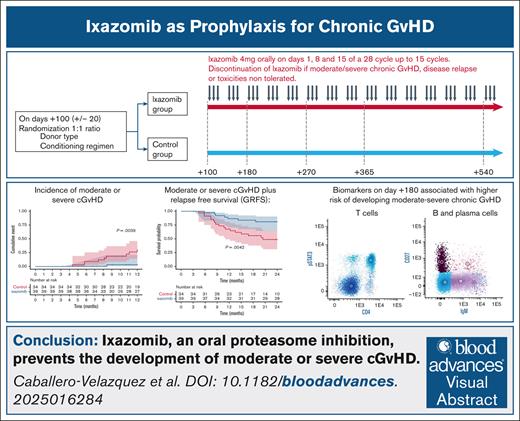
Key Points
Ixazomib, an oral proteasome inhibitor, prevents the development of moderate/severe cGVHD.
Patterns of immune system on T and B cells studied by flow cytometry predict the development of cGVHD.
Visual Abstract
Chronic graft-versus-host disease (cGVHD) is the leading cause of long-term morbidity and mortality after allogeneic hematopoietic stem cell transplantation. We hypothesize that it is possible to decrease its risk by manipulating the immune response in late phases of transplantation. We performed a prospective randomized trial including 73 patients. Patients in the treatment arm received 4 mg of ixazomib (IXZ) every 28 days from day +100. With a median follow-up of 24 months, the cumulative incidence of moderate/severe cGVHD in the IXZ vs control groups at 1 and 2 years were: 3.23% vs 30.2% (hazard ratio [HR], 0.089; P = .02) and 13% vs 43% (HR, 0.23; P = .01), respectively. Estimates for cGVHD and relapse-free survival at 2 years were 81% for IXZ and 49% for the control group (HR, 0.30). Increased STAT3 and p38 phosphorylation in T cells, and higher proportion of B cells that have undergone immunoglobulin isotype switching and circulating plasma cells on day +180 were associated with a significantly higher risk of developing moderate/severe cGVHD. The administration of IXZ decreases the risk of moderate/severe cGVHD. It is possible to identify biological patterns by flow cytometry to predict the risk of cGVHD. This trial was registered at www.clinicaltrials.gov as #NCT03225417.
Introduction
Graft-versus-host disease (GVHD) is a leading cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Classically, 3 stages have been described in the pathophysiology of acute GVHD (aGVHD), which, finally, lead to donor alloreactive T-cell activation and migration into target organs in which they induce their cytotoxic effect.1 All these processes do occur very early in the posttransplant period. Most strategies currently used to prevent GVHD are based on the blockade of ≥1 of these steps using different immunosuppressive drugs.2
Regarding chronic GVHD (cGVHD), a 3-step model has also been proposed3: in a first step, inflammatory cytokines and Toll-like receptor agonists are released in response to aGVHD or infections. Step 2 is characterized by the activation of the adaptive immune system. Thymic injury engendered during steps 1 and 2 has deleterious effects on central tolerance, whereas decreased numbers and/or dysfunctional regulatory cells hampers peripheral tolerance. In step 3, propagation of tissue injury occurs because of the trigger of aberrant repair mechanisms based on the release of growth factors such as transforming growth factor β by tissue-repair macrophages, which, in turn, activate fibroblasts growth, collagen deposition, and fibrosis.
The design of novel strategies to prevent GVHD should consider the different mechanisms involved in the pathophysiology of aGVHD and cGVHD. As far as the latter is concerned, we should not only target the first steps, which are “aGVHD related” but also immune cell subpopulations that play a longer-term role in the development of immunotolerance. Remarkably, preclinical models have shown that proteasome inhibition (PI) has immunomodulatory properties by targeting selective depletion of proliferating alloreactive T cells, reduction of T-helper type 1 cytokines, and blocking of antigen-presenting cell activation without a negative effect on regulatory T cells (Tregs).4-6 Moreover, the use of bortezomib has already been explored to prevent or treat GVHD.7-11 Nevertheless, these studies have mostly focused on the use of PI in the early posttransplant period.
In a preclinical model we have shown that ixazomib (IXZ), an oral proteasome inhibitor, can effectively target all immune cell subpopulations involved in the development of cGVHD. Interestingly, IXZ increases Tregs and decreases effector donor T cells. In addition, IXZ-treated mice have a faster recovery of B cells and a lower number of neutrophils in cGVHD target organs than a control group. In summary, delayed administration of IXZ ameliorates cGVHD while preserving graft-versus-leukemia and promotes a protolerogenic immune response after allo-HSCT.12 Interestingly, Pai et al described similar results using bortezomib in the cGVHD setting, both in preclinical models and in the clinical setting.13
Some biomarkers have been described which allow the identification of patients with a different risk of developing aGVHD and even stratify patients with different grades of severity and probabilities to respond to steroids, such as regenerating family member 3 alpha or suppression of tumorigenicity 2.14,15 In contrast to aGVHD, few biomarkers have been identified and none is extensively used in the transplant community to predict cGVHD, becoming an unmet medical need. The JAK/STAT pathway is essential in the activation of the immune system, regulating the phosphorylation and recruitment of different STAT proteins. In this sense, a more relevant role in the development of Tregs has been attributed to STAT5 compared with STAT3.16 Likewise, different mitogen-activated protein kinases, such as p38 and extracellular signal-regulated kinases, have been studied in the context of cGVHD. For example, it has been proposed that fibroblasts from patients with systemic sclerosis have increased phosphorylation of p38 MAPK.17
With this background, we designed a prospective phase 2 randomized trial evaluating delayed administration of IXZ as cGVHD prophylaxis after allo-HSCT recipients and we searched for novel biomarkers of cGVHD.
