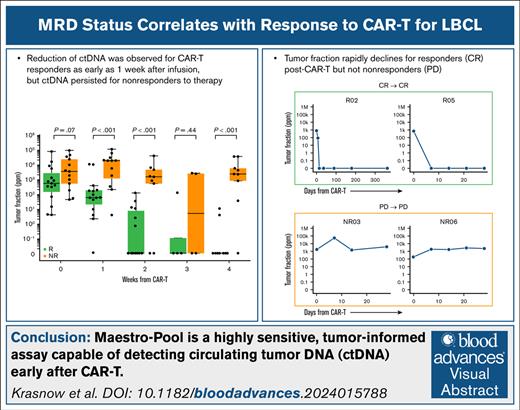
Key Points
MAESTRO-Pool is a highly sensitive, tumor-informed assay capable of detecting ctDNA early after chimeric antigen receptor T cells.
Reduction of ctDNA was observed for CAR-T cell responders as early as 1 week after infusion, but ctDNA persisted for nonresponders to therapy.
Visual Abstract
Despite responses of chimeric antigen receptor (CAR)-T cells in patients with relapsed/refractory large B-cell lymphoma (LBCL), over half of patients eventually relapse. Methods to detect early disease persistence are needed to identify patients at high risk of treatment failure. We recently developed MAESTRO, an ultrasensitive, tumor-informed measurable residual disease (MRD) assay, which can detect parts-per-million (ppm) levels of circulating tumor DNA (ctDNA) using minimal sequencing. We applied MAESTRO to 140 samples from 28 patients (15 durable responders at 12 months and 13 nonresponders) to identify treatment failure after axicabtagene ciloleucel (axi-cel), administered at our institution between 2018 and 2022. Responder and nonresponder patients had similar baseline tumor burden. By 1 week after infusion, responders had marked ctDNA reduction compared to nonresponders (P < .001). At weeks 2 and 4, responders had ctDNA levels approaching 0 ppm, whereas nonresponders had persistence of ctDNA (each P < .001). At day 0, up to 21% of patients had ctDNA fractions <0.01%; hence, these individuals would not have qualified for ctDNA monitoring with a less sensitive test. Our results confirm the feasibility of highly sensitive MRD detection by ctDNA for early identification of patients at high risk of disease progression from axi-cel.
Introduction
Chimeric antigen receptor T cells (CAR-Ts) targeting CD19 have transformed the treatment landscape for relapsed/refractory large B-cell lymphoma (LBCL). Nevertheless, long-term remission is only achieved in ∼40% of patients.1 Methods for early detection of disease persistence are needed to facilitate the identification of patients at high risk of treatment failure and prompt further therapeutic intervention.
Approximately one-third of patients will have up-front response 1 month after CAR-Ts by positron emission tomography/computed tomography (PET/CT) imaging with FDG (2-deoxy-2-[18F]fluoro-D-glucose) but subsequently experience progressive disease.2 Highly sensitive circulating tumor DNA (ctDNA) detection methods may predict longer-term outcome, particularly in this subset of patients with transient responses. ctDNA shows potential for sensitive and early detection of disease persistence or relapse. In patients with lymphoma, ctDNA can be assayed in peripheral blood samples and has already shown promise as a biomarker for detection of patients at high risk of treatment failure after first-line chemoimmunotherapy or after CAR-T.3-6
We recently developed MAESTRO, a highly sensitive, tumor-informed mutation enrichment sequencing assay for measurable residual disease (MRD), which can detect at or below parts-per-million (ppm) levels of ctDNA using minimal sequencing.7,8 Given its high efficiency, bespoke MAESTRO tests can also be pooled and applied to many patient samples (MAESTRO-Pool), conferring the advantages of being able to simultaneously assess MRD in patient-matched samples and confirm specificity in patient-unmatched samples.6,9 Here, we evaluated whether this method could detect MRD in nonresponder (NR) patients before the first clinical evaluation and explored its ability to predict response in this small cohort.
Methods
All patients provided informed written consent to institutional review board–approved protocols at the Dana-Farber/Harvard Cancer Center, allowing access to clinical data for research purposes and evaluation of clinical samples.
