrombus (complete or partial), (B) postthrombotic syndrome, (C) mortality (all-cause mortality), (D) recurrence of thrombus, (E) MB, (F) clinically relevant non major bleeding. M-H, Mantel-Haenszel.
Forest plots showing individual study and pooled estimates for outcomes comparing thrombolysis followed by AC vs AC alone in patients with extremity DVT. (A) Resolution of thrombus (complete or partial), (B) postthrombotic syndrome, (C) mortality (all-cause mortality), (D) recurrence of thrombus, (E) MB, (F) clinically relevant non major bleeding. M-H, Mantel-Haenszel.
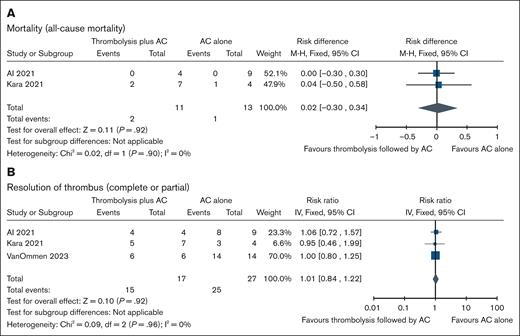
RAT
We included 3 comparative nonrandomized studies (N = 44 patients) addressing the use of thrombolysis followed by AC (n = 17) vs AC alone (n = 27) in pediatric patients with RAT.22,27,28 Compared with AC alone, thrombolytic therapy followed by AC may be associated with little to no effect on risk of thrombus resolution (complete or partial) with absolute risk of 9 more per 1000 (95% CI, 148 fewer to 204 more) but might be associated with higher risk of major bleeds, and mortality with absolute risk of 95 more per 1000 (95% CI, 59 fewer to 1000 more), and 20 more per 1000 (95% CI, 300 fewer to 340 more), respectively. There were 2 events of bleeding (unspecified) among 4 individuals in the thrombolysis followed by AC arm vs no events in 9 individuals in AC-alone arm. For CRNMB and thrombus recurrence, no events occurred in either arm. The certainty of evidence for all outcomes is very low owing to concerns about the risk of bias and imprecision. Table 5 summarizes the findings for thrombolysis followed by AC vs AC alone in pediatric patients with RAT. Forest plots showing individual study data and pooled estimates for these outcomes are presented in the Figure 5.
GRADE summary of evidence for thrombolysis followed by AC vs AC alone in patients with RAT
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 227,28 | Nonrandomized studies | 2/11 (18.2) | 1/13 (7.7) | RD, 0.02 (−0.30 to 0.34) | 20 more per 1000 (300 fewer to 340 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (assessed with: complete or partial resolution) | |||||||
| 322,27,28 | Nonrandomized studies | 15/17 (88.2) | 25/27 (92.6) | RR, 1.01 (0.84-1.22) | 9 more per 1000 (148 fewer to 204 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 122 | Nonrandomized studies | 0/6 (0.0) | 0/14 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified) | |||||||
| 127 | Nonrandomized studies | 2/4 (50.0) | 0/9 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Major bleeding | |||||||
| 122 | Nonrandomized studies | 1/6 (16.7) | 1/14 (7.1) | RR, 2.33 (0.17-31.46) | 95 more per 1000 (59 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Clinically relevant non major bleeding | |||||||
| 122 | Nonrandomized studies | 0/6 (0.0) | 0/14 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality) | |||||||
| 227,28 | Nonrandomized studies | 2/11 (18.2) | 1/13 (7.7) | RD, 0.02 (−0.30 to 0.34) | 20 more per 1000 (300 fewer to 340 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (assessed with: complete or partial resolution) | |||||||
| 322,27,28 | Nonrandomized studies | 15/17 (88.2) | 25/27 (92.6) | RR, 1.01 (0.84-1.22) | 9 more per 1000 (148 fewer to 204 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence | |||||||
| 122 | Nonrandomized studies | 0/6 (0.0) | 0/14 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (assessed with: unspecified) | |||||||
| 127 | Nonrandomized studies | 2/4 (50.0) | 0/9 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Major bleeding | |||||||
| 122 | Nonrandomized studies | 1/6 (16.7) | 1/14 (7.1) | RR, 2.33 (0.17-31.46) | 95 more per 1000 (59 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Clinically relevant non major bleeding | |||||||
| 122 | Nonrandomized studies | 0/6 (0.0) | 0/14 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
Risk of bias, assessed using ROBINS-I, was judged to be serious because of selection bias without adjustment for confounders.
Imprecision because of small number of included patients and patients with events in the included studies.