Methods
Study design and participants
This was a prospective randomized phase 2, open label, multicenter trial performed within the Grupo Español de Trasplante Hematopoyético y Terapia Celular evaluating the impact of the delayed/or no administration of IXZ on day +100 after allo-HSCT on the incidence of moderate-severe cGVHD (ClinicalTrials.gov identifier: NCT03225417).
Eligible patients were candidates aged ≥18 years who had received myeloablative or reduced-intensity conditioning allo-HSCT from a related donor matched 6/6 for HLA-A, HLA-B, and HLA-DRB1; or unrelated donor who was HLA matched in 7/8 or 8/8 at HLA-A, HLA-B, HLA-C, and HLA-DRB1 at high resolution using DNA-based typing. Any GVHD prophylaxis scheme was accepted except antithymocyte globulin, cyclophosphamide, or any in vitro or in vivo T-cell depletion protocol. Absolute neutrophil count of >1 × 109/L and platelets of >75 × 109/L were eligibility requirements.
Exclusion criteria included hepatitis B or C, HIV infection, poor performance status, other cancer diagnosis, creatinine clearance of <30 mL/min, moderate alteration of liver tests, major surgery or systemic treatment with potent CYP3A inducers in the 14 days before inclusion, uncontrolled infection, uncontrolled cardiovascular problems, psychiatric illness that could interfere with compliance with treatment, known allergy to any of the study drugs, and grade 1 peripheral neuropathy with pain or grade ≥2 with or without pain. Participation in other clinical trials within 30 days before inclusion was not allowed. Other exclusion criteria were previous treatment with IXZ, active disease, and active microangiopathy. Patients with previous aGVHD were allowed to be included in the trial if they were in complete remission and were receiving a steroid dose of <0.25 mg/kg per day.
Screening and randomization
Patients were approached for this study after the decision to proceed to allo-HSCT was made and a suitable HLA-matched donor identified. On days +100 ± 20 after transplant, patients were included in the trial once inclusion/exclusion criteria were confirmed. All enrolled patients were included in the analysis. Ineligible patients were off study, and no further follow-up was obtained. Randomization was performed in a 1:1 ratio using permuted blocks with a random block size, stratified by donor type (HLA-matched sibling vs matched unrelated vs mismatched unrelated) and conditioning regimen (reduced intensity or myeloablative conditioning).
Procedures
In the intervention group, IXZ was administered after day 100 at a once-weekly dose of 4 mg orally on days 1, 8, and 15 of a 28-day cycle (3 weeks on, 1 week off) up to 15 cycles. The dosing and schedule were based on previous studies, which demonstrated adequate tolerability and safety, and that dosing was independent of body surface area.18 Before each cycle, patients required an absolute neutrophil count of ≥1 × 109/L, platelet count of ≥75 × 109/L, and all other nonhematologic toxicities attributed as probably or definitely related to IXZ to be resolved to grade ≤1 or to the patient’s baseline condition. The study included dose adjustments for hematologic and nonhematologic toxicities. If the patient failed to meet the aforementioned criteria for initiation of any cycle, or any of the criteria specified in Table 6.2, dosing was delayed and the patient evaluated weekly for up to 4 weeks. The patient was reevaluated weekly to determine whether the criteria to restart treatment had been met. If the patient continued to fail to meet criteria to receive the next cycle after a maximum of 4 weeks of delay, the criteria for dose-limiting toxicity would have been reached and, in such cases, the treatment would have been stopped and the patient removed from the trial. cGVHD signs were assessed at each study visit, every 4 weeks during the treatment period and subsequently every 3 months.
Patients who had early discontinuation of IXZ because of toxicity remained under study for follow-up until 2 years after allo-HSCT was reached. Primary immunosuppression taper was conducted according to institutional practice. Control group patients made the same schedule of visits as those in the IXZ group.
Study end points and definitions
The primary end point was the incidence of moderate or severe cGVHD.
Secondary end points included the incidence of any grade of cGVHD 2 years after transplant irrespective of whether the drug had to be stopped because of toxicity or any other reason; GVHD-free, relapse-free survival (GRFS); overall survival (OS), and differences in primary immunosuppression tapering.
cGVHD was diagnosed and evaluated as per 2005 National Institutes of Health consensus conference criteria.19 Toxicities were defined as the development of grade >1 according to the common terminology criteria for adverse events (version 4). Infections included the number of events, grade, and organism group (bacterium, virus, or fungus). GRFS included disease relapse, moderate or severe cGVHD, and death, as events.
Biomarker studies
Flow cytometry studies
Immunological characterization by multiparameter flow cytometry was performed on the peripheral blood (PB) at days +100, +180, +270, +365, +540, and +720 after transplant. The immune cell subpopulations were quantified on samples of PB collected in EDTA. These samples were stained immediately using the combination of monoclonal antibodies that is listed in supplemental Table 1. Standard protocols were followed for membrane (stain, lyse, and wash) and intracellular antigens (fixation and permeabilization). Samples were acquired using an FACSLyric (FACSuite software, BD Biosciences) and FACSCantoII (FACS Diva software, BD Biosciences). Data analysis was performed using Infinicyt software 2.0 (Cytognos, S.L.). Analysis of the T-cell receptor (TCR) Vβ repertoire was performed according to the manufacturer’s instructions (IOTest Beta Mark kit). Expression of T-cell costimulatory and coinhibitory molecules: 4-IBB (CD137, tumor necrosis factor receptor superfamily TNFRSF member 9), programmed death 1, OX40 (CD134 or TNFRSF4), cytotoxic T-lymphocyte antigen 4, and TNFRSF18 in the CD4+ and CD8+ compartments was measured under resting conditions and after a 4-hour incubation with ionomycin (0.91 μg/mL) and phorbol myristate acetate (20 μg/mL). Phosphoflow analysis of extracellular signal–regulated kinases (Pt202/204), p38 (Pt180/y182), STAT3 (Py705), and STAT5 (Py694) were performed using the Phosflow T-cell activation kit (BD Biosciences; catalog no. 560750). Median fluorescence intensity (MFI) and percentage were determined for each marker in global CD3+ cells, CD3+CD4+CD8− cells and CD3+CD4−CD8+ cells (supplemental Figure 1). To assess the expression of the BAFF receptor, the MFI of this marker was determined and the difference in expression between days +100 and +180 was calculated with the following formula: ΔCD268 (BAFF-R) = (MFI on day +180 – MFI on day +100). Likewise, supplemental Tables 2 and 3 describe the phenotype used to identify the different subsets of immune cells.