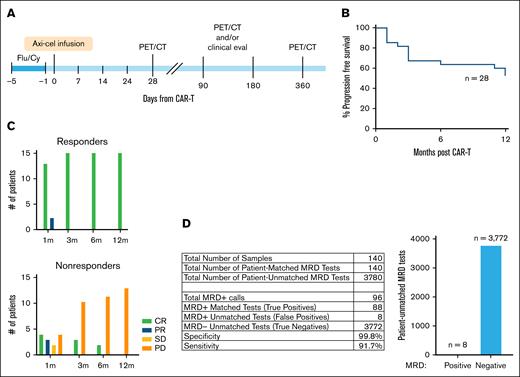
Response was assessed by PET/CT at 1, 3, 6, and 12 months after CAR-Ts and determined according to the 2014 Lugano criteria (Figure 1A).10 Responders (Rs) were defined as those achieving complete metabolic response (CR) or partial response (PR) that continued through 12 months, whereas NRs were defined as those having stable disease or progressive disease (PD) at any FDG-PET/CT evaluation before 12 months (Figure 1B-C; supplemental Figure 1A). Tumor and normal DNA, isolated from individual tumor tissue and peripheral blood mononuclear cells, respectively, were used to create personalized tumor mutation fingerprints. ctDNA was quantified in plasma and/or serum samples from at least 3 time points within 1 month of CAR-T therapy infusion and, where available, from additional time points to 1 year.
Patient characteristics and study design. (A) Schematic of treatment timeline and response assessments. (B) Kaplan-Meier curve showing progression-free survival of patients included in the analysis. (C) Number of patients with complete response (CR; green bars), PR (blue bars), SD (yellow bars), or PD (orange bars) at each response assessment time point by PET/CT scan for patients who are classified as durable Rs at 12 months (top) or NRs at or before 12 months (bottom). (D) Table shows MRD detection in patient-matched and -unmatched tests. Bar chart depicts the number of patient-unmatched MRD tests that were positive vs negative for MRD. Axi-cel, axicabtagene ciloleucel; Flu/Cy, fludarabine/cyclophosphamide; SD, stable disease.
Patient characteristics and study design. (A) Schematic of treatment timeline and response assessments. (B) Kaplan-Meier curve showing progression-free survival of patients included in the analysis. (C) Number of patients with complete response (CR; green bars), PR (blue bars), SD (yellow bars), or PD (orange bars) at each response assessment time point by PET/CT scan for patients who are classified as durable Rs at 12 months (top) or NRs at or before 12 months (bottom). (D) Table shows MRD detection in patient-matched and -unmatched tests. Bar chart depicts the number of patient-unmatched MRD tests that were positive vs negative for MRD. Axi-cel, axicabtagene ciloleucel; Flu/Cy, fludarabine/cyclophosphamide; SD, stable disease.
Whole genome sequencing, sample preparation, and MAESTRO-Pool assays were performed as previously described.9,11,12 Briefly, tumor and germ line whole genome sequencing were performed to identify patient-specific tumor somatic single-nucleotide variants (SNVs), which were used to design patient-specific MAESTRO probes tracking up to 5000 SNVs per patient. Cell-free DNA (cfDNA) was isolated from patient plasma samples. MAESTRO probes for all patients were pooled together to form a MAESTRO-Pool panel, which was applied to all samples from all patients. Tumor fraction (TFx) corresponds to the fraction of cancer-derived vs total cfDNA. After filtering (supplemental Methods), MRD status, TFx, and the limit of detection (LOD95) were calculated as previously described.9 LOD95 is the lowest TFx at which ctDNA detection would be predicted to occur with 95% likelihood; it is not the lowest TFx that can be reliably detected. TFx fold changes were computed relative to baseline. When ctDNA was undetectable at a subsequent time point, the LOD95 was substituted for the TFx to reflect a minimum fold change as previously described.8
