Key Points
A targeted approach to block enzymes in the trichloroacetic acid cycle had limited efficacy in the treatment of patients with relapsed BL.
The unique biology of the 2 diseases in the 2 responding patients may have played a role.
Visual Abstract
Patients with primary refractory or relapsed (R/R) MYC-driven aggressive B-cell lymphomas, such as Burkitt lymphoma (BL), plasmablastic lymphoma (PBL), and double-hit lymphoma (DHL)/triple-hit lymphoma (THL), have a dismal prognosis with few survivors. The deregulated MYC protein drives proliferation with dependence on tricarboxylic acid cycle intermediates as biosynthetic precursors. Devimistat disrupts mitochondrial production of adenosine triphosphate and biosynthetic intermediates. We conducted a phase 2 trial to explore the efficacy of devimistat in R/R-BL, R/R-PBL, and R/R-DHL, administered daily for 5 days every 14 days for 2 cycles, followed by maintenance for 5 days every 21 days until progression, toxicity, or transplant. No responses were seen in the 2 enrolled patients with R/R-PBL and 9 with R/R-DHL/THL. Among the 13 patients with R/R-BL, 2 had a complete response (CR), resulting in a CR rate (CRR) of 15%, whereas the others experienced rapid disease progression. The median follow-up time for patients with R/R-BL was 1 month (range, 0-47). Among the 2 patients with R/R-BL CR, the response duration was 8 months in 1 and ongoing at the time of data cutoff, 17 months after study entry. Overall, 3 patients had grade 3 events at least possibly related to the study drug: neutropenia, thrombocytopenia, headache, noncardiac chest pain, and increase of troponin. Considering the dismal prognosis of R/R MYC-driven aggressive B-cell lymphomas with the current standard of care and low CRR with devimistat, efforts should be made to improve the outcome of these malignancies with their first line of therapy and explore novel therapies in the R/R setting. This trial was registered at www.ClinicalTrials.gov as #NCT03793140.
Introduction
We aimed to investigate a novel agent targeting the tricarboxylic acid (TCA) cycle in patients with relapsed or refractory (R/R) MYC-driven lymphomas, for which few, if any, therapeutic options are available. Burkitt lymphoma (BL) is a rare and highly aggressive MYC-driven non-Hodgkin lymphoma (NHL), representing ∼1% of mature NHLs in the United States.1,2 Patients with primary refractory BL have a dismal prognosis, with few survivors.3-6 No specific standard second-line or higher therapeutic approach exists. Plasmablastic lymphoma (PBL) is a similarly rare, aggressive large B-cell lymphoma (LBCL) commonly seen in patients with immunosuppression or HIV infection.7,8,MYC is expressed in ∼50% of cases and correlates with MYC translocations and amplifications.9,10 As with R/R-BL, patients with R/R-PBL,11 have no standard therapeutic options beyond first line, and a poor prognosis. Finally, patients with high-grade BCL with rearrangements of MYC and BCL2 and/or BCL6 (double-hit lymphoma [DHL] or triple-hit lymphoma [THL]) have an inferior prognosis compared with diffuse LBCL (DLBCL), not otherwise specified.12 High-grade BCL (HGBCL), not otherwise specified, excludes DHL and has worse survival outcomes than those historically reported for patients with DLBCL.13 The presence of MYC rearrangement (MYCr) is associated with nonstatistically significant worse progression-free survival (PFS).13
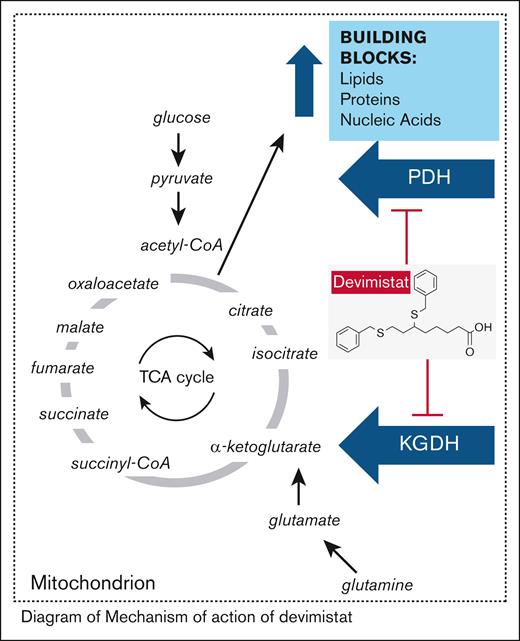
The characteristic hallmark of all these malignancies is a deregulated MYC oncogene, leading to accelerated proliferation and reprogrammed cell metabolism that relies on TCA cycle intermediates as biosynthetic precursors. Devimistat (CPI-613) is a non–redox-active analog of lipoic acid, a required cofactor for 2 key mitochondrial enzymes of the TCA cycle: pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. Devimistat shuts down adenosine triphosphate and biosynthetic production, leading to cancer cell death (Figure 1).14-17 In the initial phase 1 trial (CL-CPI-613-009; n = 26), a patient with multiply refractory BL achieved partial response (PR), that was sustained for over 1 year on ongoing devimistat monotherapy (2940 mg/m2), consolidated by surgical resection. She remained alive 7 years later without relapse.15 The US Food and Drug Administration (FDA) has granted Orphan Drug designation to CPI-613 for the treatment of BL.18 We conducted a phase 2 trial to further explore efficacy in 2 cohorts of R/R MYC-driven lymphomas, separated by the absence or presence of concurrent BCL2 translocation.19
Mechanism of action of devimistat. acetyl-CoA, acetyl coenzyme A; KGDH, α-ketoglutarate dehydrogenase; PDH, pyruvate dehydrogenase; succinyl-CoA, succinyl coenzyme A.
