TO THE EDITOR:
Clonal hematopoiesis (CH) is the clonal expansion of hematopoietic stem and progenitor cells harboring somatic mutations, and carries an increased risk of myeloid neoplasia and all-cause mortality.1 Exposure to cancer therapies, including radiotherapy (RT), can select and promote the expansion of CH clones, particularly those carrying mutations in DNA damage response (DDR) genes, including TP53.2 Notably, TP53-mutant CH is the most common precursor to therapy-related myeloid neoplasms (tMNs) and understanding how RT affects CH can provide important insights into the relationship of RT with tMN risk.3 However, RT is not a uniform treatment modality and can be delivered with variable doses and techniques to distinct anatomic compartments. It remains unknown how these parameters affect CH and subsequent risk of tMN.
Here, CH mutations were identified among patients who underwent targeted, deep-coverage sequencing from paired peripheral blood and solid tumor samples (MSK-IMPACT, ie, Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets) after receiving RT.2 CH was defined as a somatic blood putative-driver mutation with a minimum variant allele frequency (VAF) of 2%, as previously described.2 Patients with active hematologic malignancy or a hematologic malignancy diagnosed within 3 years of sequencing were excluded from analysis. CH mutations in the DDR genes PPM1D, TP53, ATM, and CHEK2 were defined as DDR-CH. To confirm that mutation calls from blood samples did not reflect circulating tumor DNA, we examined the VAF of mutations in the blood relative to mutations from matched tumor sequencing. We defined somatic CH mutations as those identified in the blood with a VAF of ≥2 times that of the tumor or ≥1.5 if tumor biopsy was collected from a lymph node.2 Clinical and RT characteristics were abstracted from medical records (Table 1). All patients received photon-based RT. To account for differences in RT dose and fractionation, equivalent radiation dose in 2 Gy fractions (EQD2) was calculated with an α/β of 3 because we considered CH a late effect. We then used logistic regression adjusted for age, race, time from diagnosis to blood draw, smoking status, and chemotherapy class (cytotoxic, immune, or targeted therapies) to test for an association between RT parameters and CH. All analyses were conducted using R version 4.3.3.
Clinical and radiation treatment characteristics
| . | All . | No CH . | Non-DDR CH . | DDR CH . |
|---|---|---|---|---|
| N = 2195 . | n = 1691 . | n = 346 . | n = 158 . | |
| Sex | ||||
| Female | 1167 (53.2%) | 926 (79.3%) | 169 (14.5%) | 72 (6.17%) |
