Key Points
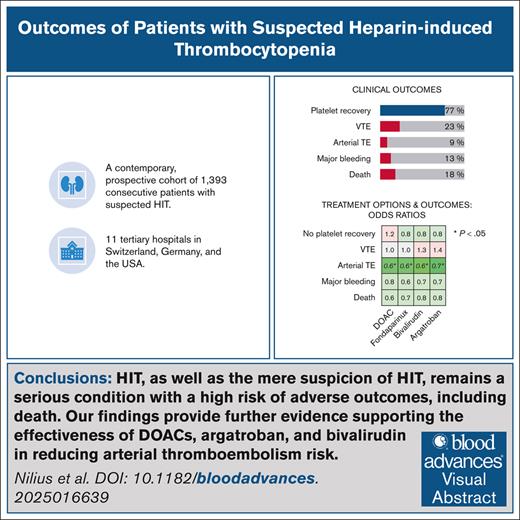
HIT, as well as the mere suspicion of HIT, remains a serious condition with a high risk of adverse outcomes, including death.
Further evidence is provided supporting the effectiveness of DOACs, argatroban, and bivalirudin in reducing arterial thromboembolism risk.
Visual Abstract
Managing patients with suspected heparin-induced thrombocytopenia (HIT) poses significant clinical challenges. Limited evidence exists on how management decisions impact clinical outcomes, leading to treatment recommendations based on low-certainty evidence. This study aimed to evaluate the treatment strategies and clinical outcomes of patients with suspected HIT in a contemporary multicenter cohort. We conducted a prospective, multicenter cohort study including consecutive patients with suspected HIT from 11 centers. Patients were stratified into 3 groups: (1) HIT confirmed, (2) HIT-negative but heparin/platelet factor 4 (PF4) antibody-positive, and (3) HIT-negative without antibodies. Clinical and laboratory data were systematically collected. HIT was diagnosed using the washed-platelet heparin-induced platelet activation test as the reference standard. Among 1393 patients (46% female, median age 67 years), HIT was confirmed in 119 (8.5%). Most patients were in intensive care (37%), or had undergone cardiac surgery (32%). Argatroban was the predominant treatment (70%), and platelet recovery occurred in 77% of patients with HIT. Among patients with HIT, subsequent venous thromboembolism occurred in 23%, arterial thromboembolism in 9%, major bleeding in 12.6%, and mortality in 18%, with no significant differences between anticoagulants. Treatment with argatroban, bivalirudin, or direct oral anticoagulants (DOACs) significantly reduced arterial thromboembolism risk. Outcomes did not differ between patients who were HIT-negative with or without heparin/PF4 antibodies. HIT, as well as the mere suspicion of HIT, remains a serious condition with a high risk of adverse outcomes, including death. Our findings provide further evidence supporting the effectiveness of DOACs, argatroban, and bivalirudin in reducing arterial thromboembolism risk.
Introduction
Despite advancements in diagnostic tests and treatment options, managing patients with suspected heparin-induced thrombocytopenia (HIT) remains a major clinical challenge.1-5 Many hospitalized patients continue to receive unfractionated heparins or low-molecular-weight heparins (LMWHs), with an estimated 12 million individuals exposed annually in the United States alone.6 A considerable proportion develop thrombocytopenia, often accompanied by thromboembolism, raising suspicion of HIT.7,8 In recent years, new clinical scenarios, such as COVID-19 and vaccine-induced immune thrombotic thrombocytopenia, have emerged, increasing the complexity of HIT diagnosis and management.9-11 Additionally, the growing use of extracorporeal membrane oxygenation in critically ill patients has further heightened the risk of thrombocytopenia and HIT.12 In this setting, clinicians face a high-stakes decision about whether to discontinue heparin, which itself carries thromboembolic risks, or to initiate an alternative anticoagulant, increasing the risk of major bleeding.13,14
Early treatment of suspected HIT aims to prevent serious thromboembolic complications.3,6,15,16 However, these complications may arise not only from HIT itself, but also from the underlying condition requiring heparin. Discontinuing heparin in patients without HIT introduces its own thromboembolic risks, while switching to alternative anticoagulants increases the likelihood of major bleeding.14 Patients with suspected HIT are particularly vulnerable due to prior cardiopulmonary surgery, thrombocytopenia, glycoprotein IIb/IIIa inhibitor therapy, and frequent postoperative complications.15,17 Moreover, the benefits of many treatment decisions remain uncertain, and current guidelines acknowledge that most recommendations are based on low-certainty evidence.6,13
In the absence of randomized controlled trials, understanding real-world clinical outcomes is essential. Early studies reported high rates of thromboembolism and mortality in patients with HIT;16,18 however, treatment approaches and patient characteristics have evolved significantly. With new diagnostic tools, treatment options, and changing patient populations, there is a need to reassess clinical outcomes.19 Additionally, many studies on alternative anticoagulants relied on composite end points, limiting the ability to assess whether new thromboembolic events could be effectively prevented.13,20,21 Furthermore, the clinical outcomes of patients with suspected HIT who test negative, either by heparin/platelet factor 4 (PF4) immunoassay or functional assays, remain essentially unknown.6 Data on bleeding risks with nonheparin anticoagulants, particularly in patients without definitive HIT, are also limited.14,20 Moreover, despite increasing interest in direct oral anticoagulants (DOACs), robust evidence on their efficacy and safety remains scarce.6,12,13
Many earlier studies have methodological limitations, including retrospective designs with unrepresentative patient selection, small sample sizes, single-center data collection, and inconsistent diagnostic criteria for HIT.14,19,22 As a result, their findings may not accurately reflect contemporary clinical practice. To address these gaps, several researchers and scientific societies have called for prospective studies that assess patient outcomes using standardized definitions and rigorous data collection methods.6,13,14,19
To address these knowledge gaps, we conducted a prospective, multicenter cohort study to comprehensively assess the clinical outcomes of patients with suspected HIT. Our study aimed to evaluate the risk of thromboembolism, major bleeding, and mortality in confirmed HIT cases, as well as in patients without HIT, stratified by heparin/PF4 antibody status. By applying strict and uniform criteria for HIT diagnosis, and ensuring complete and accurate data collection, we sought to generate robust evidence to inform clinical decision-making.
Methods
Study design, setting, and patient population
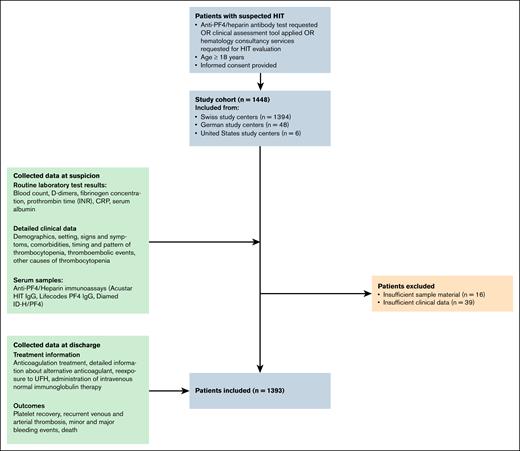
The TORADI-HIT study is a prospective, multicenter cohort study that included 1393 patients with suspected HIT from 11 centers in Switzerland, Germany, and the United States (Figure 1).23-26 Patients were enrolled consecutively between January 2018 and May 2021, but not all study centers were actively recruiting at all time points. Inclusion criteria were: (1) suspected HIT, defined by at least 1 of the following: heparin/PF4 immunoassay ordered, application of a clinical assessment tool, or hematology consultation requested; (2) age >18 years; and (3) provision of informed consent. Patients were excluded if sample material was missing or if clinical data were insufficient.
Patients were recruited from a well-established study network encompassing university and tertiary hospitals. Depending on the study center, either general informed consent or individual study-specific consent was obtained.