Mechanism of action of devimistat. acetyl-CoA, acetyl coenzyme A; KGDH, α-ketoglutarate dehydrogenase; PDH, pyruvate dehydrogenase; succinyl-CoA, succinyl coenzyme A.
Methods
Patient selection
This single-arm, open-label multicenter trial (ClinicalTrials.gov identifier: NCT03793140) was conducted at 4 referral centers (supplemental Table 1) and enrolled patients simultaneously in 2 separate cohorts.
Cohort 1 included patients without BCL2 translocation, specifically those with R/R-BL, R/R-HGBCL-MYCr, and R/R-PBL. Cohort 2 included patients with concurrent BCL2 translocation, specifically those with R/R-DHL/THL. Eligibility criteria were at least 1 previous therapy or refusing standard of care at least 3 months after previous transplant. Previous leptomeningeal disease allowed with negative cerebral spinal fluid for >4 weeks at enrollment and ongoing maintenance intrathecal therapy. The complete list of inclusion and exclusion criteria is outlined in supplemental Table 2.
Treatment
In the initial phase 1 trial (CL-CPI-613-009), devimistat was administered via central line over 2 hours on days 1 and 4 of each week. The starting dose was 420 mg/m2, and the highest dose tested was 3789 mg/m2.15 The maximum tolerated dose was determined to be 2940 mg/m2 when administered IV over a 2-hour period. The dose-limiting toxicity for devimistat identified in this study was reversible renal failure, which was encountered in 3 patients treated at 3000 mg/m2.
The trial dose of 2500 mg/m2 per day was given on day 1 through day 5 during the 2-week induction cycles, and delivered a total dose of 12 500 mg/m2 over 2 weeks for a total of 2 cycles. During maintenance cycles, this dose was be delivered over 3 weeks. This was similar to the phase 1 single-agent trials dosing of 3000 mg/m2 given on day 1 and day 4 weekly for a total dose of 12 000 mg/m2 over 2 weeks. Furthermore, this was identical to the dose delivered at the maximum tolerated dose in combination with high-dose cytarabine and mitoxantrone. Therefore, the similar starting dose of 2500 mg/m2 used in the phase 2 trial was expected to be safe.
A positron emission tomography–computed tomography scan was performed after 3 cycles. If this did not show disease progression, 3-week cycles were continued until progression, toxicity, or off-trial transplant (supplemental Figure 1). According to the prespecified definition, patients were deemed evaluable for response if they received at least 4 infusions over 5 days in the first cycle. Devimistat was administered via a central line.
End points, assessments, and statistical analysis
Statistical analysis used the Simon 2-stage design for each cohort,20 requiring 1 response among the first 9 treated patients to expand to 17. The cohorts were analyzed separately. This design yielded a type-1 error rate of 0.05 and power of 80%. The response rate to any currently single or combination available agent is essentially nonexistent without this intervention, and any response observed would be beneficial to the patient population. Therefore, we assume the null hypothesis that the current rate is 0.05 (∼0), which was be tested against a 1-sided alternative of 25%.
The primary objective of this phase 2 trial was to determine the efficacy of devimistat in patients with R/R-BL, R/R-PBL, R/R-HGBCL-MYCr, and R/R-DHL/THL analyzed separately in the cohort without BCL2 translocation (BL, PBL, and HGBCL-MYCr) or with the BCL2 translocation (DHL/THL). The primary end point was the objective response rate (ORR), with a 95% confidence interval (CI) estimated via the Clopper-Pearson exact method.
