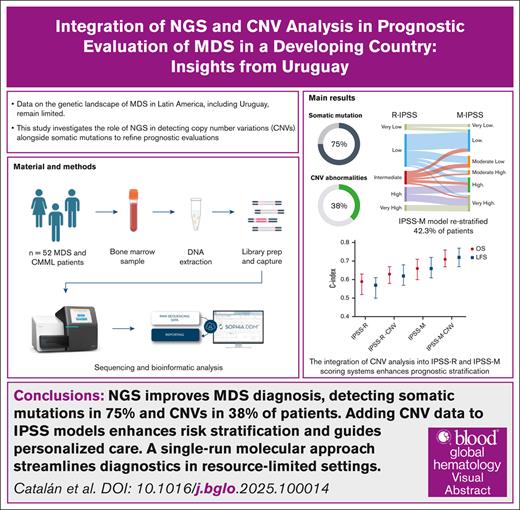
Key Points
NGS improves MDS diagnosis, detecting somatic mutations in 75% and CNVs in 38% of patients.
Adding CNV data to International Prognostic Scoring System models enhances risk stratification and guides personalized care.
Visual Abstract
Myelodysplastic syndromes (MDSs) are clonal disorders of the bone marrow characterized by ineffective hematopoiesis, cytopenia, and an increased risk of progression to acute myeloid leukemia. The phenotypic variability of the disease, driven by genetic abnormalities, poses challenges to its management and prognosis. Although traditional cytogenetic analysis detects chromosomal abnormalities in ∼50% of cases, next-generation sequencing (NGS) identifies genetic mutations in 70% to 90% of patients, providing critical prognostic insights. However, data on the genetic landscape of MDS in Latin America, including Uruguay, remain limited. This study investigates the role of NGS in detecting copy number variants (CNVs) alongside somatic mutations to refine prognostic evaluations. Among 52 patients analyzed, NGS-based CNV detection identified abnormalities in 38% of cases, including deletions on chromosomes 5 and 7 and gains on chromosome 8, many of which were missed by conventional karyotyping in real-world settings. Integrating CNV data with karyotyping into established risk models significantly improved prognostic accuracy. Furthermore, somatic mutation profiling revealed mutations in 75% of patients, with TP53, DNMT3A, TET2, ASXL1, and SF3B1 being the most frequently identified. Notably, TP53 mutations were strongly correlated with poor clinical outcomes. The International Prognostic Scoring System–Molecular model restratified 42.3% of patients, offering enhanced risk assessment and prognostic precision. By integrating CNV analysis with somatic mutation profiling, this study highlights the feasibility and utility of a NGS single-run approach that improves diagnostic accuracy and optimizes workflows, particularly in resource-constrained settings.
Introduction
Myelodysplastic syndromes (MDS) are clonal bone marrow disorders marked by abnormal hematopoiesis, blood cytopenias, and a heightened risk of progression to acute myeloid leukemia.1 Clinical presentations vary widely in cytopenia severity, bone marrow cellularity, blast count, disease progression, survival, and treatment response, largely driven by diverse genetic abnormalities. This variability poses significant challenges in disease management.2
Conventional cytogenetic analysis is essential for evaluating genetic aberrations in MDS, offering key prognostic insights on therapy response, relapse risk, and survival. However, chromosomal abnormalities are detected in only ∼50% of cases, leaving many with a normal karyotype and limited genetic information.3-5 These detected aberrations are predominantly unbalanced, resulting in copy number variants (CNVs) due to partial or complete losses or gains of chromosomal material.6 Common chromosomal alterations in MDS include abnormalities of chromosome 7, trisomy 8 and 21, loss of Y, and complex karyotypes. Typical CNVs include del(5q), del(20q), del(12p), and del(17p)/i(17)(q10), whereas reciprocal translocations are rare, occurring in 2% to 3% of cases.3-6
Next-generation sequencing (NGS) has transformed MDS diagnosis by detecting mutations in 70% to 90% of cases, establishing somatic mutations as crucial prognostic biomarkers.7-13 The International Prognostic Scoring System (IPSS)–Molecular (IPSS-M), a validated scoring system incorporating clinical, cytogenetic, and molecular data from 31 genes, stratifies patients into 6 risk groups, enhancing prognosis and guiding treatment decisions.14
Recent advancements in NGS now allow for the simultaneous detection of CNVs and point mutations within a single assay, offering greater sensitivity and resolution for CNV detection compared with traditional cytogenetics. Integrating CNV analysis with NGS has demonstrated significant improvements in risk stratification and therapeutic planning15,16
Despite the global shift toward molecular testing as a standard practice, implementing NGS in regions such as Latin America remains challenging owing to financial constraints, leading to limited data on the molecular landscape of MDS within these populations.17-19 This study seeks to bridge the existing gap by exploring the prevalence and diversity of somatic genetic variants and CNVs in Uruguayan patients with MDS. Using a single-run, targeted NGS approach, it aims to unravel the intricate relationships between molecular mutation profiles, CNV patterns, and clinical outcomes, providing valuable insights into the genetic landscape and its impact on patient care in this population.