Data collection and study procedures
A standardized protocol for data collection was developed and approved by the ethics committee. Specially trained study nurses collected clinical and laboratory data, and entered them into an electronic case report form within the REDCap database. The study workflow is outlined in Figure 1.
To ensure high-quality data collection, training sessions were conducted at each study site. Data were retrieved from hospital information systems at 2 key time points: (1) at the time of HIT suspicion, and (2) at hospital discharge. Predefined data collection forms were integrated into routine clinical workflows. Attending physicians were contacted to resolve missing or inconsistent data. In cases requiring further clarification, an expert committee, consisting of the local hematologist and the center hematologist, reviewed the data.
Baseline data included demographic characteristics, clinical setting, laboratory values, and HIT probability scores.23 Follow-up data at discharge included anticoagulation management (continuation, discontinuation, or switch to an alternative anticoagulant), details of any alternative anticoagulant used, instances of re-exposure to unfractionated heparins, administration of IV immunoglobulin, platelet count at discharge, platelet recovery status (no recovery, <50% increase, >50% increase, or >100 × 109/L), imaging-confirmed venous and arterial thromboembolism, major and minor bleeding events, mortality, and length of hospital stay. Major bleeding was defined according to the most widely accepted definition of the International Society on Thrombosis and Haemostasis: clinically overt bleeding associated with a hemoglobin drop of ≥20 g/L, transfusion of ≥2 units of red blood cells, bleeding in a critical site, or a fatal outcome.27
Definition of HIT
Patients were classified as having HIT if they tested positive in the washed-platelet heparin-induced platelet activation (HIPA) test. Washed platelet assays (ie, HIPA and serotonin release assay [SRA]), demonstrated an adequate diagnostic sensitivity and specificity.6,15,28-34 Clinical studies demonstrated a high agreement with clinical HIT,35,36 and HIPA and SRA are both regarded as reference gold standard for the diagnosis of HIT by the American Society of Hematology guidelines,6 the British Committee for Standards in Haematology,34 and many authors.6,15,28-30,34,37 The analytical performance and all methodological details of the in-house HIPA assay were validated in prior studies.31,32
The HIPA test was performed using washed platelets from 4 different donors under the following conditions: (1) with buffer, (2) with LMWH (0.2 IU/mL), and (3) with unfractionated heparin (100 IU/mL). A test was considered positive if platelet aggregation occurred in at least 2 donors within 30 minutes in the presence of 0.2 IU/mL heparin, but not in the presence of 100 IU/mL heparin. Each test plate included both positive and negative controls.
Statistical analysis
Patients were categorized into 3 groups: (1) HIT-confirmed, (2) HIT-negative but heparin/PF4 antibody-positive, and (3) HIT-negative without antibodies. Patient characteristics, treatment patterns, and clinical outcomes were summarized using medians with interquartile ranges for continuous variables and counts with percentages for categorical variables.
For patients with confirmed HIT, we used multivariable logistic regression to assess risk factors for adverse outcomes, including incomplete platelet recovery, major bleeding, venous thromboembolism, arterial thromboembolism, and mortality. Models were adjusted for sex, age, clinical setting, sepsis, chemotherapy, hemoglobin concentration, white blood cell count, platelet nadir, heparin/PF4 antibody levels, and anticoagulation regimen.
To evaluate differences in outcomes among patients who were HIT-negative with or without heparin/PF4 antibodies, we conducted additional multivariable logistic regression analyses, adjusting for the same covariates. All statistical tests were 2-tailed, and a P value < .05 was considered statistically significant. Analyses were performed using R version 4.3.1.
Ethical approval was granted by the responsible committees (Kantonale Ethikkommission Bern, 2017-01073), and the study was conducted in accordance with the Declaration of Helsinki.
Results
Baseline characteristics of patients with suspected HIT
A total of 1393 patients from 11 study centers were included in the analysis. The median age was 67 years, and 46% of patients were female. Most patients were in intensive care units (37%), or had undergone cardiovascular surgery (32%). Other clinical settings included internal medicine (20%), general surgery (10%), and major trauma (1%).
Sepsis was present in 49% of patients, and 7% had a confirmed severe acute respiratory syndrome coronavirus 2 infection. Unfractionated heparin was administered to 79% of patients, and LMWH to 43% of patients. The median 4Ts score was 3 (interquartile range, 2-5). The platelet nadir was lower in patients with confirmed HIT compared with those without HIT (median: 52 × 109/L vs 60 × 109/L). Heparin/PF4 immunoassay results and additional patient characteristics are summarized in Table 1.
Baseline characteristics of patients with suspected HIT
| Characteristics . | HIT negative . | HIT positive . | Missing data . | |
|---|---|---|---|---|
| H/PF4-ab negative . | H/PF4-ab positive . | HIPA positive . | ||
| n | 1201 | 73 | 119 | |
| Male sex, n (%) | 765 (63.9) | 51 (69.9) | 71 (59.7) | |
| Age, median (IQR) | 67.25 (58.05-75.19) | 61.31 (54.23-75.88) | 64.65 (55.50-74.48) | |
| Setting, n (%) | 1 (0.1) | |||
| ICU | 443 (36.9) | 36 (49.3) | 40 (33.6) | |
| Cardiovascular surgery | 376 (31.3) | 20 (27.4) | 47 (39.5) | |
| Internal medicine | 246 (20.5) | 11 (15.1) | 16 (13.4) | |
| General surgery | 118 (9.8) | 5 (6.8) | 9 (7.6) | |
| Major trauma | 4 (0.3) | 0 (0.0) | 6 (5.0) | |
| Other | 13 (1.1) | 1 (1.4) | 1 (0.8) | |
| Sepsis, n (%) | 578 (48.1) | 42 (57.5) | 57 (47.9) | 0 (0.0) |
| CRP, median (IQR), mg/L | 89 (35-176) | 64 (20-150) | 87 (44-146) | 86 (6.2) |
| SARS-CoV-2 infection, n (%) | 67 (5.6) | 15 (20.8) | 7 (5.9) | 9 (0.6) |
| Unfractionated heparin, n (%) | 934 (77.8) | 61 (83.6) | 103 (86.6) | 0 (0.0) |
| 4Ts score, median (IQR) | 3 (2-4) | 4 (3-5) | 5 (4-6) | 0 (0.0) |
| Platelet nadir, median (IQR), ×109/L | 60 (38-85) | 76 (46-115) | 52 (32-73) | 22 (1.6) |
| CLIA, median (IQR), U/mL | 0.0 (0.00-0.09) | 2.27 (1.48-4.90) | 10.35 (3.76-24.59) | 75 (5.4) |
| Characteristics . | HIT negative . | HIT positive . | Missing data . | |
|---|---|---|---|---|
| H/PF4-ab negative . | H/PF4-ab positive . | HIPA positive . | ||
| n | 1201 | 73 | 119 | |
| Male sex, n (%) | 765 (63.9) | 51 (69.9) | 71 (59.7) | |
| Age, median (IQR) | 67.25 (58.05-75.19) | 61.31 (54.23-75.88) | 64.65 (55.50-74.48) | |
| Setting, n (%) | 1 (0.1) | |||
| ICU | 443 (36.9) | 36 (49.3) | 40 (33.6) | |
| Cardiovascular surgery | 376 (31.3) | 20 (27.4) | 47 (39.5) | |
| Internal medicine | 246 (20.5) | 11 (15.1) | 16 (13.4) | |
| General surgery | 118 (9.8) | 5 (6.8) | 9 (7.6) | |
| Major trauma | 4 (0.3) | 0 (0.0) | 6 (5.0) | |
| Other | 13 (1.1) | 1 (1.4) | 1 (0.8) | |
| Sepsis, n (%) | 578 (48.1) | 42 (57.5) | 57 (47.9) | 0 (0.0) |
| CRP, median (IQR), mg/L | 89 (35-176) | 64 (20-150) | 87 (44-146) | 86 (6.2) |
| SARS-CoV-2 infection, n (%) | 67 (5.6) | 15 (20.8) | 7 (5.9) | 9 (0.6) |
| Unfractionated heparin, n (%) | 934 (77.8) | 61 (83.6) | 103 (86.6) | 0 (0.0) |
| 4Ts score, median (IQR) | 3 (2-4) | 4 (3-5) | 5 (4-6) | 0 (0.0) |
| Platelet nadir, median (IQR), ×109/L | 60 (38-85) | 76 (46-115) | 52 (32-73) | 22 (1.6) |
| CLIA, median (IQR), U/mL | 0.0 (0.00-0.09) | 2.27 (1.48-4.90) | 10.35 (3.76-24.59) | 75 (5.4) |
This table presents demographic, clinical, and laboratory characteristics of 1393 consecutive patients included in a prospective, multicenter cohort study. Patients were stratified into 3 groups: (1) HIT-negative without heparin/PF4 antibodies, (2) HIT-negative with heparin/PF4 antibodies, and (3) HIT-positive, defined by a positive washed-platelet HIPA test.