Forest plots showing individual study and pooled estimates for outcomes comparing thrombolysis followed by AC vs AC alone in patients with RAT. (A) Mortality (all-cause mortality), and (B) resolution of thrombus (complete or partial). M-H, Mantel-Haenszel.
Forest plots showing individual study and pooled estimates for outcomes comparing thrombolysis followed by AC vs AC alone in patients with RAT. (A) Mortality (all-cause mortality), and (B) resolution of thrombus (complete or partial). M-H, Mantel-Haenszel.
CSVT
One study (N = 10 patients) addressed the outcomes of thrombolytic therapy followed by AC vs AC alone in pediatric patients with CSVT.29 The study reported the outcomes for 6 pediatric patients receiving systemic thrombolysis followed by AC and 4 patients who received AC alone. All patients had nephrotic syndrome and CSVT. The outcomes reported were mortality, thrombus resolution (complete or partial), and thrombus recurrence. The absolute effect of thrombolysis followed by AC compared with AC alone for complete resolution was 248 more per 1000 (95% CI, 210 fewer to 1000 more). There were no deaths or thrombus recurrences in either group. These results were based on very low certainty evidence because of the very small sample size, leading to imprecision, in addition to high risk of bias. Table 6 summarizes the findings for thrombolysis followed by AC vs AC alone in pediatric patients with CSVT.
GRADE summary of findings for thrombolysis followed by AC vs AC alone in patients with CSVT
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality; follow-up: mean, 3.5 years) | |||||||
| 129 | Nonrandomized studies | 0/6 (0.0) | 0/4 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (complete; follow-up: mean, 3.5 years; assessed with: imaging) | |||||||
| 129 | Nonrandomized studies | 6/6 (100.0) | 3/4 (75.0) | RR, 1.33 (0.72-2.44) | 248 more per 1000 (210 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (follow-up: mean, 3.5 years; assessed with: complete or partial resolution) | |||||||
| 129 | Nonrandomized studies | 6/6 (100.0) | 4/4 (100.0) | RR, 1.00 (0.70-1.43) | 0 fewer per 1000 (300 fewer to 430 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence (follow-up: mean, 3.5 years) | |||||||
| 129 | Nonrandomized studies | 0/6 (0.0) | 0/4 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (assessed with: all-cause mortality; follow-up: mean, 3.5 years) | |||||||
| 129 | Nonrandomized studies | 0/6 (0.0) | 0/4 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (complete; follow-up: mean, 3.5 years; assessed with: imaging) | |||||||
| 129 | Nonrandomized studies | 6/6 (100.0) | 3/4 (75.0) | RR, 1.33 (0.72-2.44) | 248 more per 1000 (210 fewer to 1000 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (follow-up: mean, 3.5 years; assessed with: complete or partial resolution) | |||||||
| 129 | Nonrandomized studies | 6/6 (100.0) | 4/4 (100.0) | RR, 1.00 (0.70-1.43) | 0 fewer per 1000 (300 fewer to 430 more) | ⊕○○○ Very low∗,† | Critical |
| Thrombus recurrence (follow-up: mean, 3.5 years) | |||||||
| 129 | Nonrandomized studies | 0/6 (0.0) | 0/4 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
Risk of bias was assessed using ROBINS-I, we have concerns because of selection bias and confounding.
We downgraded twice for imprecision because of the small sample size and small number of events.
RVT
Two observational studies (N = 10 patients) assessing thrombolysis followed by AC (n = 7) vs AC alone (n = 3) in pediatric patients with RVT were included.30,31 In the thrombolysis followed by AC group, 1 event of proteinuria was reported among 4 patients, compared with none (0/3) in the AC-alone group. Of 7 patients who received thrombolysis followed by AC, 1 had chronic kidney disease and high blood pressure on follow-up compared with 0 of 3 in the AC arm. When assessed by kidney scintigraphy, all 3 assessed patients in the thrombolysis followed by AC group had atrophic kidney. Of 7 patients in the thrombolysis followed by AC group, 5 (71%) had complete or partial thrombus resolution, compared with 3 of 3 patients (100%) in the AC-alone group. There was 1 death among 3 patients in the AC-alone arm compared with 0 of 4 in the thrombolysis followed by AC arm. There were no patients with a thrombus recurrence in either group. Overall, the certainty of these estimated effects is very low owing to the critical risk of bias and very serious imprecision in the included studies. Table 7 summarizes the findings for thrombolysis followed by AC vs AC alone in patients with RVT.