Material and methods
Patients
This study was conducted at the University Hospital, Hospital de Clínicas, Uruguay. Inclusion criteria: patients aged ≥18 years diagnosed with MDS or chronic myelomonocytic leukemia (CMML) who had not received treatment or had bone marrow samples available before treatment initiation. Diagnoses and subgrouping of patients were performed by experienced hematologists in accordance with World Health Organization 2016 classification criteria.20 Risk stratification was conducted using IPSS-Revised (IPSS-R) score.21 Data collected included blood counts, biochemical parameters, bone marrow blast counts, cytogenetic findings, treatment regimens, and patient outcomes. Conventional cytogenetic and fluorescence in situ hybridization (FISH) studies were performed at an external diagnostic service outside the hospital through the referral of the sample.
DNA extraction and targeted sequencing
Genomic DNA was isolated from whole bone marrow by using the QIAamp DNA Blood Mini kit (Qiagen, Germany). Before sequencing, the DNA concentration was determined using a Qubit fluorometer using a DNA High Sensitivity kit (Invitrogen, ThermoFisher Scientific, USA, Inc) and the DNA quality by spectrophotometry NanoDrop ND-1000.
A genomic DNA library was prepared using Myeloid Solutions panel and Panmyeloid-v1 panel SOPHiA Genetics kit (SOPHiA GENETICS, Switzerland) in accordance with the manufacturer’s instructions. The sequencing process was performed on Illumina MiSeq system (Illumina Inc, San Diego, CA). The NGS analysis was performed on generated FASTQ sequencing files using the SOPHiA DDM platform that allows for detection, annotation, and preclassification of genomic mutations (single nucleotide variants [SNVs], indels, and CNVs) through its SOPHiA artificial intelligence. The Myeloid Solutions panel was designed to sequenced 30 genes implicated in hematological malignancies, including hot spot regions and the complete coding sequences of 10 genes with ± 25 base pairs flanking into the intron and including 5′ untranslated region exons that contain coding sequence, and the panmyeloid-V1 panel used was designed to sequenced 63 genes for all protein-coding exons for all transcripts of a given gene for the following genes with ± 25 base pairs flanking into the intron and including 5′ untranslated region exons that contain coding sequence (supplemental Tables 1 and 2).
The Myeloid Solutions panel and Panmyeloid-V1 panel were designed to sequence 30 and 63 genes, respectively, targeting coding regions, hot spot areas, and flanking intronic regions. NGS analysis showed robust performance, with an average of 42 million reads per run, 98.8% mapping rate, and 98.95% on-target high coverage (>1000 reads). Uniformity of coverage reached 99.82%, ensuring consistent results. Variants with minor allele frequency of <0.01 and functional impact on splicing or proteins were analyzed if their variant allele frequency (VAF) was >2% and supported by ≥20 reads. Variants were classified and reviewed using IGV, with pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, and benign, according to the criteria of the American College of Medical Genetics and Genomics, International Agency for Research on Cancer TP53 mutation database, and Palomo et al.22 VUS were excluded from the analysis.