ab, antibody; CLIA, chemiluminescent immunoassay capturing antibodies against heparin/PF4 complexes; CRP, C-reactive protein; ICU, intensive care unit; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
HIT was confirmed in 119 patients (8.5%) based on the HIPA test. Among patients who were HIT-positive, 33% were in intensive care, and 40% had undergone cardiovascular surgery. The prevalence of heparin/PF4 antibodies was higher in patients who were HIT-positive than in patients who were HIT-negative (median CLIA value: 10.35 U/mL vs 0.00 U/mL). Five patients with HIT (HIPA+) had a CLIA result <1 U/L. Of these 5 patients, 1 also had a negative HIT immunoglobulin G enzyme-linked immunosorbent assay result.
Treatment strategies and clinical outcomes
Alternative anticoagulation was initiated in 299 patients (21.5%), with most receiving argatroban (56%), followed by fondaparinux (20%) and rivaroxaban (8%). IV immunoglobulin was administered to 5% of patients. Among patients with HIT, 94% received an alternative anticoagulant, whereas 9% of patients who were HIT-negative were also treated with nonheparin anticoagulants.
Complete platelet recovery was observed in 77% of patients with HIT, but was considerably lower in patients who were HIT-negative (Table 2). Subsequent venous thromboembolism occurred in 23% of patients who were HIT-positive, while arterial thromboembolism was observed in 9%. Major bleeding was reported in 12.6% of patients who were HIT-positive, and 12.9% of patients who were HIT-negative. The overall mortality rate was 18% in patients who were HIT-positive, and 21% in patients who were HIT-negative.
Treatment and outcomes of patients with suspected HIT
| Treatment/outcomes . | HIT negative . | HIT positive . | Missing data . | |
|---|---|---|---|---|
| H/PF4-ab negative . | H/PF4-ab positive . | HIPA positive . | ||
| Treatment | ||||
| IVIG, n (%) | 30 (2.6) | 0 (0.0) | 6 (5.1) | 34 (2.4) |
| Alternative anticoagulant started, n (%) | 111 (9.3) | 65 (89.0) | 112 (94.1) | 11 (0.7) |
| Argatroban, n (%) | 43 (3.6) | 47 (64.4) | 83 (69.7) | |
| Bivalirudin, n (%) | 5 (0.4) | 5 (6.8) | 12 (10.1) | |
| Danaparoid, n (%) | 2 (0.2) | 0 (0.0) | 0 (0.0) | |
| Fondaparinux, n (%) | 40 (3.3) | 9 (12.3) | 14 (11.8) | |
| Rivaroxaban, n (%) | 15 (1.2) | 2 (2.7) | 9 (7.6) | |
| Apixaban, n (%) | 6 (0.5) | 3 (4.1) | 2 (1.7) | |
| Edoxaban, n (%) | 3 (0.2) | 1 (1.4) | 1 (0.8) | |
| Dabigatran, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.8) | |
| Others, n (%) | 2 (0.2) | 1 (1.4) | 4 (3.4) | |
| Outcomes | ||||
| Platelet recovery, n (%) | 53 (3.8) | |||
| Not recovered | 159 (13.8) | 6 (8.5) | 7 (6.2) | |
| Partially recovered | 303 (26.2) | 25 (35.2) | 19 (16.8) | |
| Fully recovered | 694 (60.0) | 40 (56.3) | 87 (77.0) | |
| Platelets at follow-up, median (IQR), ×109/L | 162 (86-274) | 194 (120-350) | 203 (110-280) | 28 (2.0) |
| Venous thromboembolism, n (%) | 66 (5.6) | 8 (11.3) | 27 (23.1) | 30 (2.2) |
| Arterial thromboembolism, n (%) | 55 (4.7) | 6 (8.5) | 11 (9.4) | 30 (2.2) |
| Major bleeding, n (%) | 159 (13.4) | 6 (8.2) | 15 (12.6) | 13 (0.9) |
| Death, n (%) | 260 (21.7) | 11 (15.1) | 21 (17.6) | 1 (0.1) |
| Treatment/outcomes . | HIT negative . | HIT positive . | Missing data . | |
|---|---|---|---|---|
| H/PF4-ab negative . | H/PF4-ab positive . | HIPA positive . | ||
| Treatment | ||||
| IVIG, n (%) | 30 (2.6) | 0 (0.0) | 6 (5.1) | 34 (2.4) |
| Alternative anticoagulant started, n (%) | 111 (9.3) | 65 (89.0) | 112 (94.1) | 11 (0.7) |
| Argatroban, n (%) | 43 (3.6) | 47 (64.4) | 83 (69.7) | |
| Bivalirudin, n (%) | 5 (0.4) | 5 (6.8) | 12 (10.1) | |
| Danaparoid, n (%) | 2 (0.2) | 0 (0.0) | 0 (0.0) | |
| Fondaparinux, n (%) | 40 (3.3) | 9 (12.3) | 14 (11.8) | |
| Rivaroxaban, n (%) | 15 (1.2) | 2 (2.7) | 9 (7.6) | |
| Apixaban, n (%) | 6 (0.5) | 3 (4.1) | 2 (1.7) | |
| Edoxaban, n (%) | 3 (0.2) | 1 (1.4) | 1 (0.8) | |
| Dabigatran, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.8) | |
| Others, n (%) | 2 (0.2) | 1 (1.4) | 4 (3.4) | |
| Outcomes | ||||
| Platelet recovery, n (%) | 53 (3.8) | |||
| Not recovered | 159 (13.8) | 6 (8.5) | 7 (6.2) | |
| Partially recovered | 303 (26.2) | 25 (35.2) | 19 (16.8) | |
| Fully recovered | 694 (60.0) | 40 (56.3) | 87 (77.0) | |
| Platelets at follow-up, median (IQR), ×109/L | 162 (86-274) | 194 (120-350) | 203 (110-280) | 28 (2.0) |
| Venous thromboembolism, n (%) | 66 (5.6) | 8 (11.3) | 27 (23.1) | 30 (2.2) |
| Arterial thromboembolism, n (%) | 55 (4.7) | 6 (8.5) | 11 (9.4) | 30 (2.2) |
| Major bleeding, n (%) | 159 (13.4) | 6 (8.2) | 15 (12.6) | 13 (0.9) |
| Death, n (%) | 260 (21.7) | 11 (15.1) | 21 (17.6) | 1 (0.1) |
This table summarizes treatment strategies and clinical outcomes in patients with suspected HIT (n = 1393). Patients were stratified into 3 groups: (1) HIT-negative without heparin/PF4 antibodies, (2) HIT-negative with heparin/PF4 antibodies, and (3) HIT-positive, defined by a positive washed-platelet HIPA test. Results are grouped by final diagnosis, which was not available at the time of initial treatment decisions.
ab, antibody; IVIG, IV immunoglobulins.