Results
We evaluated samples from 35 patients with relapsed/refractory LBCL treated with CAR-T (axicabtagene ciloleucel) between 2018 and 2022 at our institution (Table 1). R and NR patients were similar in terms of baseline (prebridging) tumor burden (supplemental Figure 1B) and other baseline characteristics, except that NRs had a shorter interval from diagnosis to CAR-T (median, 12.8 vs 39.6 months; P = .008). We applied MAESTRO-Pool to all patient samples, comprising a collection of patient-specific MAESTRO tests targeting 143 to 5000 SNVs per patient (median, 1895), across 179 cfDNA samples (supplemental Figure 1C). Four patients were excluded due to having undetectable ctDNA at baseline, most likely due to their tests being underpowered with LOD95 values exceeding 1000 ppm. Three of these patients had very low ichorCNA TFx/tumor purity (0, 0.03, and 0.05), which indicates that we were very limited in the number of somatic variants we could derive from the tumor samples for these patients to design fingerprints. The fourth patient was dropped because we did not have detectable ctDNA at baseline, which precludes downstream comparisons using log-fold TFx change. Two patients did not have baseline plasma available, and 1 patient died from non–cancer-related causes within 1 year of CAR-T (supplemental Figure 1D). Thus, 28 patients were included in the final analysis (15 Rs and 13 NRs). Five patients had PR at the first FDG-PET/CT assessment, with 2 converting to CR and 3 to PD before 12 months. Three patients with initial CR subsequently progressed by 1 year. MAESTRO-Pool demonstrated remarkable specificity for individual patient tumor fingerprints; of the 3780 patient-unmatched MRD tests, only 8 were ctDNA positive, yielding a false positive rate of 0.2% (Figure 1D). Notably, one plasma sample from NR01 was responsible for 3 of the 8 false positive calls.
Patient characteristics by response at month 12
| . | Total . | Month 12 response . | P value . | |
|---|---|---|---|---|
| CR . | PD/NCR . | |||
| N = 28 (%) . | n = 15 (54%) . | n = 13 (46%) . | ||
| Sex | ||||
| Female | 14 (50) | 7 (47) | 7 (54) | >.99∗ |
| Male | 14 (50) | 8 (53) | 6 (46) | |
| Age at diagnosis, y | ||||
| Median (range) | 64 (37-77) | 67 (47-77) | 61 (37-73) | .14† |
| ≤60 | 10 (36) | 5 (33) | 5 (38) | >.99∗ |
| >60 | 18 (64) | 10 (67) | 8 (62) | |
| Diagnosis | ||||
| DLBCL | 17 (61) | 7 (47) | 10 (77) | .12∗ |
| High-grade B-cell lymphoma, triple hit | 1 (4) | 1 (7) | ||
| PMBCL | 1 (4) | — | 1 (8) | |
| Transformed follicular | 6 (21) | 5 (33) | 1 (8) | |
| Transformed marginal zone | 1 (4) | 1 (7) | — | |
| Transformed SLL | 1 (4) | — | 1 (8) | |
| Transformed Waldenström macroglobulinemia | 1 (4) | 1 (7) | — | |
| Subtype | ||||
| EBV+ | 1 (4) | — | 1 (8) | .55∗ |
| GCB-Type | 10 (36) | 6 (40) | 4 (31) | |