Positron emission tomography–computed tomography scans were performed at study entry, after cycle 3, and then after every 4 cycles of maintenance thereafter until the first year of study treatment, until disease progression, or other off-study event. Patients were considered to have disease evaluable for response if they received at least 4 of the 5 planned doses in cycle 1. Response and progression were evaluated using the RECIL criteria for response assessment in lymphoma and/or bone marrow biopsy (depending on sites of disease indicated by the treating physician).
PFS, duration of response, and overall survival (OS) were estimated using the product-limit Kaplan-Meier method.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
The study was approved by each relevant institutional review board, and all participants provided written informed consent.
Results
Patient characteristics
Between January 2019 and August 2023, a total of 15 patients were enrolled in cohort 1: 13 with R/R-BL, 2 with R/R-PBL, and none with R/R-HGBCL-MYCr. In addition, 9 patients were enrolled in cohort 2 with R/R-DHL/THL (Table 1). The last dose of therapy was administered in November 2023.
Patient characteristics
| Patient Characteristics . | Cohort 1∗ . | Cohort 2 . | ||
|---|---|---|---|---|
| R/R-BL . | R/R-PBL . | R/R-HGBCL with rearrangements of MYC and BCL2 and/or BCL6 . | ||
| Characteristic . | Overall, n (%) . | Evaluable, n (%) . | Overall,† n (%) . | Overall,† n (%) . |
| No. of patients | 13 (100) | 9 (100) | 2 (100) | 9 (100) |
| Sex | ||||
| Male | 10 (77) | 7 (78) | 2 (100) | 7 (78) |
| Female | 3 (23) | 2 (22) | 0 | 2 (22) |
| Race | ||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 |
| Asian | 1 (8) | 1 (11) | 0 | 0 |
| Black or African American | 0 | 0 | 0 | 0 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 |
| White | 12 (92) | 8 (89) | 2 (100) | 8 (89) |
| Data not available | 1 (11) | |||
| Ethnicity | ||||
| Hispanic or Latino | 3 (23) | 2 (22) | 2 (100) | 2 (22) |
| Not Hispanic or Latino | 10 (77) | 7 (78) | 0 | 6 (67) |
| Data not available | 1 (11) | |||
| Age at enrollment, y | ||||
| Median | 35 | 40 | 47 | 65 |
| Range | 21-55 | 21-55 | 33-60 | 50-81 |
| ECOG performance status | ||||
| 0-1 | 7 (54) | 6 (67) | 0 | 7 (78) |
| >1 | 6 (46) | 3 (33) | 2 (100) | 2 (22) |
| LDH | ||||
| Normal | 2 (15) | 2 (22) | 1 (50) | 2 (22) |
| Elevated | 11 (85) | 7 (78) | 1 (50) | 7 (78) |
| Data not available | 0 | |||
| Previous line of systemic therapy | ||||
| Median | 3 | 3 | 3 | 4 |
| Range | 1-9 | 1-9 | 2-3 | 2-7 |
| Previous CAR T cells | 5 (38) | 4 (44) | 0 | 4 (44) |
| Previous allo-SCT | 2 (15) | 2 (22) | 0 | 0 |
| Previous auto-SCT | 2 (15) | 1 (11) | 0 | 1 (11) |
| History of CNS disease | ||||
| No | 11 (85) | 7 (78) | 2 (100) | 8 (89) |
| Yes | 2 (15) | 2 (22) | 0 | 1 (11) |
| HIV status at enrollment | ||||
| Negative | 11 (85) | 7 (78) | 1 (50) | 9 (100) |
| Positive | 2 (15) | 2 (22) | 1 (50) | 0 |
| Median HIV viral load | <30 | 0 | ||
| Range HIV viral load | <20 to 23 | <20 to 23 | NA | 0 |
| Median CD4 count | 312 | NA | ||
| Range CD4 count | 20 to >200 | 20 to >200 | NA | NA |
| Patient Characteristics . | Cohort 1∗ . | Cohort 2 . | ||
|---|---|---|---|---|
| R/R-BL . | R/R-PBL . | R/R-HGBCL with rearrangements of MYC and BCL2 and/or BCL6 . | ||