Cytokines studies
Serum samples were collected in serum separation tubes and stored at −80°C until subsequent analysis. BAFF and interferon beta were measured by Milliplex human cytokine/chemokine magnetic bead panel IV (Merck Millipore; reference: HCYP4MAG-64K) according to the manufacturer’s instructions.
Statistical analysis
Analyses were performed using International Business Machines Statistical Package for the Social Sciences 29 software and R studio 4.3.2. Sample normality was examined using the Shapiro-Wilk test and the Q-Q graphical method. Comparisons between the values of different variables were analyzed using the Student t test (for normal variables) or the Mann-Whitney U test (for nonnormal variables) and Pearson χ2 for qualitative variables. The Fisher exact test was considered in 2 × 2 contingency tables when the expected observations were <5 in at least 20%. When the tables were of an order more than 2 × 2, the likelihood ratio was also observed. TCR Vβ repertoire diversity was quantified using a Gini-like diversity.20 In general, a P value < .05 was considered statistically significant. The cumulative incidence of cGVHD was performed with competitive risk. Cumulative event was determinate using Kaplan-Meier analysis and statistical significance was determined using the log-rank (Mantel-Cox) test. Cox proportional hazards regression was used to estimate cGVHD. The proportional hazards assumption was validated using the cox.zhp function of the R (survival) library. In contrast, visualization techniques such as swimmers plots and balloon plots were used. The trial was designed to randomize 130 patients across IXZ or control group (n = 65 each). The incidence rates of cGVHD and relapses were calculated for each group using the cumulative incidence estimator, along with 95% confidence intervals (CIs), event-free survival, and OS were estimated using the Kaplan-Meier method along with 95% CIs. To calculate sample size, assuming a power of 80% and a significance level of 5% and considering a risk of moderate plus severe cGVHD of 60% for the control arm (as previously mentioned in our own experience using standar risk level/TCR) and 30% for the treatment arm, the number of patients required in each group should be 58. Assuming 12% patient loss, the number of patients included in each group should be 65.
For the confirmation of biomarkers, a multivariate Cox regression analysis was performed adjusted to the use or not of IXZ.
The study was approved by the institutional review board at each institution and all patients provided written informed consent before being admitted to the trial.
Results
Patients
From 13 March 2019 through 31 March 2022, 73 patients (39 to IXZ and 34 to the control cohort) from 5 Spanish centers were enrolled. Median age was 52.2 (range, 29-69) and 54.9 years (range, 20-69) for IXZ and control groups, respectively. Patient characteristics were balanced between the 2 groups (Table 1).
Patient baseline characteristics
| . | IXZ, n = 39 (53%) . | Control, n = 34 (57%) . | P value . |
|---|---|---|---|
| Age, y, median (range) | 52.2 (29-69) | 54.9 (20-69) | .89 |
| Sex, male/female, n (%) | 24 (61.5)/15 (38.5) | 17 (50)/17 (50) | .32 |
| Diagnosis, n (%) | .87 | ||
| Lymphoma | 8 (20.5) | 6 (17.6) | |
| AML | 14 (35.9) | 14 (41.2) | |
| ALL | 2 (5.1) | 2 (5.9) | |
| Myelodysplastic syndrome | 11 (28.2) | 7 (20.6) | |
| Multiple myeloma | 4 (10.3) | 4 (11.8) | |
| CMPD | 0 | 1 (2.9) | |
| Donor, n (%) | .96 | ||
| MRD | 29 (74.4) | 18 (52.9) | |
| MURD | 9 (23.1) | 14 (41.3) | |
| MMRD | 1 (2.6) | 0 | |
| MMURD | 0 | 2 (5.9) | |
| GVHD prophylaxis, n (%) | .46 | ||
| Tk-MTX | 9 (23.1) | 5 (14.7) | |
| Tk/CsA-siro | 6 (20.5) | 11 (32.3) | |
| Tk-siro-MMF | 22 (56.4) | 18 (52.9) | |
| Conditioning, n (%) | .61 | ||
| Myeloablative | 10 (25.6) | 7 (20.6) | |
| Reduced intensity | 29 (74.4) | 27 (79.4) | |
| Previous aGVHD, n (%) | .37 | ||
| Yes | 34 (86.8) | 27 (78.8) | |
| No | 5 (13.2) | 7 (21.2) |
| . | IXZ, n = 39 (53%) . | Control, n = 34 (57%) . | P value . |
|---|---|---|---|
| Age, y, median (range) | 52.2 (29-69) | 54.9 (20-69) | .89 |
| Sex, male/female, n (%) | 24 (61.5)/15 (38.5) | 17 (50)/17 (50) | .32 |
| Diagnosis, n (%) | .87 | ||
| Lymphoma | 8 (20.5) | 6 (17.6) | |
| AML | 14 (35.9) | 14 (41.2) | |
| ALL | 2 (5.1) | 2 (5.9) | |
| Myelodysplastic syndrome | 11 (28.2) | 7 (20.6) | |
| Multiple myeloma | 4 (10.3) | 4 (11.8) | |
| CMPD | 0 | 1 (2.9) | |
| Donor, n (%) | .96 | ||
| MRD | 29 (74.4) | 18 (52.9) | |
| MURD | 9 (23.1) | 14 (41.3) | |
| MMRD | 1 (2.6) | 0 | |
| MMURD | 0 | 2 (5.9) | |
| GVHD prophylaxis, n (%) | .46 | ||
| Tk-MTX | 9 (23.1) | 5 (14.7) | |
| Tk/CsA-siro | 6 (20.5) | 11 (32.3) | |
| Tk-siro-MMF | 22 (56.4) | 18 (52.9) | |
| Conditioning, n (%) | .61 | ||
| Myeloablative | 10 (25.6) | 7 (20.6) | |
| Reduced intensity | 29 (74.4) | 27 (79.4) | |
| Previous aGVHD, n (%) | .37 | ||
| Yes | 34 (86.8) | 27 (78.8) | |
| No | 5 (13.2) | 7 (21.2) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CMPD, chronic myeloproliferative disease; MMRD, mismatched related donor; MMURD, mismatched unrelated donor; MRD, matched related donor; MURD, matched unrelated donor; Tk/CsA-siro, tacrolimus/cyclosporine-sirolimus; Tk-MTX, tacrolimus-methotrexate; Tk-siro-MMF, tacrolimus-sirolimus-mycophenolate mofetil.