For CNV detection, a manufacturer-developed secondary analysis module was used that uses intrarun sample normalization based on region coverage from at least 8 samples. Reference samples were selected automatically for normalization, and CNV calling was performed using a hidden Markov model algorithm to determine the most probable copy number for each target region, along with confidence levels. Samples were categorized as rejected, medium noise, or low noise based on residual coverage noise, with no CNV results reported for rejected samples. Target regions were classified as high confidence, medium confidence, or undetermined.
Statistical analysis
Statistical analysis was performed using SPSS 21 and GraphPad Software. Qualitative data were summarized as percentages, and quantitative data as medians with interquartile ranges (IQRs). Nonparametric tests (Mann-Whitney U test, χ2, or Fisher exact test) evaluated differences between variables. Overall survival (OS) and leukemia-free survival (LFS) were calculated using Kaplan-Meier analysis, with differences assessed by log-rank tests. Multivariate Cox proportional hazards models determined hazard ratios (HRs) and 95% confidence intervals (CIs), whereas the Harrell Concordance index (C-index) evaluated prognostic accuracy. Statistical significance was set at P value <.05.
Ethical consideration
Informed consent was obtained from patients enrolled in this study. The protocol was approved by the institutional review board in accordance with the Declaration of Helsinki.
Results
Demographics and clinical data of patients with MDS
As shown in Table 1, the study analyzed 52 patients with MDS or CMML, primarily older adults (median age, 67 years) and a slight female predominance. According to World Health Organization 2016 criteria, patients exhibited diverse MDS subtypes, including 5 CMML cases. Key features included ∼15% with hypocellular bone marrow and >50% requiring transfusions. Cytogenetic analysis revealed a normal karyotype in 69.2% of cases, with 67% of these having <20 metaphases available for evaluation. Risk stratification using the IPSS-R highlighted a heterogeneous distribution across risk categories, reflecting the variability in disease severity and prognostic outcomes within the cohort.
Clinical and laboratory features of patients with MDS at diagnosis
| . | All patients, N = 52 . |
|---|---|
| Age, median (IQR), y | 67 (57-76) |
| Female-to-male ratio | 1.6:1 |
| MDS according to the WHO 2016 classification, n (%) | |
| MDS-SLD | 4 (7.7) |
| MDS-MLD | 21 (40.4) |
| MDS with isolated 5q | 4 (7.7) |
| MDS-RS-SLD | 1 (1.9) |
| MDS-RS-MLD | 3 (5.8) |
| MDS EB-1 | 2 (3.8) |
| MDS EB-2 | 11 (21.2) |
| MDS/MPN-RS-T | 1 (1.9) |
| CMML | 5 (9.6) |