| Characteristic . | Overall, n (%) . | Evaluable, n (%) . | Overall,† n (%) . | Overall,† n (%) . |
| No. of patients | 13 (100) | 9 (100) | 2 (100) | 9 (100) |
| Sex | ||||
| Male | 10 (77) | 7 (78) | 2 (100) | 7 (78) |
| Female | 3 (23) | 2 (22) | 0 | 2 (22) |
| Race | ||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 |
| Asian | 1 (8) | 1 (11) | 0 | 0 |
| Black or African American | 0 | 0 | 0 | 0 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 |
| White | 12 (92) | 8 (89) | 2 (100) | 8 (89) |
| Data not available | 1 (11) | |||
| Ethnicity | ||||
| Hispanic or Latino | 3 (23) | 2 (22) | 2 (100) | 2 (22) |
| Not Hispanic or Latino | 10 (77) | 7 (78) | 0 | 6 (67) |
| Data not available | 1 (11) | |||
| Age at enrollment, y | ||||
| Median | 35 | 40 | 47 | 65 |
| Range | 21-55 | 21-55 | 33-60 | 50-81 |
| ECOG performance status | ||||
| 0-1 | 7 (54) | 6 (67) | 0 | 7 (78) |
| >1 | 6 (46) | 3 (33) | 2 (100) | 2 (22) |
| LDH | ||||
| Normal | 2 (15) | 2 (22) | 1 (50) | 2 (22) |
| Elevated | 11 (85) | 7 (78) | 1 (50) | 7 (78) |
| Data not available | 0 | |||
| Previous line of systemic therapy | ||||
| Median | 3 | 3 | 3 | 4 |
| Range | 1-9 | 1-9 | 2-3 | 2-7 |
| Previous CAR T cells | 5 (38) | 4 (44) | 0 | 4 (44) |
| Previous allo-SCT | 2 (15) | 2 (22) | 0 | 0 |
| Previous auto-SCT | 2 (15) | 1 (11) | 0 | 1 (11) |
| History of CNS disease | ||||
| No | 11 (85) | 7 (78) | 2 (100) | 8 (89) |
| Yes | 2 (15) | 2 (22) | 0 | 1 (11) |
| HIV status at enrollment | ||||
| Negative | 11 (85) | 7 (78) | 1 (50) | 9 (100) |
| Positive | 2 (15) | 2 (22) | 1 (50) | 0 |
| Median HIV viral load | <30 | 0 | ||
| Range HIV viral load | <20 to 23 | <20 to 23 | NA | 0 |
| Median CD4 count | 312 | NA | ||
| Range CD4 count | 20 to >200 | 20 to >200 | NA | NA |
allo-SCT, allogeneic SCT; auto-SCT, autologous SCT; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NA, not applicable.
No patients were accrued to the cohort 1 R/R-HGBCL with rearrangements of MYC without BCL2.
All the patients were evaluable.
Among the 13 patients with R/R-BL, 4 were deemed nonevaluable. Specifically, 2 patients received <4 doses of devimistat before coming off study because of progression of disease (PD). One patient was removed from the study before devimistat treatment but after enrollment because of active infection and PD, whereas another patient withdrew consent after receiving <4 doses of devimistat (supplemental Figure 2).
Patient characteristics of the 9 patients with R/R-BL evaluable for response included predominantly non-Hispanic White men, with a median age of 40 years (range, 21-55) and a median of 3 previous systemic lines of treatment. Among them, 2 patients were HIV positive, and 2 had a history of central nervous system (CNS) disease (1 patient with leptomeningeal disease, then parenchymal disease; 1 patient with leptomeningeal disease alone), inactive at enrollment as required.
Efficacy
In cohort 1, 2 of 9 patients with R/R-BL achieved a complete response (CR), whereas neither of the 2 patients with R/R-PBL showed any response (Table 2). The study used the Simon 2-stage design, which initially involved 9 evaluable patients for stage 1. The study would expand if at least 1 patient responded. Because there were 2 responders in cohort 1, the study could expand to 17 patients, but it was terminated early because of low accrual.