Primary end point
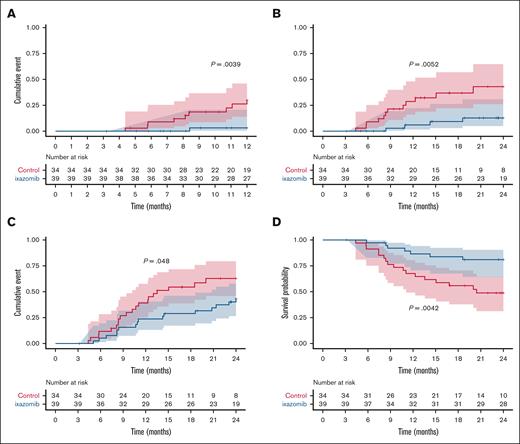
Adjusted Kaplan-Meier estimates for moderate or severe incidence of cGVHD at 1 year were 3.23% (95% CI, 0-9.25) for IXZ, and 30.2% (95% CI, 11.2-45.1) for control, P = .0039 (Figure 1A); with hazard ratio (HR) of 0.089.
Incidences of cGVHD. Incidence of moderate or severe cGVHD at 1 year during clinical trial follow-up (that is, until event as defined in “Methods” or drug suspension occurred) (A) and during the extended 2-year follow-up (B). (C) Incidence of overall cGVHD. (D) Moderate or severe GRFS. The cumulative incidence of cGVHD was performed using Kaplan-Meier analysis, and statistical significance was determined using the log-rank (Mantel-Cox) test.
Incidences of cGVHD. Incidence of moderate or severe cGVHD at 1 year during clinical trial follow-up (that is, until event as defined in “Methods” or drug suspension occurred) (A) and during the extended 2-year follow-up (B). (C) Incidence of overall cGVHD. (D) Moderate or severe GRFS. The cumulative incidence of cGVHD was performed using Kaplan-Meier analysis, and statistical significance was determined using the log-rank (Mantel-Cox) test.
Secondary end points
The incidences of moderate or severe cGVHD in the extended 2-year follow-up was 13% (95% CI, 0-24) for IXZ and 43% (95% CI, 19-60) for control; HR, 0.23 (Figure 1B). For overall cGVHD, the respective values at 1 year were 23.7% (95% CI, 8.9-36.1) for IXZ and 45.2% (95% CI, 25.3-59.8) for control; P = .062; HR, 0.46. Likewise, overall cGVHD in the extended 2-year follow-up was 43% (95% CI: 25-57) for IXZ and 63% (95% CI, 31-77) for control (Figure 1C). Median number of months from transplant to the onset of cGVHD was 10.5 in the IXZ group vs 8.2 months in the control group. With a median follow-up of 24 months (range, 3-24), Kaplan-Meier estimates for GRFS at 2 years were 81% (95% CI, 70-95) for IXZ and 49% (95% CI, 34-70) for the control group; P = .004; HR, 0.30 (Figure 1D). We did observe a higher use of corticosteroids in patients in the control group, in relation to a higher incidence of moderate or severe cGVHD (12 vs 4; P = .01).
Disease relapse and survival
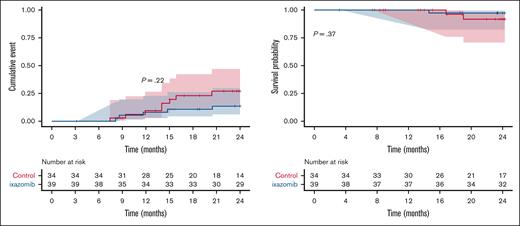
With a median follow-up of 2 years after transplantation, there were no significant differences in the risk of relapse between the 2 groups although there was a trend toward a lower incidence of relapse in the IXZ group (27% vs 13.6% in the IXZ vs control group; P = .22; Figure 2A). The proportion of patients who were alive after 2 years after randomization were 97% (95% CI, 92-100) and 92% (95% CI, 82-100) in the IXZ and control groups, respectively (Figure 2B). This difference was also not statistically significant. All patients who died (IXZ, n = 1 [3%] and control, n = 2 [8%]), died from disease progression during the second year after allo-HSCT.
Disease relapse and survival. Incidence of relapse (A) and OS (B). The cumulative incidence of relapse and OS were determined using Kaplan-Meier analysis, and statistical significance was determined using the log-rank (Mantel-Cox) test.
Disease relapse and survival. Incidence of relapse (A) and OS (B). The cumulative incidence of relapse and OS were determined using Kaplan-Meier analysis, and statistical significance was determined using the log-rank (Mantel-Cox) test.