| MYC and BCL2 and/or BCL6 rearrangements “double hit” | 1 (4) | 1 (7) | — | |
| Non-GCB | 10 (36) | 4 (27) | 6 (46) | |
| Triple hit | 1 (4) | 1 (7) | - | |
| Missing | 5 (18) | 3 (20) | 2 (15) | |
| ECOG PS | ||||
| 0 | 10 (36) | 6 (40) | 4 (31) | .87∗ |
| 1 | 15 (54) | 8 (53) | 7 (54) | |
| 2 | 3 (11) | 1 (7) | 2 (15) | |
| Ann Arbor stage | ||||
| I | 3 (11) | 1 (7) | 2 (15) | .59∗ |
| II | 1 (4) | — | 1 (8) | |
| III | 9 (32) | 6 (40) | 3 (23) | |
| IV | 15 (54) | 8 (53) | 7 (54) | |
| Time from Dx to CAR-T, median (range), mo | 20.0 (4.6-121.7) | 39.6 (4.9-121.7) | 14.3 (4.6-98.5) | .013† |
| No. of previous treatments, median (range) | 3 (1-11) | 3 (1-11) | 3 (2-5) | .42† |
| Previous autologous hematopoietic stem cell transplant | ||||
| No | 19 (68) | 8 (53) | 11 (85) | .11∗ |
| Yes | 9 (32) | 7 (47) | 2 (15) | |
| Bridging therapy | ||||
| No | 17 (61) | 9 (60) | 8 (62) | >.99∗ |
| Yes | 11 (39) | 6 (40) | 5 (38) | |
| . | Total . | Month 12 response . | P value . | |
|---|---|---|---|---|
| CR . | PD/NCR . | |||
| N = 28 (%) . | n = 15 (54%) . | n = 13 (46%) . | ||
| Sex | ||||
| Female | 14 (50) | 7 (47) | 7 (54) | >.99∗ |
| Male | 14 (50) | 8 (53) | 6 (46) | |
| Age at diagnosis, y | ||||
| Median (range) | 64 (37-77) | 67 (47-77) | 61 (37-73) | .14† |
| ≤60 | 10 (36) | 5 (33) | 5 (38) | >.99∗ |
| >60 | 18 (64) | 10 (67) | 8 (62) | |
| Diagnosis | ||||
| DLBCL | 17 (61) | 7 (47) | 10 (77) | .12∗ |
| High-grade B-cell lymphoma, triple hit | 1 (4) | 1 (7) | ||
| PMBCL | 1 (4) | — | 1 (8) | |
| Transformed follicular | 6 (21) | 5 (33) | 1 (8) | |
| Transformed marginal zone | 1 (4) | 1 (7) | — | |
| Transformed SLL | 1 (4) | — | 1 (8) | |
| Transformed Waldenström macroglobulinemia | 1 (4) | 1 (7) | — | |
| Subtype | ||||
| EBV+ | 1 (4) | — | 1 (8) | .55∗ |
| GCB-Type | 10 (36) | 6 (40) | 4 (31) | |
| MYC and BCL2 and/or BCL6 rearrangements “double hit” | 1 (4) | 1 (7) | — | |
| Non-GCB | 10 (36) | 4 (27) | 6 (46) | |
| Triple hit | 1 (4) | 1 (7) | - | |
| Missing | 5 (18) | 3 (20) | 2 (15) | |
| ECOG PS | ||||
| 0 | 10 (36) | 6 (40) | 4 (31) | .87∗ |
| 1 | 15 (54) | 8 (53) | 7 (54) | |
| 2 | 3 (11) | 1 (7) | 2 (15) | |
| Ann Arbor stage | ||||
| I | 3 (11) | 1 (7) | 2 (15) | .59∗ |
| II | 1 (4) | — | 1 (8) | |
| III | 9 (32) | 6 (40) | 3 (23) | |
| IV | 15 (54) | 8 (53) | 7 (54) | |
| Time from Dx to CAR-T, median (range), mo | 20.0 (4.6-121.7) | 39.6 (4.9-121.7) | 14.3 (4.6-98.5) | .013† |
| No. of previous treatments, median (range) | 3 (1-11) | 3 (1-11) | 3 (2-5) | .42† |
| Previous autologous hematopoietic stem cell transplant | ||||
| No | 19 (68) | 8 (53) | 11 (85) | .11∗ |
| Yes | 9 (32) | 7 (47) | 2 (15) | |
| Bridging therapy | ||||
| No | 17 (61) | 9 (60) | 8 (62) | >.99∗ |
| Yes | 11 (39) | 6 (40) | 5 (38) | |
DLBCL, Diffuse Large B-cell Lymphoma; Dx, Diagnosis; EBV+, Epstein-Barr Virus; ECOG PS, Eastern Cooperative Oncology Group Performance Status; GCB-type, Germinal center B-cell-like-type; NCR, non-complete response; PMBCL, Primary Mediastinal B-cell Lymphoma; SLL, Small lymphocytic lymphoma.
Fisher exact test.
Wilcoxon rank sum test.