GRADE summary of findings for thrombolysis followed by AC vs AC alone in patients with RVT
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (follow-up: range, 6 months to 5.7 years; assessed with: all-cause mortality) | |||||||
| 131 | Nonrandomized studies | 0/4 (0.0) | 1/3 (33.3) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (follow-up: median, 5.7 years; assessed with: not specified) | |||||||
| 131 | Nonrandomized studies | 3/4 (75.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,†,‡ | Critical |
| Thrombus recurrence (follow-up: mean, 5.7 years) | |||||||
| 131 | Nonrandomized studies | 0/4 (0.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Thrombus progression (follow-up: mean, 6 months) | |||||||
| 130 | Nonrandomized studies | 1/3 (33.3) | — | — | — | ⊕○○○ Very low∗,† | Critical |
| Proteinuria (follow-up: median, 5.7 years) | |||||||
| 131 | Nonrandomized studies | 1/4 (25.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Chronic kidney disease (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| High blood pressure (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 0/3 (0.0) | Not estimable | ⊕○○○ Very low∗,† | Critical | |
| Long-term pathological kidney features (follow-up: median, 5.7 years; assessed with: pathological kidney features, defined as proteinuria, kidney atrophy, hypertension, or chronic kidney disease) | |||||||
| 131 | Nonrandomized studies | 3/4 (75.0) | 2/3 (66.7) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Atrophic nonfunctioning kidney (follow-up: mean, 6 months; assessed with: renal scintigraphy) | |||||||
| 130 | Nonrandomized studies | 3/3 (100.0) | — | — | — | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (follow-up: range, 6 months to 5.7 years; assessed with: complete or partial resolution) | |||||||
| 230,31 | Nonrandomized studies | 5/7 (71.4) | 3/3 (100.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Important |
| Complete clot resolution (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 1/3 (33.3) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Important |
| No. of studies . | Study design . | No. of patients (%) . | Effect . | Certainty . | Importance . | ||
|---|---|---|---|---|---|---|---|
| Thrombolysis followed by AC . | AC alone . | Relative (95% CI) . | Absolute (95% CI) . | ||||
| Mortality (follow-up: range, 6 months to 5.7 years; assessed with: all-cause mortality) | |||||||
| 131 | Nonrandomized studies | 0/4 (0.0) | 1/3 (33.3) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Bleeding (follow-up: median, 5.7 years; assessed with: not specified) | |||||||
| 131 | Nonrandomized studies | 3/4 (75.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,†,‡ | Critical |
| Thrombus recurrence (follow-up: mean, 5.7 years) | |||||||
| 131 | Nonrandomized studies | 0/4 (0.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Thrombus progression (follow-up: mean, 6 months) | |||||||
| 130 | Nonrandomized studies | 1/3 (33.3) | — | — | — | ⊕○○○ Very low∗,† | Critical |
| Proteinuria (follow-up: median, 5.7 years) | |||||||
| 131 | Nonrandomized studies | 1/4 (25.0) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Chronic kidney disease (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 0/3 (0.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| High blood pressure (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 0/3 (0.0) | Not estimable | ⊕○○○ Very low∗,† | Critical | |
| Long-term pathological kidney features (follow-up: median, 5.7 years; assessed with: pathological kidney features, defined as proteinuria, kidney atrophy, hypertension, or chronic kidney disease) | |||||||
| 131 | Nonrandomized studies | 3/4 (75.0) | 2/3 (66.7) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Critical |
| Atrophic nonfunctioning kidney (follow-up: mean, 6 months; assessed with: renal scintigraphy) | |||||||
| 130 | Nonrandomized studies | 3/3 (100.0) | — | — | — | ⊕○○○ Very low∗,† | Critical |
| Thrombus resolution (follow-up: range, 6 months to 5.7 years; assessed with: complete or partial resolution) | |||||||
| 230,31 | Nonrandomized studies | 5/7 (71.4) | 3/3 (100.0) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Important |
| Complete clot resolution (follow-up: range, 6 months to 5.7 years) | |||||||
| 230,31 | Nonrandomized studies | 1/7 (14.3) | 1/3 (33.3) | Not estimable | Not estimable | ⊕○○○ Very low∗,† | Important |
F/U, follow up.
Risk of bias was assessed using ROBINS-I, we have concerns because of selection bias and confounding.
We downgraded twice for imprecision because of the small sample size and small number of events.
Niada et al30: dilated lateral ventricles on F/U: 1 of 3 probably secondary to an intraventricular hemorrhage.