Toxicities
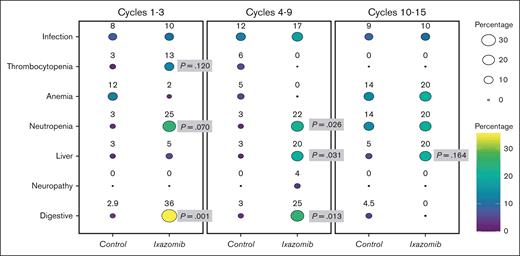
Toxicities were more frequent in the experimental group. Overall, 14 patients (35.9%) in IXZ group experienced grades 3 and 4 toxicities, compared with 6 patients (17.6%) in control group. The most common toxicities were gastrointestinal (IXZ, n = 14 [35.9%] vs control, n = 1 [2.9%] during the first 3 cycles; IXZ, n = 6 [25.0%] vs control group, n =1 [3.0%] during cycles 4-9; and IXZ, n = 0 [0%] vs control group, n = 1 [4.5%] during cycles 10-15) and hematologic (incidence of neutropenia was significantly higher in the IXZ group than the control group: 10 [25.6%] vs 1 [2.9%] during cycles 1-3; 5 [20.8%] vs 1 [3%], during cycles 4-9; and 2 [20%] vs 3 [13.6%] during last 6 cycles; respectively). Other toxicities and infections occurring among patients enrolled in the clinical trial are summarized in Table 2 and Figure 3.
Incidence of adverse events
| . | Cycles 1-3 . | P value . | Cycles 4-9 . | P value . | Cycles 10-15 . | P value . | |||
|---|---|---|---|---|---|---|---|---|---|
| IXZ, N = 39 . | Control, N = 34 . | IXZ, N = 24 . | Control, N = 33 . | IXZ, N = 10 . | Control, N = 22 . | ||||
| Digestive toxicity, n (%) | 14 (35.9) | 1 (2.9) | .001 | 6 (25.0) | 1 (3) | .013 | 0 (0) | 1 (4.5) | .490 |
| Grade 2 | 7 (17.9) | 0 (0) | 5 (20.8) | 1 (3) | 1 (4.5) | ||||
| Grade 3 | 7 (17.9) | 1 (2.9) | 1 (4.2) | 0 (0) | 0 (0) | ||||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Peripheral neuropathy, n (%) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) | .230 | 0 (0) | 0 (0) | ||
| Grade 2 | 0 (0) | ||||||||
| Grade 3 | 1 (4.2) | ||||||||
| Grade 4 | 0 (0) | ||||||||
| Liver toxicity, n (%) | 2 (5.1) | 1 (2.9) | .632 | 5 (20.8) | 1 (3) | .031 | 2 (20) | 1 (4.5) | .164 |
| Grade 2 | 2 (5.1) | 1 (2.9) | 3 (12.5) | 1 (3) | 1 (10) | 1 (4.5) | |||
| Grade 3 | 0 (0) | 0 (0) | 2 (8.3) | 0 (0) | 1 (10) | 0 (0) | |||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Neutropenia, n (%) | 10 (25.6) | 1 (2.9) | .070 | 5 (20.8) | 1 (3) | .026 | 2 (20) | 3 (13.6) | .472 |
| Grade 2 | 5 (12.8) | 1 (2.9) | 3 (12.5) | 1 (3) | 1 (10) | 1 (4.5) | |||
| Grade 3 | 3 (7.7) | 0 (0) | 1 (4.2) | 0 | 1 (10) | 2 (9.1) | |||
| Grade 4 | 2 (5.1) | 0 (0) | 1 (4.2) | 0 | |||||
| Anemia, n (%) | 1 (2.6) | 4 (11.8) | .055 | 0 (0) | 1 (3) | .420 | 2 (20) | 3 (13.6) | .472 |
| Grade 2 | 0 | 3 (8.8) | 1 (3) | 1 (10) | 1 (4.5) | ||||
| Grade 3 | 1 (2.6) | 1 (2.9) | 0 | 1 (10) | 2 (9.1) | ||||
| Grade 4 | 0 | 0 | |||||||
| Thrombocytopenia, n (%) | 5 (12.8) | 1 (2.9) | .120 | 0 (0) | 2 (6.1) | .230 | 0 (0) | 0 (0) | 0 |
| Grade 2 | 4 (10.3) | 1 (2.9) | 1 (3) | ||||||
| Grade 3 | 1 (2.6) | 0 (0) | 1 (3) | ||||||
| Grade 4 | 0 (0) | 0 (0) | |||||||
| Infections, n (%) | 4 (10.3) | 3 (8.8) | .836 | 4 (16.7) | 4 (12.1) | .620 | 1 (10) | 2 (9.1%) | .935 |
| Grade 2 | 4 (10.3) | 1 (2.9) | 0 (0) | 3 (9.1) | 1 (10%) | 1 (4.5%) | |||
| Grade 3 | 0 (0) | 1 (2.9) | 3 (12.5) | 0 (0) | 1 (4.5%) | ||||
| Grade 4 | 0 (0) | 1 (2.9) | 1 (4.2) | 1 (3) | |||||
| . | Cycles 1-3 . | P value . | Cycles 4-9 . | P value . | Cycles 10-15 . | P value . | |||
|---|---|---|---|---|---|---|---|---|---|
| IXZ, N = 39 . | Control, N = 34 . | IXZ, N = 24 . | Control, N = 33 . | IXZ, N = 10 . | Control, N = 22 . | ||||
| Digestive toxicity, n (%) | 14 (35.9) | 1 (2.9) | .001 | 6 (25.0) | 1 (3) | .013 | 0 (0) | 1 (4.5) | .490 |
| Grade 2 | 7 (17.9) | 0 (0) | 5 (20.8) | 1 (3) | 1 (4.5) | ||||
| Grade 3 | 7 (17.9) | 1 (2.9) | 1 (4.2) | 0 (0) | 0 (0) | ||||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Peripheral neuropathy, n (%) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) | .230 | 0 (0) | 0 (0) | ||
| Grade 2 | 0 (0) | ||||||||
| Grade 3 | 1 (4.2) | ||||||||
| Grade 4 | 0 (0) | ||||||||