Notably, any nonheparin anticoagulant use was strongly associated with a lower risk of subsequent arterial thromboembolism, but did not significantly affect venous thromboembolism rates. Among treatment strategies, fondaparinux (P = .01) and argatroban (P = .02) were associated with an increased risk of major bleeding.
Risk factors for adverse outcomes
We analyzed potential risk factors for major adverse outcomes, including incomplete platelet recovery, subsequent venous and arterial thromboembolism, major bleeding, and mortality in patients with HIT (Table 3). Most patient characteristics were not significantly associated with these outcomes. However, male sex was linked to a higher risk of venous thromboembolism (P = .006), and intensive care unit admission or major trauma status was marginally associated with major bleeding (P = .05 and P = .04, respectively).
Risk factors for adverse outcomes in patients with confirmed HIT
| Characteristic . | Not fully recovered platelets . | Major bleeding . | Venous thromboembolism . | Arterial thrombosis . | Death . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 102 . | Exp (Beta) . | 95% CI . | P value . | n = 106 . | Exp (Beta) . | 95% CI . | P value . | n = 105 . | Exp (Beta) . | 95% CI . | P value . | n = 105 . | Exp (Beta) . | 95% CI . | P value . | n = 106 . | Exp (Beta) . | 95% CI . | P value . | |
| Sex | ||||||||||||||||||||
| Female | 40 | — | — | — | 43 | — | — | — | 42 | — | — | — | 42 | — | — | — | 43 | — | — | — |
| Male | 62 | 1.09 | 0.92-1.31 | .3 | 63 | 1.05 | 0.91-1.20 | .5 | 63 | 1.29 | 1.08-1.53 | .006 | 63 | 1.02 | 0.91-1.15 | .7 | 63 | 1.00 | 0.85-1.17 | >.9 |
| Age less than median | 102 | 1.08 | 0.91-1.30 | .4 | 106 | 0.97 | 0.85-1.12 | .7 | 105 | 1.06 | 0.89-1.26 | .5 | 105 | 0.98 | 0.87-1.10 | .7 | 106 | 1.14 | 0.97-1.34 | .10 |
| Setting | ||||||||||||||||||||
| Postoperative general surgery and orthopedics | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — |
| Postoperative cardiac and vascular surgery | 40 | 0.92 | 0.63-1.34 | .7 | 42 | 0.80 | 0.59-1.08 | .14 | 42 | 1.05 | 0.72-1.52 | .8 | 42 | 1.18 | 0.91-1.52 | .2 | 42 | 0.99 | 0.70-1.39 | >.9 |
| Internal medicine | 13 | 1.23 | 0.80-1.88 | .3 | 13 | 0.85 | 0.61-1.20 | .4 | 13 | 0.86 | 0.56-1.32 | .5 | 13 | 1.02 | 0.76-1.36 | >.9 | 13 | 1.00 | 0.68-1.47 | >.9 |
| ICU | 37 | 1.18 | 0.81-1.73 | .4 | 38 | 0.74 | 0.55-0.99 | .049 | 37 | 1.14 | 0.78-1.66 | .5 | 37 | 1.03 | 0.80-1.33 | .8 | 38 | 0.98 | 0.69-1.38 | .9 |
| Major trauma | 6 | 0.84 | 0.51-1.38 | .5 | 6 | 0.65 | 0.44-0.97 | .037 | 6 | 0.75 | 0.45-1.23 | .3 | 6 | 0.92 | 0.65-1.30 | .6 | 6 | 0.88 | 0.56-1.38 | .6 |
| Other | 1 | 1.21 | 0.48-3.09 | .7 | 1 | 1.08 | 0.57-2.04 | .8 | 1 | 1.15 | 0.49-2.71 | .7 | 1 | 1.15 | 0.49-2.71 | .7 | ||||
| Sepsis | ||||||||||||||||||||
| No | 49 | — | — | — | 52 | — | — | — | 51 | — | — | — | 51 | — | — | — | 52 | — | — | — |
| Yes | 53 | 1.05 | 0.88-1.26 | .6 | 54 | 1.15 | 1.01-1.32 | .043 | 54 | 1.02 | 0.86-1.22 | .8 | 54 | 1.01 | 0.89-1.13 | >.9 | 54 | 1.14 | 0.97-1.33 | .11 |
| Chemotherapy | ||||||||||||||||||||
| No | 98 | — | — | — | 102 | — | — | — | 101 | — | — | — | 101 | — | — | — | 102 | — | — | — |
| Yes | 4 | 0.65 | 0.41-1.01 | .060 | 4 | 0.91 | 0.64-1.30 | .6 | 4 | 0.77 | 0.49-1.21 | .3 | 4 | 0.99 | 0.73-1.34 | >.9 | 4 | 1.05 | 0.70-1.57 | .8 |
| Hb >12 g/L | 102 | 0.94 | 0.64-1.38 | .7 | 106 | 0.92 | 0.67-1.25 | .6 | 105 | 0.88 | 0.60-1.30 | .5 | 105 | 0.92 | 0.70-1.20 | .5 | 106 | 1.05 | 0.74-1.50 | .8 |
| WBC >10 × 109/L | 102 | 1.12 | 0.93-1.35 | .2 | 106 | 1.05 | 0.91-1.21 | .5 | 105 | 1.01 | 0.84-1.22 | .9 | 105 | 1.02 | 0.90-1.16 | .7 | 106 | 1.11 | 0.94-1.30 | .2 |
| Platelet nadir >50 × 109/L | 102 | 1.02 | 0.86-1.21 | .8 | 106 | 1.01 | 0.89-1.16 | .8 | 105 | 0.97 | 0.82-1.14 | .7 | 105 | 0.99 | 0.89-1.11 | .9 | 106 | 0.87 | 0.75-1.01 | .068 |
| AcuStar HIT per U/mL | 102 | 1.00 | 1.00-1.00 | .2 | 106 | 1.00 | 1.00-1.00 | >.9 | 105 | 1.00 | 1.00-1.00 | .3 | 105 | 1.00 | 1.00-1.00 | .7 | 106 | 1.00 | 1.00-1.00 | .6 |
| Anticoagulation therapy | ||||||||||||||||||||
| No alternative anticoagulant | 6 | — | — | — | 7 | — | — | — | 6 | — | — | — | 6 | — | — | — | 7 | — | — | — |
| DOAC only | 3 | 1.15 | 0.63-2.10 | .6 | 4 | 0.79 | 0.52-1.21 | .3 | 4 | 0.96 | 0.56-1.65 | .9 | 4 | 0.59 | 0.41-0.86 | .007 | 4 | 0.63 | 0.39-1.01 | .058 |
| Fondaparinux only | 8 | 0.83 | 0.52-1.33 | .4 | 10 | 0.63 | 0.45-0.89 | .011 | 10 | 1.04 | 0.66-1.64 | .9 | 10 | 0.62 | 0.45-0.84 | .003 | 10 | 0.73 | 0.49-1.09 | .13 |
| Bivalirudin | 9 | 0.81 | 0.51-1.29 | .4 | 9 | 0.70 | 0.49-1.00 | .054 | 9 | 1.31 | 0.82-2.08 | .3 | 9 | 0.60 | 0.44-0.83 | .002 | 9 | 0.76 | 0.51-1.15 | .2 |
| Argatroban | 76 | 0.79 | 0.55-1.15 | .2 | 76 | 0.71 | 0.54-0.93 | .017 | 76 | 1.38 | 0.96-2.00 | .085 | 76 | 0.67 | 0.52-0.86 | .002 | 76 | 0.80 | 0.58-1.09 | .2 |