| Male | 1028 (46.8%) | 765 (74.4%) | 177 (17.2%) | 86 (8.37%) |
| Age, y | 60.7 (51.2-69.1) | 58.2 (48.4-66.7) | 68.4 (60.0-73.4) | 69.0 (62.3-74.7) |
| Primary tumor subtype | ||||
| Breast carcinoma | 395 (18.0%) | 325 (82.3%) | 52 (13.2%) | 18 (4.56%) |
| Cancer of unknown primary | 66 (3.01%) | 44 (66.7%) | 12 (18.2%) | 10 (15.2%) |
| Colorectal cancer | 155 (7.06%) | 126 (81.3%) | 21 (13.5%) | 8 (5.16%) |
| Endometrial cancer | 72 (3.28%) | 47 (65.3%) | 12 (16.7%) | 13 (18.1%) |
| Esophagogastric carcinoma | 93 (4.24%) | 68 (73.1%) | 14 (15.1%) | 11 (11.8%) |
| Glioma | 162 (7.38%) | 141 (87.0%) | 17 (10.5%) | 4 (2.47%) |
| Head and neck carcinoma | 138 (6.29%) | 108 (78.3%) | 20 (14.5%) | 10 (7.25%) |
| Non–small cell lung cancer | 325 (14.8%) | 234 (72.0%) | 65 (20.0%) | 26 (8.00%) |
| Other | 399 (18.2%) | 310 (77.7%) | 65 (16.3%) | 24 (6.02%) |
| Pancreatic cancer | 83 (3.78%) | 61 (73.5%) | 13 (15.7%) | 9 (10.8%) |
| Prostate cancer | 160 (7.29%) | 113 (70.6%) | 34 (21.2%) | 13 (8.12%) |
| Soft tissue sarcoma | 97 (4.42%) | 81 (83.5%) | 12 (12.4%) | 4 (4.12%) |
| Thyroid cancer | 50 (2.28%) | 33 (66.0%) | 9 (18.0%) | 8 (16.0%) |
| Days between RT and blood draw | 267 (36.0-834) | 254 (29.5-808) | 355 (59.0-101) | 212 (60.0-565) |
| Modality | ||||
| IMRT | 787 (35.9%) | 608 (77.3%) | 121 (15.4%) | 58 (7.37%) |
| 3D | 308 (14.0%) | 238 (77.3%) | 41 (13.3%) | 29 (9.42%) |
| Conventional | 46 (2.10%) | 37 (80.4%) | 6 (13.0%) | 3 (6.52%) |
| VMAT | 263 (12.0%) | 200 (76.0%) | 42 (16.0%) | 21 (7.98%) |
| Multiple | 560 (25.5%) | 438 (78.2%) | 85 (15.2%) | 37 (6.61%) |
| Unknown | 231 (10.5%) | 170 (73.6%) | 51 (22.1%) | 10 (4.33%) |
| Anatomic site | ||||
| Abdomen | 124 (5.65%) | 95 (76.6%) | 17 (13.7%) | 12 (9.68%) |
| Arm/leg | 49 (2.23%) | 40 (81.6%) | 6 (12.2%) | 3 (6.12%) |
| Brain | 226 (10.3%) | 189 (83.6%) | 25 (11.1%) | 12 (5.31%) |
| Chest wall/breast | 282 (12.8%) | 220 (78.0%) | 48 (17.0%) | 14 (4.96%) |
| Head/neck | 262 (11.9%) | 199 (76.0%) | 44 (16.8%) | 19 (7.25%) |
| Multiple | 429 (19.5%) | 323 (75.3%) | 66 (15.4%) | 40 (9.32%) |
| Pelvis | 303 (13.8%) | 230 (75.9%) | 50 (16.5%) | 23 (7.59%) |
| Prostate | 77 (3.51%) | 58 (75.3%) | 16 (20.8%) | 3 (3.90%) |
| Rib/scapula/sternum | 7 (0.32%) | 5 (71.4%) | 1 (14.3%) | 1 (14.3%) |
| Spine | 118 (5.38%) | 102 (86.4%) | 13 (11.0%) | 3 (2.54%) |
| Thorax | 174 (7.93%) | 118 (67.8%) | 31 (17.8%) | 25 (14.4%) |
| Unknown | 144 (6.56%) | 112 (77.8%) | 29 (20.1%) | 3 (2.08%) |
| Chemotherapy modality | ||||
| Alkylating agent | 600 (27.33%) | 490 (81.66%) | 77 (12.83%) | 33 (5.50%) |
| Antimetabolite | 769 (35.03%) | 571 (74.25%) | 131 (17.03%) | 67 (8.71%) |