Risk of bias and quality of evidence assessment
All the included studies were judged to have high risk of bias on the ROBINS-I tool. This is due to selection bias, because none of the studies adjusted for potential confounders, and patients were referred to thrombolysis based on the physician’s discretion and baseline characteristics. There were also concerns about imprecision because of the small number of events and sample size, which led to wide CIs. The overall certainty of evidence was judged as very low.
Discussion
Summary of results
This systematic review evaluated the benefits and risks of using thrombolytic therapy followed by AC compared with AC alone for the management of acute VTE in pediatric patients across various thrombus locations, including PE, DVT, RAT, CSVT, and RVT. Our review demonstrated that high-quality comparative studies investigating the efficacy and safety of thrombolysis followed by AC compared with AC therapy in children are still lacking.
Overall, compared with AC therapy alone, thrombolytic therapy may be associated with higher rates of MB, CRNMB, and unspecified bleeding events in children with VTE. Although bleeding rates were reported for each VTE type and might be higher among patients receiving thrombolysis across all VTE types, the small sample sizes within these groups limit the ability to draw definitive conclusions regarding the safety profile by VTE subtype. With the exception of patients with RAT, thrombolytic therapy was not associated with an increased risk of all-cause mortality compared with AC alone.
This analysis also identified potential clinical scenarios in which thrombolytic therapy may be beneficial, especially in patients with PE with hemodynamic compromise for whom it may result in a decreased risk of mortality. In the setting of submassive PE, progression to massive PE was evaluated in only 1 study with a limited number of patients in both arms, making it challenging to determine the true effect of thrombolysis on preventing progression to massive PE. Among pediatric patients with an extremity DVT, thrombolysis therapy might be associated with an increased rate of thrombus recurrence compared with AC alone with little to no effect on thrombus resolution, and all-cause mortality. Our analysis suggests that thrombolysis may be associated with a decreased risk for the development of PTS; however, this benefit was modest and came at the expense of an increased risk of bleeding. This finding may be explained by the fact that the studies included in our review were not randomized. Patients who underwent thrombolysis were likely more symptomatic, with extensive, proximal thrombosis compared with those treated with AC alone, explaining our results.
Our analysis showed that thrombolysis might have little to no benefit on the risk of thrombus resolution in patients with RAT with an increased risk of MB, unspecified bleeding events, and all-cause mortality. Lastly, the very small number of patients with CSVT and RVT in the included studies limited our ability to draw any definitive conclusions regarding the efficacy of thrombolytic therapy in these thromboses types.
Comparison to adults
We performed a focused literature search and identified 3 systematic reviews investigating the use of thrombolysis in adults. In the study by Izcovich et al, the evidence suggests that thrombolytic therapy reduces short-term mortality in patients with submassive or intermediate-risk PE.32 Additionally, low-certainty evidence suggests that thrombolytic therapy might reduce PTS in patients with proximal DVT at the expense of an increase in MB episodes which align with our study results.32
Another systematic review on the use of thrombolysis in patients with PE published in 2014 by Chatterjee et al suggests that thrombolysis is associated with lower rates of all-cause mortality and increased risks of MB and intracranial hemorrhage.33 A more recent systematic review (Mathew et al) found no significant difference in in-hospital mortality or the overall risk of MB in patients with intermediate-risk PE.34 However, thrombolysis appeared to reduce the need for vasopressor use and secondary or rescue thrombolysis.
Therefore, in adults, the ASH guideline panel recommends thrombolytic therapy followed by AC for patients with massive PE, and suggests AC alone for patients with proximal DVT, or submassive PE.35
Potential limitations
Limitations of this analysis include the overall small sample size of the included studies, which led to very wide CIs and imprecision in the effect estimate. In addition, for many outcomes we were not able to calculate the relative or absolute effect estimate. Furthermore, given that all included studies were nonrandomized studies of intervention, there is a high risk for selection bias and confounding by indication. It is possible that patients perceived to have more extensive thrombotic burden or symptomatic thrombosis at baseline were treated with thrombolysis, and patients deemed to be at increased risk of bleeding were treated with AC therapy alone, resulting in clinical heterogeneity between treatment arms and potential impact on treatment outcomes. We were unable to perform subgroup analysis comparing outcomes by therapy mode (ie, systemic vs CDT) because of the small sample size of the included studies and because many studies did not differentiate between the 2 treatment modalities. In addition, there was significant heterogeneity in the dosing protocols of thrombolysis used across the included studies, limiting our ability to perform a dose comparison between studies. Although the included studies have methodological limitations, we assessed the certainty of the evidence using the GRADE method and highlighted the very low certainty of the evidence.