| Characteristic . | Not fully recovered platelets . | Major bleeding . | Venous thromboembolism . | Arterial thrombosis . | Death . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 102 . | Exp (Beta) . | 95% CI . | P value . | n = 106 . | Exp (Beta) . | 95% CI . | P value . | n = 105 . | Exp (Beta) . | 95% CI . | P value . | n = 105 . | Exp (Beta) . | 95% CI . | P value . | n = 106 . | Exp (Beta) . | 95% CI . | P value . | |
| Sex | ||||||||||||||||||||
| Female | 40 | — | — | — | 43 | — | — | — | 42 | — | — | — | 42 | — | — | — | 43 | — | — | — |
| Male | 62 | 1.09 | 0.92-1.31 | .3 | 63 | 1.05 | 0.91-1.20 | .5 | 63 | 1.29 | 1.08-1.53 | .006 | 63 | 1.02 | 0.91-1.15 | .7 | 63 | 1.00 | 0.85-1.17 | >.9 |
| Age less than median | 102 | 1.08 | 0.91-1.30 | .4 | 106 | 0.97 | 0.85-1.12 | .7 | 105 | 1.06 | 0.89-1.26 | .5 | 105 | 0.98 | 0.87-1.10 | .7 | 106 | 1.14 | 0.97-1.34 | .10 |
| Setting | ||||||||||||||||||||
| Postoperative general surgery and orthopedics | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — | 6 | — | — | — |
| Postoperative cardiac and vascular surgery | 40 | 0.92 | 0.63-1.34 | .7 | 42 | 0.80 | 0.59-1.08 | .14 | 42 | 1.05 | 0.72-1.52 | .8 | 42 | 1.18 | 0.91-1.52 | .2 | 42 | 0.99 | 0.70-1.39 | >.9 |
| Internal medicine | 13 | 1.23 | 0.80-1.88 | .3 | 13 | 0.85 | 0.61-1.20 | .4 | 13 | 0.86 | 0.56-1.32 | .5 | 13 | 1.02 | 0.76-1.36 | >.9 | 13 | 1.00 | 0.68-1.47 | >.9 |
| ICU | 37 | 1.18 | 0.81-1.73 | .4 | 38 | 0.74 | 0.55-0.99 | .049 | 37 | 1.14 | 0.78-1.66 | .5 | 37 | 1.03 | 0.80-1.33 | .8 | 38 | 0.98 | 0.69-1.38 | .9 |
| Major trauma | 6 | 0.84 | 0.51-1.38 | .5 | 6 | 0.65 | 0.44-0.97 | .037 | 6 | 0.75 | 0.45-1.23 | .3 | 6 | 0.92 | 0.65-1.30 | .6 | 6 | 0.88 | 0.56-1.38 | .6 |
| Other | 1 | 1.21 | 0.48-3.09 | .7 | 1 | 1.08 | 0.57-2.04 | .8 | 1 | 1.15 | 0.49-2.71 | .7 | 1 | 1.15 | 0.49-2.71 | .7 | ||||
| Sepsis | ||||||||||||||||||||
| No | 49 | — | — | — | 52 | — | — | — | 51 | — | — | — | 51 | — | — | — | 52 | — | — | — |
| Yes | 53 | 1.05 | 0.88-1.26 | .6 | 54 | 1.15 | 1.01-1.32 | .043 | 54 | 1.02 | 0.86-1.22 | .8 | 54 | 1.01 | 0.89-1.13 | >.9 | 54 | 1.14 | 0.97-1.33 | .11 |
| Chemotherapy | ||||||||||||||||||||
| No | 98 | — | — | — | 102 | — | — | — | 101 | — | — | — | 101 | — | — | — | 102 | — | — | — |
| Yes | 4 | 0.65 | 0.41-1.01 | .060 | 4 | 0.91 | 0.64-1.30 | .6 | 4 | 0.77 | 0.49-1.21 | .3 | 4 | 0.99 | 0.73-1.34 | >.9 | 4 | 1.05 | 0.70-1.57 | .8 |
| Hb >12 g/L | 102 | 0.94 | 0.64-1.38 | .7 | 106 | 0.92 | 0.67-1.25 | .6 | 105 | 0.88 | 0.60-1.30 | .5 | 105 | 0.92 | 0.70-1.20 | .5 | 106 | 1.05 | 0.74-1.50 | .8 |
| WBC >10 × 109/L | 102 | 1.12 | 0.93-1.35 | .2 | 106 | 1.05 | 0.91-1.21 | .5 | 105 | 1.01 | 0.84-1.22 | .9 | 105 | 1.02 | 0.90-1.16 | .7 | 106 | 1.11 | 0.94-1.30 | .2 |
| Platelet nadir >50 × 109/L | 102 | 1.02 | 0.86-1.21 | .8 | 106 | 1.01 | 0.89-1.16 | .8 | 105 | 0.97 | 0.82-1.14 | .7 | 105 | 0.99 | 0.89-1.11 | .9 | 106 | 0.87 | 0.75-1.01 | .068 |
| AcuStar HIT per U/mL | 102 | 1.00 | 1.00-1.00 | .2 | 106 | 1.00 | 1.00-1.00 | >.9 | 105 | 1.00 | 1.00-1.00 | .3 | 105 | 1.00 | 1.00-1.00 | .7 | 106 | 1.00 | 1.00-1.00 | .6 |
| Anticoagulation therapy | ||||||||||||||||||||
| No alternative anticoagulant | 6 | — | — | — | 7 | — | — | — | 6 | — | — | — | 6 | — | — | — | 7 | — | — | — |
| DOAC only | 3 | 1.15 | 0.63-2.10 | .6 | 4 | 0.79 | 0.52-1.21 | .3 | 4 | 0.96 | 0.56-1.65 | .9 | 4 | 0.59 | 0.41-0.86 | .007 | 4 | 0.63 | 0.39-1.01 | .058 |
| Fondaparinux only | 8 | 0.83 | 0.52-1.33 | .4 | 10 | 0.63 | 0.45-0.89 | .011 | 10 | 1.04 | 0.66-1.64 | .9 | 10 | 0.62 | 0.45-0.84 | .003 | 10 | 0.73 | 0.49-1.09 | .13 |
| Bivalirudin | 9 | 0.81 | 0.51-1.29 | .4 | 9 | 0.70 | 0.49-1.00 | .054 | 9 | 1.31 | 0.82-2.08 | .3 | 9 | 0.60 | 0.44-0.83 | .002 | 9 | 0.76 | 0.51-1.15 | .2 |
| Argatroban | 76 | 0.79 | 0.55-1.15 | .2 | 76 | 0.71 | 0.54-0.93 | .017 | 76 | 1.38 | 0.96-2.00 | .085 | 76 | 0.67 | 0.52-0.86 | .002 | 76 | 0.80 | 0.58-1.09 | .2 |
This table presents multivariable regression models identifying risk factors for major adverse outcomes during the clinical course, including incomplete platelet recovery, venous and arterial thromboembolism, major bleeding, and mortality. Regression coefficients and 95% CIs are reported (a coefficient of 1 indicates no effect). Treatment with alternative anticoagulants was significantly associated with a lower risk of subsequent arterial thromboembolism.
CI, confidence interval; Hb, hemoglobin; ICU, intensive care unit; WBC, white blood cell.
Clinical outcomes in patients who were HIT-negative
We assessed risk factors for adverse outcomes in patients who were HIT-negative to determine whether the presence of heparin/PF4 antibodies influenced clinical events (Table 4). As expected, established risk factors in hospitalized patients, such as intensive care unit admission, sepsis, low hemoglobin, high white blood cell count, and chemotherapy, were significantly associated with adverse outcomes.