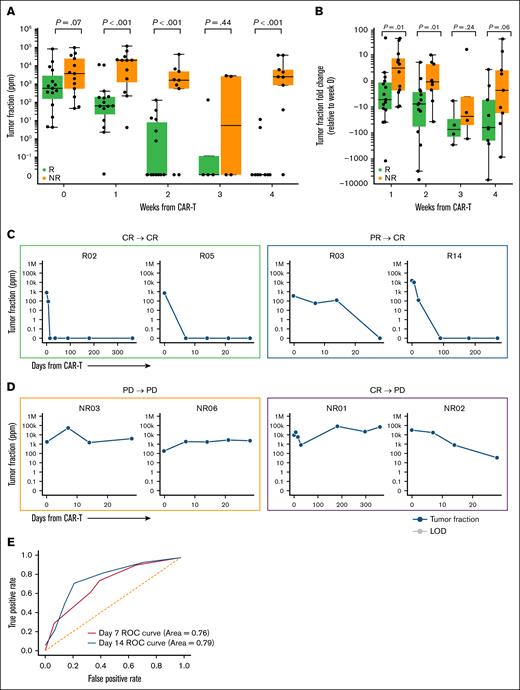
We examined the association between response to CAR-T treatment and ctDNA dynamics from MAESTRO-Pool. All patients had detectable ctDNA at baseline (day 0; ie, the day of CAR-T infusion), with a median TFx of 872.26 ppm (range, 3.92-87 361.24). At baseline, 21% of patients had ctDNA fractions <100 ppm, and we observed a trend toward an increase in TFx in NRs compared to Rs (P = .07; Figure 2A). At 1 week, all but 1 patient had detectable ctDNA, with a median TFx of 199.98 ppm (range, 0-104 504.29). At weeks 1 and 2, ctDNA levels were markedly lower in Rs, approaching 0 ppm, than in NRs (P < .001, at both time points; Figure 2A). By 1 week, Rs demonstrated lower TFx than NRs (P < .001), and the median TFx increased by 2.96-fold for NRs and decreased by 5.90-fold for Rs relative to baseline (P = .01). By 2 weeks, the TFx decreased by 1.22-fold for NRs and 8.60-fold for Rs relative to baseline (P = .01). At week 3, fewer patient samples were available, limiting interpretation. By week 4, TFx continued to decline, reaching 24.5-fold and 2.58-fold decreases in NRs, respectively; and 76.1-fold and 69.5-fold decreases in Rs, respectively (P = .24 and .06, respectively; Figure 2B).
TFx and TFx fold change levels over time. (A) TFx levels observed during CAR-T treatment categorized by response status. (B) TFx fold change observed relative to baseline categorized by response status. For week 1 and onward samples with undetectable ctDNA, LOD95 was substituted for TFx to reflect a minimum fold change in TFx. (C) TFx with corresponding LOD95 for 2 example patients after CAR-T achieving CR at 1 month and maintaining response through 1 year (green box; left) and 2 patients achieving PR at 1 month and subsequently converting to CR (blue box; right). Of note, LOD95 reflects the lowest TFx at which there is 95% likelihood of detection, as opposed to the lowest detectable TFx. (D) TFx with corresponding LOD95 for 2 example patients after CAR-T with PD at 1 month (orange box, left) and 2 example patients achieving CR or PR at 1 month and subsequently progressing (purple box; right). For panels C-D, blue line indicates TFx. (E) Overall performance of predicting patient response status at week 1 and week 2 using TFx fold change from baseline. ROC, Receiver Operating Characteristic.
TFx and TFx fold change levels over time. (A) TFx levels observed during CAR-T treatment categorized by response status. (B) TFx fold change observed relative to baseline categorized by response status. For week 1 and onward samples with undetectable ctDNA, LOD95 was substituted for TFx to reflect a minimum fold change in TFx. (C) TFx with corresponding LOD95 for 2 example patients after CAR-T achieving CR at 1 month and maintaining response through 1 year (green box; left) and 2 patients achieving PR at 1 month and subsequently converting to CR (blue box; right). Of note, LOD95 reflects the lowest TFx at which there is 95% likelihood of detection, as opposed to the lowest detectable TFx. (D) TFx with corresponding LOD95 for 2 example patients after CAR-T with PD at 1 month (orange box, left) and 2 example patients achieving CR or PR at 1 month and subsequently progressing (purple box; right). For panels C-D, blue line indicates TFx. (E) Overall performance of predicting patient response status at week 1 and week 2 using TFx fold change from baseline. ROC, Receiver Operating Characteristic.