| Carboplatin | 461 (21.00%) | 321 (69.63%) | 70 (15.18%) | 70 (15.18%) |
| Cisplatin | 447 (20.36%) | 338 (75.61%) | 66 (14.76%) | 43 (9.61%) |
| Immune therapy | 175 (7.97%) | 131 (74.85%) | 29 (16.57%) | 15 (8.57%) |
| Microtubule damaging | 133 (6.05%) | 114 (85.71%) | 15 (11.27%) | 4 (3.00%) |
| No chemotherapy | 436 (19.86%) | 344 (78.90%) | 82 (18.81%) | 10 (2.29%) |
| Other cytotoxic therapy | 24 (1.09%) | 22 (91.66%) | 2 (8.33%) | 0 (0.00%) |
| Oxaliplatin | 273 (12.43%) | 208 (76.19%) | 43 (15.75%) | 22 (8.05%) |
| Radiopharmaceutical | 39 (1.77%) | 24 (61.53%) | 8 (20.51%) | 7 (17.94%) |
| Targeted therapy | 558 (25.42%) | 443 (79.39%) | 77 (13.79%) | 38 (6.81%) |
| Taxane | 764 (34.80%) | 575 (75.26%) | 111 (14.52%) | 78 (10.20%) |
| Topoisomerase I inhibitor | 186 (8.47%) | 140 (75.26%) | 23 (12.36%) | 23 (12.36%) |
| Topoisomerase II inhibitor | 542 (24.69%) | 418 (77.12%) | 80 (14.76%) | 44 (08.11%) |
| . | All . | No CH . | Non-DDR CH . | DDR CH . |
|---|---|---|---|---|
| N = 2195 . | n = 1691 . | n = 346 . | n = 158 . | |
| Sex | ||||
| Female | 1167 (53.2%) | 926 (79.3%) | 169 (14.5%) | 72 (6.17%) |
| Male | 1028 (46.8%) | 765 (74.4%) | 177 (17.2%) | 86 (8.37%) |
| Age, y | 60.7 (51.2-69.1) | 58.2 (48.4-66.7) | 68.4 (60.0-73.4) | 69.0 (62.3-74.7) |
| Primary tumor subtype | ||||
| Breast carcinoma | 395 (18.0%) | 325 (82.3%) | 52 (13.2%) | 18 (4.56%) |
| Cancer of unknown primary | 66 (3.01%) | 44 (66.7%) | 12 (18.2%) | 10 (15.2%) |
| Colorectal cancer | 155 (7.06%) | 126 (81.3%) | 21 (13.5%) | 8 (5.16%) |
| Endometrial cancer | 72 (3.28%) | 47 (65.3%) | 12 (16.7%) | 13 (18.1%) |
| Esophagogastric carcinoma | 93 (4.24%) | 68 (73.1%) | 14 (15.1%) | 11 (11.8%) |
| Glioma | 162 (7.38%) | 141 (87.0%) | 17 (10.5%) | 4 (2.47%) |
| Head and neck carcinoma | 138 (6.29%) | 108 (78.3%) | 20 (14.5%) | 10 (7.25%) |
| Non–small cell lung cancer | 325 (14.8%) | 234 (72.0%) | 65 (20.0%) | 26 (8.00%) |
| Other | 399 (18.2%) | 310 (77.7%) | 65 (16.3%) | 24 (6.02%) |
| Pancreatic cancer | 83 (3.78%) | 61 (73.5%) | 13 (15.7%) | 9 (10.8%) |
| Prostate cancer | 160 (7.29%) | 113 (70.6%) | 34 (21.2%) | 13 (8.12%) |
| Soft tissue sarcoma | 97 (4.42%) | 81 (83.5%) | 12 (12.4%) | 4 (4.12%) |
| Thyroid cancer | 50 (2.28%) | 33 (66.0%) | 9 (18.0%) | 8 (16.0%) |
| Days between RT and blood draw | 267 (36.0-834) | 254 (29.5-808) | 355 (59.0-101) | 212 (60.0-565) |
| Modality | ||||
| IMRT | 787 (35.9%) | 608 (77.3%) | 121 (15.4%) | 58 (7.37%) |
| 3D | 308 (14.0%) | 238 (77.3%) | 41 (13.3%) | 29 (9.42%) |
| Conventional | 46 (2.10%) | 37 (80.4%) | 6 (13.0%) | 3 (6.52%) |
| VMAT | 263 (12.0%) | 200 (76.0%) | 42 (16.0%) | 21 (7.98%) |