Risk factors for adverse outcomes in patients who were HIT-negative
| Characteristic . | Not fully recovered platelets . | Major bleeding . | Venous thromboembolism . | Arterial thrombosis . | Death . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1144 . | Exp (Beta) . | 95% CI . | P value . | n = 1172 . | Exp (Beta) . | 95% CI . | P value . | n = 1150 . | Exp (Beta) . | 95% CI . | P value . | n = 1150 . | Exp (Beta) . | 95% CI . | P value . | n = 1175 . | Exp (Beta) . | 95% CI . | P value . | |
| Sex | ||||||||||||||||||||
| Female | 403 | — | — | — | 412 | — | — | — | 403 | — | — | — | 403 | — | — | — | 412 | — | — | — |
| Male | 741 | 1.00 | 0.94-1.06 | >.9 | 760 | 1.05 | 1.01-1.10 | .015 | 747 | 1.01 | 0.98-1.04 | .5 | 747 | 0.99 | 0.97-1.02 | .5 | 763 | 0.98 | 0.93-1.03 | .4 |
| Age less than median | 1144 | 1.05 | 0.99-1.11 | .11 | 1172 | 0.96 | 0.92-1.00 | .044 | 1150 | 0.97 | 0.95-1.00 | .058 | 1150 | 1.00 | 0.97-1.02 | .8 | 1175 | 1.05 | 1.00-1.10 | .034 |
| Setting | ||||||||||||||||||||
| Postoperative general surgery and orthopedics | 108 | — | — | — | 110 | — | — | — | 105 | — | — | — | 105 | — | — | — | 110 | — | — | — |
| Postoperative cardiac and vascular surgery | 377 | 1.05 | 0.94-1.17 | .4 | 383 | 1.03 | 0.96-1.11 | .4 | 374 | 0.93 | 0.88-0.98 | .012 | 374 | 1.03 | 0.98-1.08 | .3 | 383 | 0.97 | 0.89-1.06 | .5 |
| Internal medicine | 219 | 1.25 | 1.12-1.39 | <.001 | 227 | 0.98 | 0.91-1.06 | .7 | 226 | 0.94 | 0.89-1.00 | .037 | 226 | 1.01 | 0.96-1.06 | .8 | 228 | 0.98 | 0.90-1.08 | .7 |
| ICU | 428 | 1.19 | 1.07-1.31 | .001 | 438 | 1.04 | 0.97-1.12 | .3 | 431 | 0.97 | 0.92-1.02 | .3 | 431 | 1.02 | 0.97-1.07 | .4 | 440 | 1.15 | 1.05-1.25 | .001 |
| Major trauma | 4 | 0.84 | 0.52-1.36 | .5 | 4 | 1.12 | 0.80-1.58 | .5 | 4 | 0.90 | 0.71-1.15 | .4 | 4 | 0.97 | 0.78-1.21 | .8 | 4 | 0.87 | 0.59-1.29 | .5 |
| Other | 8 | 1.19 | 0.85-1.68 | .3 | 10 | 0.93 | 0.74-1.16 | .5 | 10 | 0.91 | 0.78-1.07 | .3 | 10 | 0.98 | 0.85-1.13 | .8 | 10 | 0.90 | 0.70-1.17 | .4 |
| Sepsis | ||||||||||||||||||||
| No | 582 | — | — | — | 600 | — | — | — | 592 | — | — | — | 592 | — | — | — | 602 | — | — | — |
| Yes | 562 | 1.01 | 0.95-1.07 | .8 | 572 | 1.02 | 0.97-1.06 | .5 | 558 | 1.03 | 1.00-1.06 | .080 | 558 | 1.01 | 0.98-1.03 | .7 | 573 | 1.04 | 0.99-1.10 | .083 |
| Chemotherapy | ||||||||||||||||||||
| No | 1037 | — | — | — | 1060 | — | — | — | 1038 | — | — | — | 1036 | — | — | — | 1062 | — | — | — |
| Yes | 107 | 1.12 | 1.01-1.24 | .026 | 112 | 0.98 | 0.91-1.05 | .5 | 112 | 0.97 | 0.92-1.02 | .2 | 112 | 0.98 | 0.93-1.02 | .3 | 113 | 1.14 | 1.05-1.23 | .001 |
| Hb >12 g/L | 1144 | 1.07 | 0.97 1.17 | .2 | 1172 | 0.92 | 0.86-0.98 | .009 | 1150 | 0.97 | 0.93-1.01 | .2 | 1150 | 0.98 | 0.94-1.02 | .2 | 1175 | 0.91 | 0.84-0.98 | .009 |
| WBC >10 × 109/L | 1144 | 0.94 | 0.88-0.99 | .024 | 1172 | 1.09 | 1.05-1.14 | <.001 | 1150 | 1.03 | 1.00-1.06 | .053 | 1150 | 1.05 | 1.03-1.08 | <.001 | 1175 | 1.13 | 1.08-1.18 | <.001 |
| Platelet nadir >50 × 109/L | 1144 | 1.17 | 1.10-1.24 | <.001 | 1172 | 0.97 | 0.93-1.01 | .11 | 1150 | 1.00 | 0.97-1.03 | .8 | 1150 | 0.98 | 0.96-1.01 | .2 | 1175 | 0.94 | 0.90-0.99 | .016 |
| AcuStar HIT | ||||||||||||||||||||
| Negative | 1076 | — | — | — | 1103 | — | — | — | 1083 | — | — | — | 1083 | — | — | — | 1106 | — | — | — |
| Positive | 68 | 1.02 | 0.87-1.18 | .8 | 69 | 0.97 | 0.87-1.07 | .5 | 67 | 0.98 | 0.91-1.06 | .7 | 67 | 1.01 | 0.94-1.08 | .9 | 69 | 0.93 | 0.83-1.06 | .3 |
| Anticoagulation therapy | ||||||||||||||||||||
| No alternative anticoagulant | 988 | — | — | — | 1012 | — | — | — | 994 | — | — | — | 994 | — | — | — | 1015 | — | — | — |
| DOAC only | 22 | 1.07 | 0.087-1.32 | .5 | 22 | 0.91 | 0.78-1.05 | .2 | 22 | 1.04 | 0.94-1.15 | .5 | 22 | 1.02 | 0.93-1.12 | .7 | 22 | 0.93 | 0.79-1.10 | .4 |
| Fondaparinux only | 43 | 0.88 | 0.76-1.02 | .10 | 44 | 0.96 | 0.86-1.06 | .4 | 43 | 0.96 | 0.89-1.04 | .3 | 43 | 0.99 | 0.93-1.06 | .8 | 44 | 0.88 | 0.78-0.99 | .036 |
| Bivalirudin | 8 | 0.97 | 0.69-1.36 | .9 | 8 | 1.00 | 0.78-1.27 | >.9 | 8 | 1.10 | 0.93-1.31 | .3 | 8 | 0.95 | 0.81-1.11 | .5 | 8 | 1.14 | 0.51-1.15 | .4 |
| Argatroban | 83 | 1.01 | 0.88-1.15 | >.9 | 86 | 0.97 | 0.88-1.06 | .5 | 83 | 1.11 | 1.03-1.18 | .003 | 83 | 1.06 | 1.00-1.13 | .046 | 86 | 1.01 | 0.91-1.13 | .8 |
| Characteristic . | Not fully recovered platelets . | Major bleeding . | Venous thromboembolism . | Arterial thrombosis . | Death . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1144 . | Exp (Beta) . | 95% CI . | P value . | n = 1172 . | Exp (Beta) . | 95% CI . | P value . | n = 1150 . | Exp (Beta) . | 95% CI . | P value . | n = 1150 . | Exp (Beta) . | 95% CI . | P value . | n = 1175 . | Exp (Beta) . | 95% CI . | P value . | |
| Sex | ||||||||||||||||||||
| Female | 403 | — | — | — | 412 | — | — | — | 403 | — | — | — | 403 | — | — | — | 412 | — | — | — |