Most Rs (11/15 [73%]) had undetectable ctDNA within 4 weeks of treatment (supplemental Figure 2). At a qualitative level, the 2 patients with an initial PR who subsequently converted to CR had ctDNA trajectories similar to patients with up-front CR (Figure 2C), whereas patients with an initial CR or PR (n = 6) who subsequently had PD had overall ctDNA trajectories more akin to patients with up-front PD (Figure 2D). This suggests that MRD detection by ctDNA may be a more accurate predictor of durable response at 1 year than FDG-PET/CT determination of response, although further validation in larger cohorts is needed. Analysis of the receiver operating characteristic curves for TFx and TFx fold change at weeks 1 and 2 revealed that ctDNA at week 2 consistently emerged as a stronger predictor of patient response status overall (Figure 2E; supplemental Figure 3A). Indeed, the Receiver Operating Characteristic (ROC) values were higher at week 2 (area under the curve, 0.97) than at week 1 (area under the curve, 0.87).
For patients with a PR at 1 month who later converted to CR, MAESTRO-Pool showed early ctDNA clearance similar to that in patients with CR at 1 month (supplemental Figure 3B-C). Conversely, the 4 patients with a 1-month PR who later progressed did not have ctDNA reduction, similar to patients with up-front PD.
Discussion
To date, few clinical biomarkers have been identified that predict response to CAR-T therapy.13,14 None have yet been successfully applied to standard clinical care for identifying patients with LBCL at high risk of relapse to allow for early intervention.
Here, we applied MAESTRO-Pool for tracking ctDNA in patients with relapsed/refractory LBCL as an early indicator of MRD and disease progression. This approach is both highly sensitive and specific, identifying differences in MRD as early as 1 week after infusion that strongly associate with a durable 12-month response to CAR-T. Within 1 week of CAR-T infusion, eventual Rs demonstrated a marked reduction in ctDNA, whereas patients who went on to have PD demonstrate persistence of ctDNA. Our results suggest that ctDNA level at week 2 or fold change from baseline can predict durable response at 1 year. Moreover, ctDNA measurement early after CAR-T infusion may better predict long-term outcomes than FDG-PET/CT evaluation, particularly for those with PR by FDG-PET/CT at 1 month; however, this observation requires more robust validation in larger cohorts. One potential caveat is that the time from diagnosis to CAR-T treatment might influence tumor burden at the time of CAR-T, which could influence initial baseline ctDNA levels. However, this appears to be unlikely, because baseline metabolic tumor burden was similar between Rs and NRs. We have additionally compared and found differences in ctDNA fold change between Rs and NRs, which is agnostic to baseline tumor burden.
MAESTRO-Pool has several important advantages. Because it enriches mutated cfDNA apart from the massive excess of nonmutated cfDNA in plasma, it enables testing of thousands of patient-specific tumor mutations with duplex sequencing using up to 100-fold fewer reads. This enables detection at or below ppm levels of ctDNA in patient-matched samples, whereas the pooled testing configuration provides empirical assessment of the specificity of each bespoke MRD assay in patient-unmatched samples. Indeed, in this study, we showed that, of 3780 unmatched MRD tests, only 8 (0.2%) were found to be MRD positive. Another technology, PhasED-seq,15 tracks phased variants (mutations co-occurring on cfDNA fragments) to achieve similar accuracies as duplex sequencing without requiring these to be found on both strands of each duplex. MAESTRO could assay phased variants, but these were not included in our study.
NR patients generally had high TFx, and it did not decrease with time. However, the high sensitivity of MAESTRO-Pool enabled monitoring of response in more patients and at greater depth than would be detectable using a less sensitive test, suggesting this could be a more viable option for disease monitoring than other less sensitive tests that are currently available. This may also aid in the differentiation between transient and durable responses by 1 week. Although this cohort is limited by relatively small sample size to draw firm conclusions, these findings suggest that ctDNA clearance can predict durable response, whereas ctDNA persistence may enable early identification of patients at the highest risk of disease progression, which may inform earlier intervention and improve outcomes. Future prospective studies in larger patient cohorts will aid in establishing the clinical prognostic utility of ctDNA detection.