| Hb, median (IQR), g/dL | 8.6 (6.9-10.5) |
| ANC, median (IQR), ×109/L | 3.1 (1.2-5.4) |
| PLT, median (IQR), ×109/L | 81 (38-187) |
| LDH, median (IQR), U/L | 236 (158-335) |
| Hypocellular bone marrow, n (%) | 8 (15.4) |
| Bone marrow fibrosis, n (%) | 2 (3.8%) |
| Secondary MDS, n (%) | 10 (19.2%) |
| Ferritin, median (IQR), mg/dL | 541 (148-896) |
| Transfusion dependence, n (%) | 27 (51.9) |
| Bone marrow blast %, median (IQR) | 1.0 (0.0-9.0) |
| Cytogenetic, n (%) | |
| Without metaphase | 3 (5.8) |
| Normal | 36 (69.2) |
| 20q− | 2 (3.8) |
| 5q− | 4 (7.6) |
| 7−/7q− | 3 (5.7) |
| Y− | 1 (1.9) |
| Complex karyotype | 1 (1.9) |
| Other abnormalities | 2 (3.8) |
| IPSS-R, n (%) | 49 |
| Very low | 4 (8.2) |
| Low | 22 (44.9) |
| Intermediate | 9 (18.4) |
| High | 10 (20.4) |
| Very high | 4 (8.1) |
| Survival, median (95% CI), mo | 94 (75.8-112.2) |
| . | All patients, N = 52 . |
|---|---|
| Age, median (IQR), y | 67 (57-76) |
| Female-to-male ratio | 1.6:1 |
| MDS according to the WHO 2016 classification, n (%) | |
| MDS-SLD | 4 (7.7) |
| MDS-MLD | 21 (40.4) |
| MDS with isolated 5q | 4 (7.7) |
| MDS-RS-SLD | 1 (1.9) |
| MDS-RS-MLD | 3 (5.8) |
| MDS EB-1 | 2 (3.8) |
| MDS EB-2 | 11 (21.2) |
| MDS/MPN-RS-T | 1 (1.9) |
| CMML | 5 (9.6) |
| Hb, median (IQR), g/dL | 8.6 (6.9-10.5) |
| ANC, median (IQR), ×109/L | 3.1 (1.2-5.4) |
| PLT, median (IQR), ×109/L | 81 (38-187) |
| LDH, median (IQR), U/L | 236 (158-335) |
| Hypocellular bone marrow, n (%) | 8 (15.4) |
| Bone marrow fibrosis, n (%) | 2 (3.8%) |
| Secondary MDS, n (%) | 10 (19.2%) |
| Ferritin, median (IQR), mg/dL | 541 (148-896) |
| Transfusion dependence, n (%) | 27 (51.9) |
| Bone marrow blast %, median (IQR) | 1.0 (0.0-9.0) |
| Cytogenetic, n (%) | |
| Without metaphase | 3 (5.8) |
| Normal | 36 (69.2) |
| 20q− | 2 (3.8) |
| 5q− | 4 (7.6) |
| 7−/7q− | 3 (5.7) |
| Y− | 1 (1.9) |
| Complex karyotype | 1 (1.9) |
| Other abnormalities | 2 (3.8) |
| IPSS-R, n (%) | 49 |
| Very low | 4 (8.2) |
| Low | 22 (44.9) |
| Intermediate | 9 (18.4) |
| High | 10 (20.4) |
| Very high | 4 (8.1) |
| Survival, median (95% CI), mo | 94 (75.8-112.2) |
ANC, absolute neutrophil count; Hb, hemoglobin; MDS EB1, MDS excess blasts type 1; MDS EB2, MDS excess blasts type 2; MDS-MLD, MDS with multilineage dysplasia; MDS/MPN-RS-T, MDS/MPN ring sideroblasts with thrombocytosis; MDS-RS-MLD, MDS ring sideroblasts and multilineage dysplasia; MDS-RS-SLD, MDS ring sideroblasts and with single lineage; MDS-SLD, MDS with single lineage dysplasia; PLT, platelet count; WHO, World Health Organization.
The median follow-up of patients was 35.8 months (IQR, 11.2-50.0). The median OS was 94.0 months (95% CI, 12.6-175.3). Of 52 patients, 7 (13.5%) progressed to acute myeloid leukemia, and the median LFS was not reached.