| Liver toxicity, n (%) | 2 (5.1) | 1 (2.9) | .632 | 5 (20.8) | 1 (3) | .031 | 2 (20) | 1 (4.5) | .164 |
| Grade 2 | 2 (5.1) | 1 (2.9) | 3 (12.5) | 1 (3) | 1 (10) | 1 (4.5) | |||
| Grade 3 | 0 (0) | 0 (0) | 2 (8.3) | 0 (0) | 1 (10) | 0 (0) | |||
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Neutropenia, n (%) | 10 (25.6) | 1 (2.9) | .070 | 5 (20.8) | 1 (3) | .026 | 2 (20) | 3 (13.6) | .472 |
| Grade 2 | 5 (12.8) | 1 (2.9) | 3 (12.5) | 1 (3) | 1 (10) | 1 (4.5) | |||
| Grade 3 | 3 (7.7) | 0 (0) | 1 (4.2) | 0 | 1 (10) | 2 (9.1) | |||
| Grade 4 | 2 (5.1) | 0 (0) | 1 (4.2) | 0 | |||||
| Anemia, n (%) | 1 (2.6) | 4 (11.8) | .055 | 0 (0) | 1 (3) | .420 | 2 (20) | 3 (13.6) | .472 |
| Grade 2 | 0 | 3 (8.8) | 1 (3) | 1 (10) | 1 (4.5) | ||||
| Grade 3 | 1 (2.6) | 1 (2.9) | 0 | 1 (10) | 2 (9.1) | ||||
| Grade 4 | 0 | 0 | |||||||
| Thrombocytopenia, n (%) | 5 (12.8) | 1 (2.9) | .120 | 0 (0) | 2 (6.1) | .230 | 0 (0) | 0 (0) | 0 |
| Grade 2 | 4 (10.3) | 1 (2.9) | 1 (3) | ||||||
| Grade 3 | 1 (2.6) | 0 (0) | 1 (3) | ||||||
| Grade 4 | 0 (0) | 0 (0) | |||||||
| Infections, n (%) | 4 (10.3) | 3 (8.8) | .836 | 4 (16.7) | 4 (12.1) | .620 | 1 (10) | 2 (9.1%) | .935 |
| Grade 2 | 4 (10.3) | 1 (2.9) | 0 (0) | 3 (9.1) | 1 (10%) | 1 (4.5%) | |||
| Grade 3 | 0 (0) | 1 (2.9) | 3 (12.5) | 0 (0) | 1 (4.5%) | ||||
| Grade 4 | 0 (0) | 1 (2.9) | 1 (4.2) | 1 (3) | |||||
Incidence of different toxicities in IXZ and control groups in cycles 1 to 3, 4 to 9, and 10 to 15 illustrated by balloon plot. The color and size of each circle means a different percentage of toxicity over time.
Incidence of different toxicities in IXZ and control groups in cycles 1 to 3, 4 to 9, and 10 to 15 illustrated by balloon plot. The color and size of each circle means a different percentage of toxicity over time.
Reduction and discontinuation of IXZ
Overall, 15 patients (38.5%) in IXZ group required reductions. Most common cause of reduction was gut toxicity (Table 3; Figure 4). A total of 31 patients (79.5%) did not complete all 15 preestablished cycles. Most common cause of treatment suspension was withdrawal of consent (n = 15 [38.5%] in the IXZ group, and n = 1 [2.9%] in the control group) because mild toxicities, which did not require discontinuation of treatment but affected the patients’ quality of life (Table 3). After 2 years of follow-up, 10 patients who had ceased IXZ developed cGVHD; interestingly it was mild in 8 of these patients.
Causes of IXZ dose reductions and suspension
| Cause of reduction . | n (%) . | Cause of suspension . | n (%) . |
|---|---|---|---|
| Gastrointestinal toxicity | 10 (25.6) | Withdrawal of consent | 15 (23) |
| Neuropathy | 1 (2.6) | Disease progression | 4 (10.3) |
| Neutropenia | 2 (5.1) | cGVHD | 1 (2.6) |
| Thrombocytopenia | 1 (2.6) | Gastrointestinal toxicity | 2 (5.2) |
| Infection | 1 (2.6) | Neutropenia | 1 (2.6) |
| Thrombocytopenia | 1 (2.6) | ||
| Neuropathy | 1 (2.6) | ||
| Liver toxicity | 1 (2.6) | ||
| Infection | 1 (2.6) | ||
| Hemolytic uremic syndrome | 1 (2.6) | ||
| Pericardial effusion | 1 (2.6) | ||
| Medical team decision | 1 (2.6) | ||
| Fever | 1 (2.6) |
| Cause of reduction . | n (%) . | Cause of suspension . | n (%) . |
|---|---|---|---|
| Gastrointestinal toxicity | 10 (25.6) | Withdrawal of consent | 15 (23) |
| Neuropathy | 1 (2.6) | Disease progression | 4 (10.3) |
| Neutropenia | 2 (5.1) | cGVHD | 1 (2.6) |
| Thrombocytopenia | 1 (2.6) | Gastrointestinal toxicity | 2 (5.2) |
| Infection | 1 (2.6) | Neutropenia | 1 (2.6) |
| Thrombocytopenia | 1 (2.6) | ||
| Neuropathy | 1 (2.6) | ||
| Liver toxicity | 1 (2.6) | ||
| Infection | 1 (2.6) | ||
| Hemolytic uremic syndrome | 1 (2.6) | ||
| Pericardial effusion | 1 (2.6) | ||
| Medical team decision | 1 (2.6) | ||
| Fever | 1 (2.6) |
Reduction and discontinuation of treatment among patients receiving IXZ. Swimmer plot.