Acknowledgments
The authors thank the Ted and Eileen Pasquarello Tissue Bank and Doreen Hearsey for prospective collection and processing of serial serum/plasma samples and the Dana-Farber Cancer Institute/Harvard Cancer Center (DFCI/HCC) cellular therapies support staff for continued care of CAR-T patients.
Funding for this study was provided by Kite, a Gilead company, and the Gerstner Family Foundation. K.M. received support from the Lubin Family Foundation. P.A. gratefully acknowledges the support of the Leukemia and Lymphoma Society as well as the Harold and Virginia Lash Foundation. C.J.W. is Lavine Family Chair for Preventive Cancer Therapies.
Authorship
Contribution: K.M., S.H.G., N.K., J.R., K.X., G.M.M., V.A.A., C.J.W., P.A., and C.J. conceived and designed the study; K.M., S.H.G., N.K., R.M., C.D., L.I.G., J.D.C., J.O.W., S.J.R., and M.McDonough identified, collected, and interpreted patient information; K.M., K.X., T.B., A.C., and L.G. designed and performed experiments; H.J. performed metabolic tumor volume quantification; R.R. and D.S.N. designed and performed statistical analysis; K.M., N.K., C.S., J.R. K.X., T.B., J.R., M.Mattie, and B.M. designed, performed, and interpreted data analysis; and all authors participated in manuscript writing and review and provided final approval of the manuscript.
Conflict-of-interest disclosure: C.J. reports consultancy for Kite/Gilead, Bristol Myers Squibb (BMS)/Celgene, Novartis, Instil Bio, ImmPACT Bio, Caribou Bio, Miltenyi, Ipsen, ADC Therapeutics, AbbVie, AstraZeneca, MorphoSys, Synthekine, and Sana; and research funding from Kite/Gilead and Pfizer. C.J.W. holds equity in BioNTech Inc; and receives research funding from Pharmacyclics. V.A.A. is a coinventor of the MAESTRO measurable residual disease (MRD) test, which has been licensed to Exact Sciences; receives research funding from Exact Sciences, which was not involved in this study; and is also a cofounder and advisor of Amplifyer Bio, which was not involved in this study. S.H.G. holds patents related to adoptive cell therapies, held by University College London and NovalGen Limited; has received honoraria, speakers' fees, travel support, and/or advisory board fees from AbbVie, BeiGene, Gilead, EUSA/Recordati, Electra Pharma, and Janssen; and has undertaken consultancy and hold patents with Freeline Therapeutics, NovalGen, and University College London Business. P.A. provides consultancy for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; and receives research funding from Kite, Merck, BMS, Adaptive, Genentech, IGM Biosciences, and AstraZeneca. H.J. receives research support (to institution) from Blue Earth Diagnostics, Inc and Lantheus; consultancy fees from Advanced Accelerator Applications and Spectrum Dynamics; royalties from Cambridge Publishing; and honoraria from Blue Earth Diagnostics, Inc and Monrol. D.S.N. has stock ownership in Madrigal Pharmaceuticals. G.M.M. is a coinventor of the MAESTRO MRD test, which has been licensed to Exact Sciences. The remaining authors declare no competing financial interests.
Correspondence: Catherine J. Wu, Dana-Faber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215; email: Catherine_wu@dfci.harvard.edu; and Viktor A. Adalsteinsson, Broad Institute of the Massachusetts Institute of Technology and Harvard, 75 Ames Street, Cambridge, MA 02142; email: viktor@broadinstitute.org.
References
Author notes
N.K., K.M., C.S., and J.R. are joint first authors.
Original data are available upon reasonable request from the corresponding authors, Viktor A. Adalsteinsson (viktor@broadinstitute.org) and Catherine J. Wu (catherine_wu@dfci.harvard.edu).
The full-text version of this article contains a data supplement.