Devimistat efficacy
| Devimistat efficacy . | Cohort 1∗ . | Cohort 2 . | ||
|---|---|---|---|---|
| R/R-BL . | R/R-PBL . | R/R-HGBCL with rearrangements of MYC and BCL2 and/or BCL6 . | ||
| Devimistat and response . | Overall, n (%) . | Evaluable, n (%) . | Overall,† n (%) . | Overall,† n (%) . |
| No. of patients | 13 (100) | 9 (100) | 2 (100) | 9 (100) |
| Median no. of cycles of devimistat | 1 | 1 | 1.4 | 1 |
| Range of no. of cycles of devimistat | 0-11 | 0.8-11 | 0.8-2 | 1-9 |
| Median follow-up time (range), mo | 1 (0-47) | 1 (0-47) | 0.5 (0-1) | 2 (0-8) |
| Best response | ||||
| CRR | 2 (15) | 2 (22) | 0 | 0 |
| PR | 0 | 0 | 0 | 0 |
| SD | 0 | 0 | 0 | 2 (22) |
| PD | 11 (85) | 7 (78) | 2 (100) | 7 (78) |
| Not evaluable | 4 (31) | 0 | 0 | 0 |
| Duration of response | Ongoing | Ongoing | 0 | 0 |
| Devimistat efficacy . | Cohort 1∗ . | Cohort 2 . | ||
|---|---|---|---|---|
| R/R-BL . | R/R-PBL . | R/R-HGBCL with rearrangements of MYC and BCL2 and/or BCL6 . | ||
| Devimistat and response . | Overall, n (%) . | Evaluable, n (%) . | Overall,† n (%) . | Overall,† n (%) . |
| No. of patients | 13 (100) | 9 (100) | 2 (100) | 9 (100) |
| Median no. of cycles of devimistat | 1 | 1 | 1.4 | 1 |
| Range of no. of cycles of devimistat | 0-11 | 0.8-11 | 0.8-2 | 1-9 |
| Median follow-up time (range), mo | 1 (0-47) | 1 (0-47) | 0.5 (0-1) | 2 (0-8) |
| Best response | ||||
| CRR | 2 (15) | 2 (22) | 0 | 0 |
| PR | 0 | 0 | 0 | 0 |
| SD | 0 | 0 | 0 | 2 (22) |
| PD | 11 (85) | 7 (78) | 2 (100) | 7 (78) |
| Not evaluable | 4 (31) | 0 | 0 | 0 |
| Duration of response | Ongoing | Ongoing | 0 | 0 |
SD, stable disease.
No patients were accrued to the cohort 1 R/R-HGBCL with rearrangements of MYC without BCL2.
All the patients were evaluable.
In addition, none of the 9 patients with R/R-DHL/THL (positive for BCL2 translocation) achieved a response, with only 2 patients with R/R-DHL/THL having stable disease as their best response. This led to the closure of cohort 2.
By intent-to-treat analysis, the ORR and CR rates (CRRs) were 2 of 15 (13%) for cohort 1, and 2 of 13 (15%) for patient with R/R-BL. The 95% CI for cohort 1 (2/13 [15.4%]) is 1.9 to 45.4.
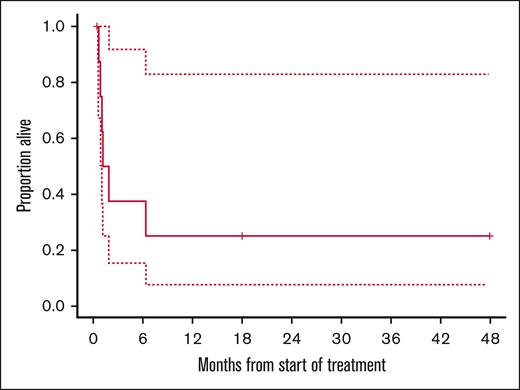
The 13 patients with R/R-BL received a median of 1 cycle of devimistat (range, 0.8-11) over a period of 1 week to 9 months. The median follow-up was 1 month (range, 0-47). Among the two patients with CR, the duration of response was 8 months and 17 months, ongoing at the time of data cutoff in October 2024. The 1-year OS rate was 22% for evaluable patients with R/R-BL (Figure 2).
The first patient with R/R-BL who achieved CR was aged 40 years at the start of the trial; HIV positive with a viral load of 23 copies per mL; and had received 4 previous lines of therapy CODOX-M/R-IVAC (PR; cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate/rituximab, ifosfamide, etoposide, and high-dose cytarabine), R-ICE (rituximab, ifosfamide, carboplatin, and etoposide; PR), pembrolizumab and acalabrutinib (CR for 11 months), an R-methotrexate cytarabine (PR). He had an isolated, disabling, bulky thigh mass, achieved a PR after the third cycle of devimistat, and a CR after the seventh cycle. He received a total of 11 cycles of devimistat, remained in CR for 5 months before disease progression, and remained alive 25 months after the start of alternative therapy R-GEMOX (rituximab, gemcitabine, and oxaliplatin).