Strengths
The strengths of this study include the rigorous systematic approach used to identify and assess the quality of evidence of the included studies. The ASH-ISTH guideline panel prioritized these questions because of their clinical significance in updating the pediatric VTE management guidelines. To our knowledge, this is the first comprehensive systematic review comparing the use of thrombolytic therapy followed by AC vs AC alone in pediatric VTE. The evidence summaries were based on comparative data except for patients with RVT, which provides the best available evidence on thrombolysis use in pediatric patients with VTE. The review assessed outcomes of thrombolysis across multiple thrombus locations, providing a broad understanding of its use in pediatric subpopulations, including those with PE, DVT, RAT, CSVT, and RVT. This allows us to better identify potential clinical situations in which thrombolysis may be beneficial, as not all VTE types have the same baseline risk for VTE complications and outcomes. Additionally, this review highlights critical knowledge gaps regarding thrombolysis use in pediatric VTE, underscoring the need for future research.
Future directions
Ideally, randomized studies are needed to evaluate the efficacy and safety of thrombolysis for the management of VTE in the pediatric population; however, recognizing the potential challenges in feasibility, large-scale studies and registries with uniform protocols, standardized outcome definitions and stratified outcomes by thromboses types are needed to better assess the use of thrombolysis in pediatric populations. Additionally, studies to investigate optimal doses, duration, and outcomes of systemic thrombolytic therapy and CDT are needed.
Conclusions
This systematic review highlights some of the key challenges in developing recommendations for the use of thrombolysis therapy in children with VTE. There is an urgent need for the design and conduct of multicenter prospective comparative studies, and registries evaluating the use of thrombolysis therapy that maximizes the homogeneity of the populations studied and use of standardized treatment protocols and outcome definitions. The certainty in the evidence of the current studies remains very low because of the risk of bias and very small sample sizes, which limit our ability to make definitive conclusions about the short- and long-term outcomes of the use of thrombolysis followed by AC compared with AC alone in children with VTE. Furthermore, the optimal way to use thrombolysis in children (dose, systemic vs local, and duration) remains unknown.
Acknowledgments
This systematic review was performed as part of the American Society of Hematology (ASH)/International Society on Thrombosis and Haemostasis (ISTH) Clinical Practice Guidelines on venous thromboembolism in the pediatric population. The authors thank the ASH and ISTH staff for their support during the guideline development process. The authors also thank Skye Bickett for designing and conducting the search strategy.
This work was funded and supported by the ASH and the ISTH.
Authorship
Contribution: R.A.M., P.M., H.K., and M.A. conceptualized the study; H.K., M.A., A.A., Q.H., H.K.A.Z., R.M., C. Tabak, P.P., and S.L.B. were responsible for data curation; H.K. and M.A. were responsible for formal analysis; H.K., M.A., A.A., Q.H., H.K.A.Z., R.M., C. Tabak, P.P., and S.L.B. performed the study investigation; R.A.M., P.M., H.K., and M.A. developed the methodology; M.A. was responsible for project administration; R.A.M. and P.M. supervised the study; M.B., R.S.B., R.B., T.B., B.R.B., L.R.B., A.K.C.C., E.V.S.F., J.J., S.J., B.A.K., N.K., R.K., C.M., M.-C.P.-M., L.R., C.M.R., S.E.S., C.M.T., C. Tarango, C.H.V.O., M.C.V., S.K.V., J.W., S.W., H.P.W., G.W., and A.Z. were responsible for validation; H.K. and M.A. were responsible for visualization; H.K., M.A., and M.B. wrote the original draft of the manuscript; R.S.B., R.B., T.B., B.R.B., L.R.B., A.K.C.C., E.V.S.F., J.J., S.J., B.A.K., N.K., R.K., C.M., M.-C.P.-M., L.R., C.M.R., S.E.S., C.M.T., C. Tarango, C.H.V.O., M.C.V., S.K.V., J.W., S.W., H.P.W., G.W., A.Z., R.A.M., P.M., A.A., Q.H., H.K.A.Z., R.M., C. Tabak, P.P., and S.L.B. reviewed and edited the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: All authors were members of the guideline panel, members of the systematic review team, or both. As such, they completed a disclosure of interest form, which was reviewed by the American Society of Hematology/International Society on Thrombosis and Haemostasis and is available in the supplemental Material.
Correspondence: Reem A. Mustafa, Division of Nephrology and Hypertension, Department of Internal Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd, MS3002, Kansas City, KS 66160; email: rmustafa@kumc.edu.
References
Author notes
H.K. and M.A. are joint first authors and contributed equally to this study.
The full-text version of this article contains a data supplement.