| Male | 741 | 1.00 | 0.94-1.06 | >.9 | 760 | 1.05 | 1.01-1.10 | .015 | 747 | 1.01 | 0.98-1.04 | .5 | 747 | 0.99 | 0.97-1.02 | .5 | 763 | 0.98 | 0.93-1.03 | .4 |
| Age less than median | 1144 | 1.05 | 0.99-1.11 | .11 | 1172 | 0.96 | 0.92-1.00 | .044 | 1150 | 0.97 | 0.95-1.00 | .058 | 1150 | 1.00 | 0.97-1.02 | .8 | 1175 | 1.05 | 1.00-1.10 | .034 |
| Setting | ||||||||||||||||||||
| Postoperative general surgery and orthopedics | 108 | — | — | — | 110 | — | — | — | 105 | — | — | — | 105 | — | — | — | 110 | — | — | — |
| Postoperative cardiac and vascular surgery | 377 | 1.05 | 0.94-1.17 | .4 | 383 | 1.03 | 0.96-1.11 | .4 | 374 | 0.93 | 0.88-0.98 | .012 | 374 | 1.03 | 0.98-1.08 | .3 | 383 | 0.97 | 0.89-1.06 | .5 |
| Internal medicine | 219 | 1.25 | 1.12-1.39 | <.001 | 227 | 0.98 | 0.91-1.06 | .7 | 226 | 0.94 | 0.89-1.00 | .037 | 226 | 1.01 | 0.96-1.06 | .8 | 228 | 0.98 | 0.90-1.08 | .7 |
| ICU | 428 | 1.19 | 1.07-1.31 | .001 | 438 | 1.04 | 0.97-1.12 | .3 | 431 | 0.97 | 0.92-1.02 | .3 | 431 | 1.02 | 0.97-1.07 | .4 | 440 | 1.15 | 1.05-1.25 | .001 |
| Major trauma | 4 | 0.84 | 0.52-1.36 | .5 | 4 | 1.12 | 0.80-1.58 | .5 | 4 | 0.90 | 0.71-1.15 | .4 | 4 | 0.97 | 0.78-1.21 | .8 | 4 | 0.87 | 0.59-1.29 | .5 |
| Other | 8 | 1.19 | 0.85-1.68 | .3 | 10 | 0.93 | 0.74-1.16 | .5 | 10 | 0.91 | 0.78-1.07 | .3 | 10 | 0.98 | 0.85-1.13 | .8 | 10 | 0.90 | 0.70-1.17 | .4 |
| Sepsis | ||||||||||||||||||||
| No | 582 | — | — | — | 600 | — | — | — | 592 | — | — | — | 592 | — | — | — | 602 | — | — | — |
| Yes | 562 | 1.01 | 0.95-1.07 | .8 | 572 | 1.02 | 0.97-1.06 | .5 | 558 | 1.03 | 1.00-1.06 | .080 | 558 | 1.01 | 0.98-1.03 | .7 | 573 | 1.04 | 0.99-1.10 | .083 |
| Chemotherapy | ||||||||||||||||||||
| No | 1037 | — | — | — | 1060 | — | — | — | 1038 | — | — | — | 1036 | — | — | — | 1062 | — | — | — |
| Yes | 107 | 1.12 | 1.01-1.24 | .026 | 112 | 0.98 | 0.91-1.05 | .5 | 112 | 0.97 | 0.92-1.02 | .2 | 112 | 0.98 | 0.93-1.02 | .3 | 113 | 1.14 | 1.05-1.23 | .001 |
| Hb >12 g/L | 1144 | 1.07 | 0.97 1.17 | .2 | 1172 | 0.92 | 0.86-0.98 | .009 | 1150 | 0.97 | 0.93-1.01 | .2 | 1150 | 0.98 | 0.94-1.02 | .2 | 1175 | 0.91 | 0.84-0.98 | .009 |
| WBC >10 × 109/L | 1144 | 0.94 | 0.88-0.99 | .024 | 1172 | 1.09 | 1.05-1.14 | <.001 | 1150 | 1.03 | 1.00-1.06 | .053 | 1150 | 1.05 | 1.03-1.08 | <.001 | 1175 | 1.13 | 1.08-1.18 | <.001 |
| Platelet nadir >50 × 109/L | 1144 | 1.17 | 1.10-1.24 | <.001 | 1172 | 0.97 | 0.93-1.01 | .11 | 1150 | 1.00 | 0.97-1.03 | .8 | 1150 | 0.98 | 0.96-1.01 | .2 | 1175 | 0.94 | 0.90-0.99 | .016 |
| AcuStar HIT | ||||||||||||||||||||
| Negative | 1076 | — | — | — | 1103 | — | — | — | 1083 | — | — | — | 1083 | — | — | — | 1106 | — | — | — |
| Positive | 68 | 1.02 | 0.87-1.18 | .8 | 69 | 0.97 | 0.87-1.07 | .5 | 67 | 0.98 | 0.91-1.06 | .7 | 67 | 1.01 | 0.94-1.08 | .9 | 69 | 0.93 | 0.83-1.06 | .3 |
| Anticoagulation therapy | ||||||||||||||||||||
| No alternative anticoagulant | 988 | — | — | — | 1012 | — | — | — | 994 | — | — | — | 994 | — | — | — | 1015 | — | — | — |
| DOAC only | 22 | 1.07 | 0.087-1.32 | .5 | 22 | 0.91 | 0.78-1.05 | .2 | 22 | 1.04 | 0.94-1.15 | .5 | 22 | 1.02 | 0.93-1.12 | .7 | 22 | 0.93 | 0.79-1.10 | .4 |
| Fondaparinux only | 43 | 0.88 | 0.76-1.02 | .10 | 44 | 0.96 | 0.86-1.06 | .4 | 43 | 0.96 | 0.89-1.04 | .3 | 43 | 0.99 | 0.93-1.06 | .8 | 44 | 0.88 | 0.78-0.99 | .036 |
| Bivalirudin | 8 | 0.97 | 0.69-1.36 | .9 | 8 | 1.00 | 0.78-1.27 | >.9 | 8 | 1.10 | 0.93-1.31 | .3 | 8 | 0.95 | 0.81-1.11 | .5 | 8 | 1.14 | 0.51-1.15 | .4 |
| Argatroban | 83 | 1.01 | 0.88-1.15 | >.9 | 86 | 0.97 | 0.88-1.06 | .5 | 83 | 1.11 | 1.03-1.18 | .003 | 83 | 1.06 | 1.00-1.13 | .046 | 86 | 1.01 | 0.91-1.13 | .8 |
This table presents multivariable regression models assessing factors associated with adverse outcomes during the clinical course in patients with suspected HIT but negative functional testing. Regression coefficients and 95% CIs are reported (a coefficient of 1 indicates no effect).
However, heparin/PF4 antibody positivity had no significant impact on thromboembolism, major bleeding, or mortality. These findings suggest that, among patients who were HIT-negative, antibody presence alone does not influence clinical outcomes.
Discussion
This prospective, multicenter cohort study systematically assessed the clinical outcomes of patients with suspected HIT. Of the 1393 patients included, 8.5% were found to have HIT (prevalence). Regardless of whether the final diagnosis was HIT or not, we observed high rates of subsequent thromboembolic complications, major bleeding, and death. In patients with HIT, treatment with argatroban, bivalirudin or DOACs was consistently associated with a reduced risk of arterial thromboembolism. However, this was not the case with regard to venous thromboembolism. Patients without HIT, regardless of heparin/PF4 antibody status, had similar clinical outcomes, suggesting that antibody positivity alone does not confer an increased risk of adverse events.