| Multiple | 560 (25.5%) | 438 (78.2%) | 85 (15.2%) | 37 (6.61%) |
| Unknown | 231 (10.5%) | 170 (73.6%) | 51 (22.1%) | 10 (4.33%) |
| Anatomic site | ||||
| Abdomen | 124 (5.65%) | 95 (76.6%) | 17 (13.7%) | 12 (9.68%) |
| Arm/leg | 49 (2.23%) | 40 (81.6%) | 6 (12.2%) | 3 (6.12%) |
| Brain | 226 (10.3%) | 189 (83.6%) | 25 (11.1%) | 12 (5.31%) |
| Chest wall/breast | 282 (12.8%) | 220 (78.0%) | 48 (17.0%) | 14 (4.96%) |
| Head/neck | 262 (11.9%) | 199 (76.0%) | 44 (16.8%) | 19 (7.25%) |
| Multiple | 429 (19.5%) | 323 (75.3%) | 66 (15.4%) | 40 (9.32%) |
| Pelvis | 303 (13.8%) | 230 (75.9%) | 50 (16.5%) | 23 (7.59%) |
| Prostate | 77 (3.51%) | 58 (75.3%) | 16 (20.8%) | 3 (3.90%) |
| Rib/scapula/sternum | 7 (0.32%) | 5 (71.4%) | 1 (14.3%) | 1 (14.3%) |
| Spine | 118 (5.38%) | 102 (86.4%) | 13 (11.0%) | 3 (2.54%) |
| Thorax | 174 (7.93%) | 118 (67.8%) | 31 (17.8%) | 25 (14.4%) |
| Unknown | 144 (6.56%) | 112 (77.8%) | 29 (20.1%) | 3 (2.08%) |
| Chemotherapy modality | ||||
| Alkylating agent | 600 (27.33%) | 490 (81.66%) | 77 (12.83%) | 33 (5.50%) |
| Antimetabolite | 769 (35.03%) | 571 (74.25%) | 131 (17.03%) | 67 (8.71%) |
| Carboplatin | 461 (21.00%) | 321 (69.63%) | 70 (15.18%) | 70 (15.18%) |
| Cisplatin | 447 (20.36%) | 338 (75.61%) | 66 (14.76%) | 43 (9.61%) |
| Immune therapy | 175 (7.97%) | 131 (74.85%) | 29 (16.57%) | 15 (8.57%) |
| Microtubule damaging | 133 (6.05%) | 114 (85.71%) | 15 (11.27%) | 4 (3.00%) |
| No chemotherapy | 436 (19.86%) | 344 (78.90%) | 82 (18.81%) | 10 (2.29%) |
| Other cytotoxic therapy | 24 (1.09%) | 22 (91.66%) | 2 (8.33%) | 0 (0.00%) |
| Oxaliplatin | 273 (12.43%) | 208 (76.19%) | 43 (15.75%) | 22 (8.05%) |
| Radiopharmaceutical | 39 (1.77%) | 24 (61.53%) | 8 (20.51%) | 7 (17.94%) |
| Targeted therapy | 558 (25.42%) | 443 (79.39%) | 77 (13.79%) | 38 (6.81%) |
| Taxane | 764 (34.80%) | 575 (75.26%) | 111 (14.52%) | 78 (10.20%) |
| Topoisomerase I inhibitor | 186 (8.47%) | 140 (75.26%) | 23 (12.36%) | 23 (12.36%) |
| Topoisomerase II inhibitor | 542 (24.69%) | 418 (77.12%) | 80 (14.76%) | 44 (08.11%) |
3D, 3-dimensional; IMRT, intensity modulated radiation therapy; VMAT, volumetric modulated arc therapy.
All patients had a tumor and blood sample (as a matched control) sequenced using MSK-IMPACT on an institutional prospective tumor-sequencing protocol (ClinicalTrials.gov identifier: NCT01775072) before 1 July 2019. This study was approved by the MSKCC institutional review board. A subset of patients underwent tumor genomic profiling as the standard of care and did not directly consent, in which case an institutional review board waiver was obtained to allow for inclusion into this study.