Reduction and discontinuation of treatment among patients receiving IXZ. Swimmer plot.
GVHD biomarkers
According to the median onset of cGVHD, the assays performed on day +180 were considered the most informative. Supplemental Tables 2 (subpopulations of leucocytes) and 3 (costimulatory and coinhibitory molecules) show median and range of the different biomarkers evaluated. Regarding T cells, on day +180, MFI for STAT33 phosphorylation in interleukin-6–stimulated CD4+CD8− T cells (HR, 6.78; P = .015), MFI for unstimulated CD4+CD8− T cells (HR, 5.34; P = .030) and MFI for phosphorylated STAT3 in interleukin-6–stimulated CD4−CD8+ T cells (HR, 5.4; P = .029) were significantly related to the risk of developing moderate/severe cGVHD. Concerning B cells, the proportion of cells that have undergone class switching (switched memory B cells) (HR, 12.8; P = .016) and the presence of circulating plasma cells (HR, 4.98; P = .042) were significantly related to the risk of developing moderate/severe cGVHD. In addition, an increased expression of BAFF receptor (ΔBAFFR; median fluorescence intensity on day 180 respect day 100) in B cells was significantly higher in patients with moderate/severe cGVHD (HR, 8.81; P = .042). All these biomarkers were confirmed in a multivariate analysis adjusted for IXZ intake except for the increase of expression of BAFF receptor (ΔBAFFR). The proportion of SMBC (16.94 vs 27.35; P = .012), ΔBAFFR (−1334.96 vs 1230.27; P = .009), and the concentration of soluble BAFF (0.03 vs 1.21 ng/mL; P = .013) were significantly different between IXZ and control groups. There were no differences between patients who developed cGVHD and those who did not in the rest of the parameters studied (data not shown).
Discussion
This phase 2 randomized clinical trial was designed to evaluate the impact of delayed administration of IXZ on the incidence of cGVHD, especially in its moderate and severe forms. Unlike most prophylaxis strategies focused on the immediate peritransplant and posttransplant period, this strategy attempted to manipulate the immune response in the “late” phase of transplantation to facilitate the generation of a long-term tolerogenic immune response. In this line, a study by Cutler eta l has recently been reported evaluating the impact of late administration of obinutuzumab (anti-CD20 monoclonal antibody). The primary end point of the aforementioned trial, the cumulative incidence of steroid-requiring cGVHD at 12 months from allo-HSCT, was 11% (95% CI, 5.8-20) among patients receiving obinutuzumab as compared with 38% (95% CI, 28-49) in the placebo arm (P = .008). The incidence of moderate-severe cGVHD at 12 months was 20% vs 35% for patients receiving obinutuzumab vs placebo (P = .069). There were no differences in terms of OS.21
Regarding the use of PI for cGVHD prophylaxis, several studies describe their effect on different cell subpopulations involved in the pathophysiology of cGVHD and the development of immunotolerance without increasing the risk of relapse. Furthermore, we, and others, have reported previously that PI might increase the graft-versus-leukemia effect.12 Interestingly, the use of PI as GVHD prophylaxis has already been tested in the clinical setting by the addition of bortezomib.8,11,13 As far as IXZ is concerned, Rodriguez et al evaluated the administration of a single cycle of IXZ after day 100 and up to day 150 after HSCT, showing its potential to reduce the incidence of cGVHD and facilitating the suspension of immunosuppressive treatment.22 Chhabra et al have also evaluated the impact of its administration between days +60 and +90 after transplant, demonstrating its safety but without a significant benefit in terms of incidence of cGVHD compared with historical cohorts.23
In this trial, delayed administration of IXZ significantly decreased the risk of overall, as well as moderate and severe, cGVHD. Its toxicity profile was acceptable, being predominantly gastrointestinal and hematologic, with a low percentage of grade >2 toxicities. Nevertheless, many patients did not complete the 15 cycles initially established; despite that, the positive impact of IXZ was confirmed, which suggests that it might not be necessary to reach such numbers of cycles and, therefore, treatment duration could be individualized according to tolerance. In fact, we did not find significant differences in terms of cGVHD incidence among patients receiving more or less of the median numbers of cycles.
The incidence of overall cGVHD for patients receiving IXZ at 1 and 2 years were 23.7% (95% CI, 8.9-36.1) and 43% (95% CI, 25-57), respectively; whereas the corresponding values reported by Sandmaier et al among patients receiving the triple combination of cyclosporine + mycophenolate mofetil + sirolimus24 were 37% (95% CI, 45-69) and 57% (95% CI, 26-48) at 1 and 4 years, respectively. Bolaños-Meade, using posttransplant cyclophosphamide (PT-Cy), described an incidence of overall cGVHD of 21.9% (95% CI, 16.4-27.9) at 1 year, similar to that reported in this study.25 Nevertheless, it is worth keeping in mind that the selection criteria of the patients included in our trial (on day +100) does preclude any solid conclusion regarding comparisons with these trials. In this regard, OS in both subgroups was surprisingly high. It is worth keeping in mind that only patients being in complete remission on day +100 were allowed to be included into the trial. In addition, those with previous aGVHD were included only if that they were in complete remission and receiving a steroid dose of <0.25 mg/kg per day, which might have avoided the inclusion of patients at a higher risk of delayed mortality. Regarding cyclophosphamide-related toxicities, such as delayed or poor engraftment, hemorrhagic cystitis, or cardiac toxicities were not observed with IXZ. Considering the different mechanisms of action and toxicity profiles, additional studies should be designed to explore the potential synergy of both strategies. In this regard, although initial studies using standard doses of PT-Cy were performed in the bone marrow transplant setting, this strategy has been widely described using PB as source of stem cells, which, in many studies, translated into a higher incidence of cGVHD26 as than pioneer studies from Baltimore, MD. Moreover, in an attempt to escape from cyclophosphamide-related toxicities, recent studies aim to explore the feasibility of reducing the doses PT-Cy. Accordingly, Hyder et at27 have recently reported their experience with PT-Cy at 25 mg/kg × 2 days among patients receiving bone marrow transplantation from an haploidentical donor and their results suggest that lowering the doses might allow for the decrease PT-Cy–related toxicity, maintaining a low risk of GVHD. Nevertheless, considering that most transplant centers use PB stem cells, these data might not be extrapolated, at least in terms of cGVHD. Furthermore, other groups28 reported a higher risk of GVHD among patients receiving lower doses of PT-Cy after PB stem cell transplantation from matched-related donors. Also, Fuji et al29 reported that, in their experience, the use of low-dose PT-Cy (25 mg/kg per day, 2 doses), was related to an unacceptably high risk of severe aGVHD. For this reason, these, and other authors,30 have combined lower doses of PT-Cy with antithymocyte globulin. In summary, although the use of PT-Cy has consolidated as a standard “backbone” for GVHD prophylaxis, there is still room for improvement. In this scenario, the combination with strategies such as delayed IXZ might allow PT-Cy dose reduction and, therefore, its toxicity profile, without increasing the risk of cGVHD, particularly among patients receiving PB progenitor cells.