The second patient with R/R-BL who obtained a CR was aged 21 years at the start of the trial, and HIV negative, with liver and bone lesions. He received 6 previous lines of therapy: (1) R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), (2) DA-R-EPOCH (dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin hydrochloride; PD), (3) R-HCVAD/R-MA (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/rituximab, methotrexate, and cytarabine), (4) and intrathecal methotrexate (CR), (5) rituximab, hyperfractionated cyclophosphamide, axicabtagene ciloleucel (CR), and (6) allogeneic stem cell transplant (SCT; CR). He had shrinkage but stable disease after the third cycle of devimistat and a CR after the sixth cycle. He required dose reduction of cycle 7 because of headache, later considered unrelated to devimistat; and of cycle 8 because of biopsy-proven nonlymphomatous hepatitis leading to study drug discontinuation. After additional follow-up, the hepatitis was adjudicated as unlikely to be related to the study drug and likely attributable to alcohol. At the last follow-up, the patient was alive and remained lymphoma-free at 17 months after study entry and 11 months after his last dose.
Safety
The grade ≥2 adverse events, at least possibly related to devimistat, are detailed in Table 3. Only 1 patient presented a transitory elevation of creatinine, possibly related to devimistat. However, no significant metabolic on-target adverse events were observed. Three patients had grade 3 events at least possibly related to the study drug: 1 neutropenia, thrombocytopenia, headache (serious adverse event), 1 noncardiac chest pain (serious adverse event), and 1 increase of troponin.
Safety data of all patients of cohorts 1 and 2
| AE . | No. of events . | Grade . | SAE . | Attribution . |
|---|---|---|---|---|
| Cardiac troponin T increased | 1 | 3 | No | Possible |
| Headache | 1 | 3 | Yes | Possible |
| Hyperbilirubinemia | 1 | 3 | No | Possible |
| Leukopenia | 1 | 3 | No | Possible |
| Neutropenia | 3 | 3 | No | Possible |
| Noncardiac chest pain | 1 | 3 | Yes | Possible |
| Thrombocytopenia | 1 | 3 | No | Definite |
| Abdominal pain | 1 | 2 | No | Possible |
| Abdominal pain | 2 | 2 | No | Probable |
| Anemia | 4 | 2 | No | Possible |
| Generalized edema | 1 | 2 | No | Possible |
| Headache | 1 | 2 | No | Possible |
| Hypokalemia | 2 | 2 | No | Possible |
| Leukopenia | 2 | 2 | No | Possible |
| Nausea | 3 | 2 | No | Definite |
| Neutropenia | 2 | 2 | No | Probable |
| Thromboembolic event | 1 | 2 | No | Possible |
| Transaminitis | 1 | 2 | No | Probable |
| Vomiting | 1 | 2 | No | Possible |
| Weight gain | 1 | 2 | No | Possible |
| Diarrhea | 1 | 2 | No | Possible |
| Creatinine increased | 1 | 2 | No | Possible |
| AE . | No. of events . | Grade . | SAE . | Attribution . |
|---|---|---|---|---|
| Cardiac troponin T increased | 1 | 3 | No | Possible |
| Headache | 1 | 3 | Yes | Possible |
| Hyperbilirubinemia | 1 | 3 | No | Possible |
| Leukopenia | 1 | 3 | No | Possible |
| Neutropenia | 3 | 3 | No | Possible |
| Noncardiac chest pain | 1 | 3 | Yes | Possible |
| Thrombocytopenia | 1 | 3 | No | Definite |
| Abdominal pain | 1 | 2 | No | Possible |
| Abdominal pain | 2 | 2 | No | Probable |
| Anemia | 4 | 2 | No | Possible |
| Generalized edema | 1 | 2 | No | Possible |
| Headache | 1 | 2 | No | Possible |
| Hypokalemia | 2 | 2 | No | Possible |
| Leukopenia | 2 | 2 | No | Possible |
| Nausea | 3 | 2 | No | Definite |
| Neutropenia | 2 | 2 | No | Probable |
| Thromboembolic event | 1 | 2 | No | Possible |
| Transaminitis | 1 | 2 | No | Probable |
| Vomiting | 1 | 2 | No | Possible |
| Weight gain | 1 | 2 | No | Possible |
| Diarrhea | 1 | 2 | No | Possible |
| Creatinine increased | 1 | 2 | No | Possible |
AE, adverse event; SAE, serious AE.
Exploratory analyses
We attempted whole-exome sequencing and RNA sequencing of the pretreatment pathology from the 2 patients with CR R/R-BL compared with 3 who had PD. Unfortunately, a paucity of tissue did not yield analyzable material even after multiple attempts at different samples.