Several earlier studies reported high thromboembolism and mortality rates in patients with HIT, but their findings were largely based on retrospective data, single-center cohorts, or outdated treatment practices.16,18,38 Our study confirms that HIT remains a serious condition with substantial risks, but it also reflects contemporary clinical management, including the increasing use of DOACs. Compared with historical cohorts, where thromboembolism rates often exceeded 50%, our findings suggest a possible improvement in patient outcomes, potentially due to more systematic HIT recognition and optimized anticoagulation strategies. We observed a lower rate of complete platelet recovery (77%) than reported in some prior studies,13 which may reflect differences in study design, patient populations, or real-world treatment conditions. While previous research has suggested that heparin/PF4 antibody positivity in patients who were HIT-negative could indicate an increased thrombotic risk, our data do not support this, adding to the growing uncertainty about the clinical significance of isolated antibody positivity.
A major strength of our study is its large sample size and prospective, multicenter design, which minimizes selection bias and enhances generalizability. By systematically applying the HIPA test as a reference standard for HIT diagnosis, we ensured a uniform classification of cases. Additionally, our structured data collection process, including predefined protocols and expert review of unclear cases, reduced the risk of misclassification and missing data. The inclusion of a large, consecutive patient cohort across different clinical settings further strengthens the applicability of our findings.
However, some limitations must be acknowledged. Despite being one of the largest prospective HIT studies to date, the sample size remains limited for certain subgroup analyses, particularly when comparing different anticoagulants. In addition, we may have missed patients with HIT whose treating physician did not express any suspicion. However, we believe that awareness is high in the study centers participating in the TORADI-HIT study, and that the risk of missing cases is therefore low. The relatively low prevalence is confirmation of this. We also believe that the key findings of the study would not be influenced by selection bias. As another limitation, 3 study centers accounted for most patients (supplemental Table 1). In such a constellation, distortions in the numerical results are possible in principle. However, we cannot envision how these could have influenced the key findings of the study. Besides, one might argue that despite the high degree of agreement between the 2 washed platelet tests SRA and HIPA, the good clinical data, and the recommendations of all major professional societies, it cannot be ruled out that SRA detects slightly more cases of HIT. We agree that it would change the numerical results somewhat. However, we cannot imagine how it would change the basic conclusions of the paper. Another finding of our study was that treatment with nonheparin anticoagulants was not associated with major bleeding. However, this contradicts previous studies, and may be due to the specific study population at hand being at risk of major bleeding for various other reasons. Finally, our findings may not be fully generalizable to settings where HIT diagnostics or treatment strategies differ systematically from those used in our study centers.
The question arises as to what these results mean for clinical practice, and for medical research. As this was not a randomized clinical trial that directly compared different treatments, nor did it have a large enough sample size in all subgroups, we cannot provide specific recommendations for salient clinical questions. However, it is one of the largest HIT cohorts, probably with the most rigorous methodology, so the results must be considered in the current state of knowledge. Firstly, patients with suspected HIT have a very high risk of complications and death, regardless of whether HIT is actually present. Thrombocytopenia and thromboembolism (presumable driver of suspicion) are manifestations and consequences of a wide variety of serious diseases, especially in critically ill patients. Secondly, nonheparin anticoagulants are consistently associated with a significantly reduced risk of arterial thromboembolism, but not with venous thromboembolism. Although we cannot completely rule out spurious results due to the moderate number of cases, we see no statistical indication of them. Therefore, we tend to assume that this is a genuine phenomenon, which could be explained by the lower efficacy of nonheparin anticoagulants on venous thromboembolism, for example. Thirdly, we see no evidence in our cohorts that DOACs are less effective than IV anticoagulants, which further supports their use in clinical practice. And fourthly, our data provide no evidence that patients without HIT but with positive H/PF4 antibodies are at higher risk of complications than patients without. This could be due to the more rigorous study design compared with previous studies, and does not support a specific treatment for these patients. As the next step in scientific inquiry, we propose, if possible in this difficult population, to conduct a randomized controlled trail comparing argatroban, as the most established nonheparin anticoagulant, with rivaroxaban, as the potentially safest and most simple drug.
In conclusion, our data indicate that despite advances in diagnosis and treatment, HIT remains a serious condition with a high risk of complications. Interestingly, the mere suspicion of HIT, presumably arising from thrombocytopenia and thromboembolism, emerges as a risk factor for serious complications, including death. Besides, our findings provide further evidence supporting the effectiveness of nonheparin anticoagulants, including DOACs, in reducing arterial thromboembolism. DOACs are a promising therapeutic option, but further research is needed to refine anticoagulation strategies, and ensure both efficacy and safety.
Acknowledgments
This study was supported by a research grant from the Swiss National Science Foundation (215574).
The funding source had no role in the design and conduct of the study, the analysis and interpretation of data, or in the preparation, review, or approval of the manuscript.
Authorship
Contribution: H.N. wrote the analysis plan, conducted the analysis, interpreted the findings, and contributed to the manuscript; E.S. contributed to the analysis, interpreted the findings, and wrote the manuscript; J.-D.S., D.A.T., A.G., A.M., A.S., W.A.W., B.G., P.V., L.G., J.A.K.H., and T.B. collected data and contributed to the interpretation; M.N. designed the study, wrote the protocol, conducted the study, contributed to the analysis plan, interpreted the findings, and wrote the manuscript; and all authors read, critically reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: J.A.K.H. reports (institutional) grant support, consultancy fees, or honoraria from SNSF, Baxter/Takeda, Bayer, CSL Behring, Novo Nordisk, Octapharma, Roche, Sobi, Roche, Sanofi, FOPH, and Swiss Hemophilia Society, outside of the current work. M.N. reports research grants and lecture fees from Viatris, outside of the current work. J.-D.S. reports lecture and advisory board honoraria from Bayer, CSL Behring, Pfizer, Sanofi, Siemens Diagnostics, Sobi, and Takeda (all unrelated to the current work). A.G. reports personal fees from Mylan Germany, outside the submitted work, Takeda Pharma, Falk Foundation e.V., Dilaflor, GTH e.V., Roche, Sanofi-Aventis, Instrumentation Laboratory, Chromatec, Aspen, and Bayer Vital; grants from European Medicines Agency, GIZ Else-Körner-Stiftung, Deutsche Forschungsgemeinschaft, Robert-Koch-Institut, DRK-BSD Baden-Würtemberg/Hessen, Blau Farmaceutica, Prosensa/BioMarin, Portola, Biokit, Rovi, Sagent, Ergomed, and Boehringer Ingelheim; grants and personal fees from Macopharma; grants and other from DRK-BSD NSTOB; nonfinancial support from Veralox, Vakzine Projekt Management GmbH, AstraZeneca, and Janssen Vaccines & Prevention B.V.; has a patent screening method for transfusion-related acute lung injury with royalties paid to EP2321644, 18.05.2011; and has a patent Verfahren und Vorrichtung zur Herstellung von Universalplasma (licensed to DE 10 2020 212 609 B3 2022.04.07). T.B. reports grant support, consultancy fees, honoraria, or support for attending meetings from DFG, Stiftung Transfusionsmedizin und Immunhämatologie e.V, DRK Blutspendedienst, Deutsche Herzstiftung, Ministerium für Wissenschaft, Forschung und Kunst Baden Würtemberg, Gesellschaft für Thrombose- und Hämostaseforschung, Berufsverband Deutscher Internisten, CoaChrom Diagnostica GmbH, Robert Bosch GmbH, Ergomed, Bayer, Bristol Myers Squibb, Doctrina Med AG, Leo Pharma GmbH, Schöchl Medical Education GmbH, Mitsubishi Tanabe GmbH, Novo Nordisk GmbH, and Swedish Orphan Biovitrium GmbH. The remaining authors declare no competing financial interests.
Correspondence: Michael Nagler, Department of Clinical Chemistry, Inselspital, Bern University Hospital, and University of Bern, Freiburgerstr 10, 3010 Bern, Switzerland; email: michael.nagler@insel.ch.
References
Author notes
Deidentified individual participant data that underlie the reported results are available on reasonable request from the corresponding author, Michael Nagler (michael.nagler@insel.ch).
The full-text version of this article contains a data supplement.