We identified 2195 patients who received RT before their blood was collected and analyzed for CH, encompassing 57 primary tumor histologies. A median of 267 days elapsed between the end of RT and blood draw. After RT, 22% of patients had at least 1 CH mutation (n = 486) and 6.4% had at least 1 DDR-CH mutation (n = 140). We found that EQD2 was associated with the presence of CH across all samples (odds ratio [OR], 1.3 per 10 Gy; P = 1.2 × 10−5), with a stronger association for DDR-CH (OR, 1.5; P = 6 × 10−6) than non-DDR-CH (OR, 1.2, P = 1.4 × 10−2). We found no association between RT modality (eg, conventional, 3-dimensional-conformal, or intensity-modulated RT) and either CH overall or DDR-CH.
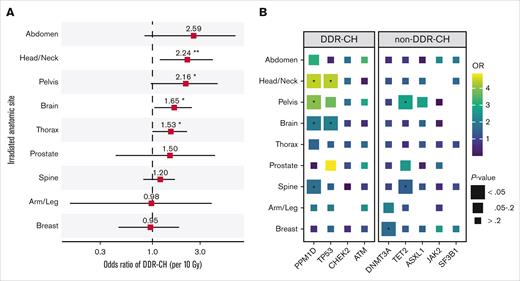
We then quantified EQD2 within irradiated anatomic sites and identified site-dependent differences in the strength of association between radiation and DDR-CH (Figure 1A-B). Specifically, we found that total dose to the head and neck (OR, 2.2, P = 9 × 10−3), pelvis (OR, 2.2, P = 5 × 10−2), brain (OR, 1.7, P = 2 × 10−2), and thorax (OR, 1.5, P = 3 × 10−2) was significantly associated with DDR-CH (Figure 1A). In gene-specific analyses, we observed significant enrichment for CH mutations in PPM1D (head/neck, pelvis, brain, and spine), TP53 (head/neck and brain), DNMT3A (breast), and TET2 (pelvis and spine; Figure 1B) in patients who received RT to these sites.
Irradiated anatomic site and risk of CH. (A) ORs with 95% confidence intervals for DDR-CH and irradiated anatomic sites in multivariable logistic regression adjusted for age, race, time from diagnosis to blood draw, smoking status, and chemotherapy class. ORs are plotted per 10-Gy dose increments in EQD2. (B) ORs between irradiated anatomic sites and individual DDR-CH mutations, adjusted via multivariable logistic regression as in panel A. ∗, P < 0.05; ∗∗, P < 0.01.
Irradiated anatomic site and risk of CH. (A) ORs with 95% confidence intervals for DDR-CH and irradiated anatomic sites in multivariable logistic regression adjusted for age, race, time from diagnosis to blood draw, smoking status, and chemotherapy class. ORs are plotted per 10-Gy dose increments in EQD2. (B) ORs between irradiated anatomic sites and individual DDR-CH mutations, adjusted via multivariable logistic regression as in panel A. ∗, P < 0.05; ∗∗, P < 0.01.
In addition to the specific mutation, high-risk CH has been linked to both an increased number of CH mutations and increased VAF.4 Thus, we next used linear regression (adjusted for the same set of potential confounders) to assess for associations between RT dose and 2 outcomes: the number of DDR-CH mutations, and the maximum VAF of a given DDR-CH mutation (supplemental Figure A-B). We found that EQD2 was significantly associated with both the number of CH mutations (B = 0.052, P = .026) and with maximum VAF (B = 0.009, P = .003). When further stratified by irradiated anatomic site, we identified significant associations between head and neck RT and number of CH mutations (B = 0.167, P = .0324) and between thoracic RT and maximum VAF (B = 0.025, P = .0082).