Discussion
In our single-arm phase 2 study, 2 of 13 patients with R/R-BL treated with devimistat achieved complete remission (ORR and CRR of 15%). Although the CR rate was low, the findings demonstrate the potential of targeting the TCA cycle in a subset of patients with R/R-BL. No response was observed in patients with R/R-PBL; only 2 of 9 patients with R/R-DHL/THL achieved stable disease as their best response. We cannot rule out whether a different dosing schedule, such as the 1 used in the phase 1 of 3000 mg/m2 on days 1, 4, 8, 11, 15, and 18 of a 28-day cycle, would have yielded different results.
The prognosis of most patients with R/R-BL with standard-of-care options is grim. Devimistat resulted in a low CRR in this heavily pretreated population. Nevertheless, both responses were clinically significant in duration, and 1 remained durable even 11 months after treatment discontinuation. We suspect the fact that both patients had multiple previous drug exposures without succumbing to BL reflects a less aggressive BL than is typical. Ideally, molecular studies, such as the expression of mitochondrial enzymes, might identify biological predictors of response or resistance to devimistat.21 We attempted to extract DNA and RNA for sequencing analyses to compare the 2 CR patients with 3 patients with PD but failed to recover sufficient nucleic acids.
Because of the rarity of BL, its highly aggressive biology, and the small proportion of those with primary treatment failure, most studies on outcomes of R/R-BL are retrospective and use standard-of-care therapies for LBCLs with poor outcomes.3-6,22,23 Short et al retrospectively identified 35 patients with R/R-BL or R/R-HGBCL treated with standard-of-care immunochemotherapy with or without SCT. The patients had a median OS of 2.8 months, with only 2 patients alive at the last follow-up time of publication, both with late relapse ≥12 months after initial diagnosis.3 Other studies6,22 reported similar outcomes, with the exception of Manji et al5 who reported 74 patients with R/R-BL treated with standard of care. The median OS of the whole cohort was 3.2 months, and the 2-year OS was 17.2%. Of 11 surviving patients, 9 received either an autologous (n = 8) or allogeneic (n = 1) SCT. More recently, in a multicenter observational cohort, Gamero et al reported the outcome of 10 patients with R/R-BL; 7 of 10 patients died within 6 ± 4 months from the date of relapse. The 3 patients who survived received a SCT and lived 2, 4, and 13 months, respectively, from the date of SCT. None of the 10 patients survived past 26 months from the date of diagnosis.4,23 Except for 1 pediatric study of sequential chimeric antigen receptor (CAR) T-cell therapy against CD19, CD22, and CD20,23 no FDA-approved CAR T-cell therapy for DLBCL has shown durable efficacy in R/R-BL.23
The study of Samples et al retrospectively analyzed 31 patients across multiple centers in patients with relapsed/refractory BL using data abstracted from the medical records. In total, 31 patients received CAR T cells after a median of 3 previous therapies (range, 1-6). Most (19 patients) received axicabtagene ciloleucel.24 The overall response rate at 1 month was 58.0%, with a 42% CR rate. However, the 6-month CR rate was only 26%, with a the median PFS of 2.3 months (95% CI, 1.0-9.0), and median OS was 6.0 months (95% CI, 1.9-11.5). Three patients (9.7%) received consolidative allogeneic SCTs, but all subsequently relapsed. Only 1 patient survived at 20 months. The efficacy of CAR T cells was therefore felt to be quite limited.
The major strength of our study was to enroll rare and highly aggressive MYC-driven NHLs and treat them with a therapy targeting their biology. Most clinical trials for NHL exclude patients with BL and PBL, those with HIV infection, or patients with a history of CNS disease, not uncommonly seen in these malignancies, leaving affected patients with a default ineffective standard-of-care therapy as their only option.
Despite few treatment options, enrollment was difficult because of the rarity of these aggressive NHLs, highly aggressive disease causing potentially rapid drop of performance status, organ dysfunction, CNS disease, and logistical constraints, leading to study closure. Nonresponding patients died rapidly, which explains the short median follow-up time. We hypothesize that the 2 patients with responding disease to CR had a unique disease biology reflected by the number of previous therapies each received. However, nucleic acid yields on their tissue samples were unsuccessful, and we could not evaluate potential predictive biomarkers of sensitivity to devimistat. Additionally, because it took >4 years to enroll 15 patients with R/R BL across 4 centers and the response rate was low, conducting a larger FDA registration study is simply infeasible. Considering the dismal prognosis of R/R-BL with the current standard of care and low CRR with devimistat, efforts should be made to improve the outcome of those with high-risk BL with their first line of therapy. Exploring novel therapies in the R/R setting will remain challenging. Indeed, we note that an ongoing pediatric European trial in R/R BL with the bispecific antibody epcoritamab (EudraCT number: 2021-004555-16) is having low accrual, similar to our study, and will be expanding to the United States (ClinicalTrials.gov identifier: NCT05206357).