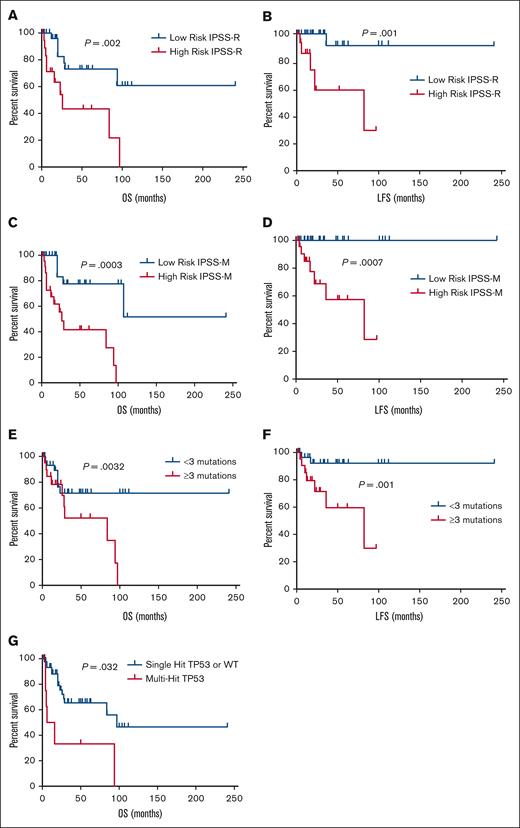
Patients were stratified according to the IPSS-R into 2 groups: low risk (≤3.5 points) and high risk (>3.5 points). As expected, IPSS-R risk group was strongly associated with OS (P = .002) and LFS (P = .001; Figure 2). In univariate analysis, several clinical factors were identified as prognosis significant for OS in our cohort: age of ≥70 years (P = .041), transfusion dependence (P = .002), hemoglobin level (P = .029), lactate dehydrogenase (LDH) level (P = .005), bone marrow blasts (P = .002), and IPSS-R scores (P = .001). Multivariate analysis revealed that transfusion dependence (HR, 1.23; 95% CI, 1.04-1.89), bone marrow blasts (HR, 1.12; 95% CI, 1.08-1.87), and IPSS-R score (HR, 1.58; 95% CI, 1.14-2.37) were independently correlated with survival. For LFS, univariate analysis highlighted hemoglobin level (P = .013), LDH level (P = .025), bone marrow blasts (P = .015), presence of fibrosis (P = .042), and IPSS-R scores (P = .004) as significant clinical factors. Multivariable analysis indicated that bone marrow blasts (HR, 1.17; 95% CI, 1.09-1.85) and IPSS-R score (HR, 1.38; 95% CI, 1.09-2.26) were independently associated with LFS.
In the low-risk IPSS-R group, 46.6% of patients were transfusion-dependent, 10% received iron chelation therapy, and 26.6% required no treatment. First-line treatments included erythropoiesis-stimulating agents (70%) and immunosuppressive therapy (6.6%), with second-line options such as luspatercept (6.6%), azacitidine (30%), and lenalidomide (16.6%). None received intensive chemotherapy, and only 1 underwent allogenic stem cell transplant (allo-SCT) after progressing to high-risk MDS. In the high-risk IPSS-R group, 68.4% were transfusion-dependent, and all received treatment, primarily azacitidine (94.7%), with 5.2% receiving intensive chemotherapy and 10.5% undergoing allo-SCT.
Somatic variants and CNV landscape
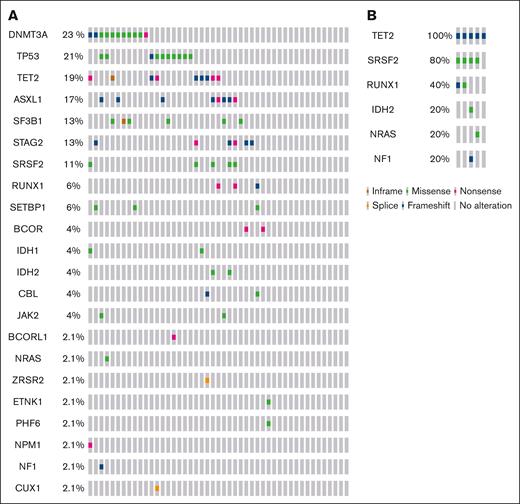
The myeloid sequencing panel identified a total of 102 pathogenic/likely pathogenic and 63 VUS variants among the 52 analyzed patients (supplemental Table 3). Genetic mutations were detected in 39 (75%) of the patients, with a median of 2 gene mutations per patient (IQR, 0-3). Figure 1 illustrates the distribution of comutations. Among patients with MDS, the most frequently affected genes were DNMT3A (23.4%), TP53 (21.2%), TET2 (19.1%), ASXL1 (17.0%), SF3B1 (12.8%), STAG2 (13.9%), SRSF2 (10.6%), RUNX1 (6.4%), BCOR (4.4%), IDH1 (4.4%), and IDH2 (4.1%), among others, as shown in Figure 1. In terms of mutation types among patients with MDS, the most mutated genes were those involved in DNA methylation mutations (53.2%), followed by RNA splicing mutations (25.5%), transcription factor mutations (23.4%), TP53 mutations (21.2%), chromatin modifier mutations (14.8%), signaling pathway mutations (12.7%), and nucleophosmin mutations (2.1%). For CMML, the frequency of mutated genes among cases was TET2 (100%), SRSF2 (80%), RUNX1 (40%), ASXL1 (20%), IDH1 (20%), and IDH2 (20%), as shown in Figure 1.