The well-established late effects of RT are defined largely by the treated site and the dose/fractionation delivered. Although RT has been linked to subsequent risk of CH,2 this is, to our knowledge, the first study to assess the risk of CH with respect to RT technique, dose, and site. Here, we found that DDR-CH was associated with higher radiation dose for head and neck, pelvic, brain, and thoracic RT. Of note, based on previous magnetic resonance imaging–based anatomic studies, we estimate that these 4 sites account for ∼60% to 70% of adult bone marrow,5 suggesting that CH risk after RT may depend more on total dose to the hematopoietic compartment than potential secondary systemic effects. RT modality was not associated with CH overall or DDR-CH, allaying concerns regarding broader low-dose exposure from modern treatment planning. Study limitations include its cross-sectional design (thus preventing assessment of baseline CH status before RT) and the diversity of systemic therapies received by patients, although potential confounders were mitigated to the extent possible via multivariable analyses.
RT is a cornerstone of oncologic treatment, administered to more than half of all patients with cancer.6 With improved outcomes and increasing long-term survivorship, efforts to reduce iatrogenic late effects of cancer treatments are crucial. Our findings carry implications for informed decision-making among several common malignancies for which long-term survivorship is common after RT (eg, breast, prostate, head and neck). Future work to characterize the relationship between RT, CH evolution, and progression to tMN is needed to evaluate the potential of CH screening to identify those at high risk of RT-related CH and tMN.
Acknowledgments: This study was supported, in part, by a National Cancer Institute (NCI) grant K08CA241318 (K.L.B.), the Lois Green Fund, Breakthrough Cancer (R.L.L.), and National Institutes of Health/NCI Cancer Center Support Grant P30CA008748 to Memorial Sloan Kettering Cancer Center.
Contribution: A.G.G., K.L.B., L.Z.B., and G.R.M. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; A.G.G., K.L.B., and L.Z.B. conceptualized and designed the study, and drafted the manuscript; A.G.G., G.R.M., K.L.B., and L.Z.B. performed statistical analysis; R.L.L., K.L.B., and L.Z.B. provided administrative, technical, or material support; K.L.B. and L.Z.B. supervised the study; and all authors critically reviewed the manuscript for important intellectual content, and were responsible for data acquisition, analysis, or interpretation.
Conflict-of-interest disclosure: K.L.B. reports grants and personal fees from Servier unrelated to this work. R.L.L. serves on the supervisory board of Qiagen (compensation/equity); is a cofounder/board member at Ajax (equity); is a scientific advisor to Mission Bio, Kurome, Anovia, Bakx, Syndax, Scorpion, Zentalis, Auron, Prelude, and C4 Therapeutics (for each aforementioned entity he receives equity/compensation); has received research support from the Cure Breast Cancer Foundation, Calico, Zentalis, and Ajax; and has consulted for Jubilant, Goldman Sachs, Incyte, AstraZeneca, and Janssen; none of these disclosures were related to this work. The remaining authors declare no competing financial interests.
Correspondence: Kelly L. Bolton, Division of Oncology, Department of Medicine, 660 South Euclid Avenue, Washington University School of Medicine, St Louis, MO 63110; email: bolton@wustl.edu; and Lior Z. Braunstein, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: braunstl@mskcc.org.
References
Author notes
A.G.G. and G.R.M. contributed equally to this study.
K.L.B. and L.Z.B. contributed equally to this study.
The minimal clinical and mutational data required to replicate the figures in this study are available on GitHub (https://github.com/kbolton-lab/XRTsite_CH). Mutation calls are available on cBioPortal (http://www.cbioportal.org/study/summary?id=msk_ch_2020). To preserve patient anonymity, data with potential patient identifiers (ie, those related to individual radiotherapy courses, individual drug names, and dates of treatment) cannot be made public. Similarly, because sequencing was obtained in a clinical capacity, raw sequencing data also cannot be publicly deposited. These data may not be used for commercial purposes in their current form. For commercial use, please contact datarequests@mskcc.org.
The full-text version of this article contains a data supplement.