Acknowledgments
The authors thank all the study investigators, patients, and their families.
The study was supported, in part, by Rafael Pharmaceuticals.
Authorship
Contribution: A.N. and S.L. conceived and designed the study; S.V. collected the data; and all authors contributed to writing and editing the manuscript.
Conflict-of-interest disclosure: R.S. received research funding from Rafael Pharmaceuticals, Pfizer/Seagen, Bristol Myers Squibb (BMS), and GlaxoSmithKline. L.N. reports employment and stock holding with Kite, a Gilead company. R.N. reports an advisory board role with ADC Therapeutics Lymphoma and 280 Bio Inc. S.K.T. reports research funding from Genmab, Genentech, Ipsen, and ADC Therapeutics; reports an advisory board role with Ipsen and Kite; and reports consulting for AbbVie. N.K. reports research support from Genentech; and reports consulting for Kyowa, ADC Therapeutics, and AbbVie. J.S.A. reports consulting for AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, BMS, Celgene, Cellectar, Foresight Diagnostics, Genentech, Gilead, Interius, Lilly, Novartis, Roche, and Seagen; and reports research support (to institution) from BMS, Celgene, Cellectis, Genentech, Merck, Mustang Bio, Regeneron, Seagen, and Takeda. S.H. reports research support from ADC Therapeutics, Affimed, Aileron, Celgene, CRISPR Therapeutics, Daiichi Sankyo, Forty Seven Inc, Kyowa Hakko Kirin, Takeda, Seattle Genetics, Trillium Therapeutics, and Secura Bio; and reports consulting for Abcuro Inc, Arvinas, Autolus, Auxilus Pharma, Corvus, Daiichi Sankyo, Dren Bio, Johnson and Johnson Medicine/Janssen Research and Development, Kyowa Hakko Kirin, March Bio, Ono Pharmaceuticals, Pfizer, Secura Bio, Shoreline Biosciences Inc, SymBio, Takeda, and Yingli Pharma Limited. M.M. reports consulting role with AbbVie, Genentech, Novartis, Roche, and Pfizer; reports an advisory board role with Allogene, Arvinas, Genmab, BMS, Genmab, Genentech, and Merck; honoraria from AbbVie, ADC Therapeutics, AstraZeneca, BMS, Epizyme, Janssen, Kite, Regeneron, Roche, Novartis, Regeneron, and Pfizer; stipends from AbbVie, ADC Therapeutics, AstraZeneca, BMS, Epizyme, Kite, and Regeneron; research funding from Janssen, Genentech, Roche, and Pfizer; consulting honoraria from Novartis; and equity ownership with Merck. I.I.R.R. reports consulting fees from Werewolf Therapeutics, Syneos, Cardinal Health, and Telix Pharmaceuticals; ownership (shareholder) in Texas Oncology and New Experimental Therapeutics LLC; and research support (to institution) from 1ST Biotherapeutics, Amgen, Apollo Therapeutics, BeiGene, Boundless Bio, Circle Pharma, Context Therapeutics, Deciphera Pharmaceuticals, EMD Serono/Merck, Flare Therapeutics, Innovent Biologics, Kumquat Biosciences, NextPoint Therapeutics, Pasithea Therapeutics, Pheon Therapeutics, Poseida Therapeutics, Regeneron Pharmaceuticals, and Zumutor Biologics. T.S.P. received research funding from Delta-Fly Pharmaceuticals, Karyopharm Therapeutics, and Rafael Pharmaceuticals; consulting for AbbVie and Genentech; and honoraria from Rafael Pharmaceuticals. S.L. is the president and chief executive officer of Rafael Pharmaceuticals. A.N. reports research funding and/or support from AbbVie, Cornerstone Pharma, and Gilead (ended 2023); consulting for ADC Therapeutics, AstraZeneca, BeiGene (2023), ClearView, Epizyme (2023), EUSA Pharma, Guidepoint Global (ended 2022), Health Advance (ended 2022), Johnson and Johnson Global, and Pfizer; honoraria from Cornerstone Pharma, DAVA Oncology, and Recordati; reports an advisory board role with Kite Pharma (2023), MorphoSys (completed 2023), and TG Therapeutics (ended 2023); and reports study drug support from Johnson and Johnson Global. The remaining authors declare no competing financial interests.
Correspondence: Ariela Noy, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: noya@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Ariela Noy (noya@mskcc.org); individual participant data will not be shared.
The full-text version of this article contains a data supplement.