Although the sample size calculated in the study design was not reached, mainly because of the low accrual during the COVID-19 pandemic, results obtained show a statistically significant difference, with a P value < .01, in the incidence of moderate-severe cGVHD between the 2 groups. Subsequent power analysis revealed a power of 0.81. Therefore, with the sample size achieved and the magnitude of the effect (HR, 0.089), our study had an 81% probability of detecting a significant difference in the incidence of moderate-severe cGVHD. The high power of the study supports the validity of our findings, suggesting that not achieving the sample size did not compromise the ability of the study to detect clinically relevant differences.
The evaluation of biomarkers to identify patients at a higher risk of developing cGVHD is an unmet medical need that could allow the anticipation of this complication by means of prophylactic measures or the avoidance of overexposure to immunosuppressive drugs among patients at a lower risk of developing cGVHD. Cytokines regulate the survival, proliferation, and differentiation of immune populations, triggering intracellular pathways such as JAK/STAT, MAPK, and phosphoinositide-3 kinase. In this regard, the STAT3 pathway has been identified as a key player in T-cell activation and subsequent development of cGVHD31 in preclinical models. Our biomarker analysis was exploratory but supports this hypothesis, to our knowledge, for the first time, in a prospective clinical trial. In this regard, patients who ended up presenting with cGVHD display significantly higher STAT3 phosphorylation at day +180. In contrast, we did not find relevant differences regarding STAT5 phosphorylation. In addition, the p38 MAPK pathway is a key regulator of inflammation and its activation plays an important role in collagen production in scleroderma diseases.32 Moreover, studies in animal models have evaluated the use of p38 inhibitors for the treatment of scleroderma-like cGVHD.33 Our study demonstrates that p38 phosphorylation in CD4+CD8− T cells is associated with a significantly higher risk of developing cGVHD, supporting its interest as a biomarker of cGVHD.
In contrast, abnormalities in B-cell populations have also been described in patients with cGVHD, including lower bone marrow precursor frequencies,34 fewer regulatory B cells,35 a skewed immunoglobulin repertoire,36 as well as significantly fewer CD27+ B cells in the PB compared with patients without cGVHD.37 The results of our study confirm the potential of B-cell immunophenotypic characterization to predict the risk of cGVHD. Specifically, a higher expression of BAFF receptor, a higher proportion of B cells that have undergone immunoglobulin heavy chain class switching, and the presence of circulating plasma cells were associated with a higher risk of developing cGVHD. These results are in agreement with previous studies exploring the use of IXZ or bortezomib in murine models.12,13
In summary, this study confirms that it is possible to reduce the incidence of cGVHD by manipulating the immune response in the “late” phase of transplantation without modifying immunosuppression in the peritransplant or immediate-posttransplant period, using proteasome inhibitors such as IXZ. A larger prospective randomized phase 3 trial would be required to compare this approach with other strategies. Finally, with respect to the biological markers, increased STAT3 and p38 phosphorylation in T cells and a higher proportion of B cells that have undergone immunoglobulin isotype switching, a higher presence of circulating plasma cells, and an increased expression of BAFF receptor were associated with an increased risk of moderate/severe cGVHD.
Acknowledgments
The authors thank all physicians, data managers, and participating patients for their contribution. The authors also thank Takeda Company for providing funding to support the contract research organization and for the study drug (protocol number X16082).
Authorship
Contribution: T.C.-V. designed the analysis of samples by flow cytometry and wrote the manuscript; J.D.S. collected and analyzed the data, and wrote the manuscript; L.L.-C., C.F.-C., D.V., I.G.-C., E.P.L., M.-J.J.L., F.M.M.-D., G.O., C.B.-G., A.C.M., and F.S.-G. recruited patients and reviewed the manuscript; E.M.-P. recruited patients and performed data analysis; C.B.G.-C. and M.d.l.R.J.-L. performed flow cytometry analysis; V.E.G. and H.A.R. performed data analysis; and J.A.P.S. designed the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José Antonio Pérez Simón, Servico de Hematologia, University Hospital Virgen del Rocio, Instituto de Biomedicina de Sevilla, Ave Manuel Siurot s/n, 41013 Sevilla, Spain; email: josea.perez.simon.sspa@juntadeandalucia.es.
References
Author notes
T.C.-V. and J.D.S. contributed equally to this study.
Data are available on request from the corresponding author, José Antonio Pérez Simón (josea.perez.simon.sspa@juntadeandalucia.es).