Genomic alterations. OncoPrint showing the distribution of genomic alterations in patients with MDS (A) and patients with CMML (B). The mutational frequency of each gene is labeled on the left.32,33
Notably, a significant proportion of patients with TP53 mutations (70%) exhibited multihit TP53 mutations, representing 14.9% of all patients with MDS. Multihit TP53 mutations were defined as having either ≥2 distinct TP53 mutations with a VAF of >10%, a single TP53 mutation coupled with a cytogenetic deletion at the TP53 locus on 17p13.1, or a single TP53 mutation with a VAF of >50%.23
We subsequently assessed CNVs using the SOPHiA DDM analytical platform in 47 patients and compared the findings with results from FISH and conventional cytogenetics, when available. CNVs were identified in 18 patients (38.3%), with a total of 23 CNVs detected. These included losses on chromosome 7 in 5 patients, losses on chromosome 5 in 4 patients, gains on chromosome 8 in 2 patients, and single cases of losses on chromosomes 20, X, 10, and 4, as well as a gain on chromosome 9. Three patients each had at least 3 CNVs. NGS confirmed 10 of 13 (76.9%) CNVs that were initially detected by conventional cytogenetics or FISH. In addition, CNVs were detected in 10 of 36 patients who exhibited normal findings or fewer alterations compared with conventional cytogenetics and FISH. The CNV analysis achieved a sensitivity of 76.92% (95% CI, 45.98-93.84) and a specificity of 77.78% (95% CI, 60.41-89.27-) when benchmarked against conventional karyotyping and/or FISH. Although conventional cytogenetics alone identified genetic abnormalities in 25% of cases, the integration of cytogenetics, FISH, and NGS-CNV analysis significantly increased the overall detection rate to 40% in patients with MDS.
Clinical impact of somatic mutations and CNVs in MDS
We assessed the clinical significance of somatic mutations and CNVs in our cohort of patients with MDS. We found that patients harboring ≥3 mutations exhibited a higher blast count (median, 7% [2-13] vs 1% [1-3]; P = .005), elevated LDH levels (median, 294 U/L [212-420] vs 220 U/L [136-269]; P = .0027), higher IPSS-R scores (median, 5 [2.50-6.0] vs 3 [2.25-3.5]; P = .013), and reduced OS (P = .0032) and LFS (P = .001) compared with patients with <3 mutations (Figure 2).
OS and LFS of patients with MDS within different risk groups. (A) OS for patients in low- and high-risk IPSS-R groups. (B) LFS for patients in low- and high-risk IPSS-R groups. (C) OS for patients in low- and high-risk IPSS-M groups. (D) LFS for patients in low- and high-risk IPSS-M groups. (E) OS for patients with <3 mutations compared with those with ≥3 mutations. (F) LFS for patients with <3 mutations compared with those with ≥3 mutations.
OS and LFS of patients with MDS within different risk groups. (A) OS for patients in low- and high-risk IPSS-R groups. (B) LFS for patients in low- and high-risk IPSS-R groups. (C) OS for patients in low- and high-risk IPSS-M groups. (D) LFS for patients in low- and high-risk IPSS-M groups. (E) OS for patients with <3 mutations compared with those with ≥3 mutations. (F) LFS for patients with <3 mutations compared with those with ≥3 mutations.
In addition, the analysis of mutation distribution across different age groups of patients with MDS showed a significantly higher frequency of gene mutations in older patients (aged >50 years) compared with younger patients (aged ≤50 years; 82.9% vs 45.4%; P = .011). Furthermore, older patients with MDS had a greater number of mutations, with a median of 2 (range, 1-3), compared with a median of 1 (range, 0-2) in the younger age group (P = .013).
As mentioned earlier, we identified a high prevalence of TP53 mutations, with their frequency varying across IPSS-R risk categories: 0% in the very low-risk group, 40% (n = 4) in the low-risk group, 10% (n = 1) in the intermediate-risk group, 20% (n = 2) in the high-risk group, and 30% (n = 3) in the very high-risk group. Among patients with TP53 variants, 20% (n = 2) were associated with del(5q) as detected through karyotyping, increasing to 30% (n = 3) when integrating karyotyping with CNV-NGS data. Only 1 patient exhibited a complex karyotype based on conventional cytogenetics. However, when combining karyotype analysis with CNV-NGS, the number of patients with complex karyotypes increased to 4 (40%), all of whom carried multihit TP53 variants. Among patients with TP53 mutations, 30% (n = 3) had secondary MDS, compared with 19% of secondary cases in patients without TP53 mutations, although this difference was not statistically significant. As expected, patients with multihit TP53 mutations had significantly lower hemoglobin levels (6.8 g/dL [range, 5.5-9.7] vs 8.8 g/dL [range, 7.5-10.6]; P = .036), a higher frequency of transfusion dependence (100% vs 44.4%), elevated ferritin levels (1235 ng/mL [range, 583-2060] vs 387 ng/mL [range, 210-786]; P = .005), and lower platelet counts (45 × 109/L [range, 23 × 109 to 89 × 109/L] vs 80 × 109/L [range, 45 × 109 to 87 × 109/L]; P = .001) compared with those with wild-type or single-hit TP53 mutations. Furthermore, patients with multihit TP53 mutations showed reduced OS (P = .032; Figure 2).
Patients with SF3B1 mutations were older (median age, 75.5 years [range, 71-83] vs 65 years [range, 55-75]; P = .02) and exhibited significantly higher ferritin levels (1282.5 ng/mL [range, 1014.3-1500.5] vs 372.2 ng/mL [range, 215.5-749.5]; P = .002), with no significant differences in hemoglobin levels or transfusion dependence frequency. In addition, these patients had a higher frequency of bone marrow ring sideroblasts (23% [range, 9.0-40.0] vs 0% [range, 0-3]; P = .0001) and a lower bone marrow blast count (1.0% [range, 1.0-1.5] vs 2.5% [range, 1.0-9.0]; P=.02). Furthermore, patients with SF3B1 mutations showed better OS, although this difference was not statistically significant.
When comparing gene variant profiles between the low-risk and high-risk groups according to the IPSS-R, we observed that the high-risk group exhibited a significantly higher number of mutations (median, 2 vs 1; P = .0001). Additionally, certain genes showed a markedly higher mutation frequency in the high-risk group compared with the low-risk group, including RUNX1 (3.3% vs 21.1%; P = .046), STAG2 (3.3% vs 26.3%; P = .017), IDH2 (0% vs 15.7%; P = .025), SRSF2 (3.3% vs 42.1%; P = .001), SETBP1 (0% vs 15.7%; P = .025), and TP53 (13.3% vs 31.5%; P = .051, not significant). In contrast, the high-risk group showed a lower frequency of SF3B1 mutations compared with the low-risk group (20% vs 0%; P = .037).
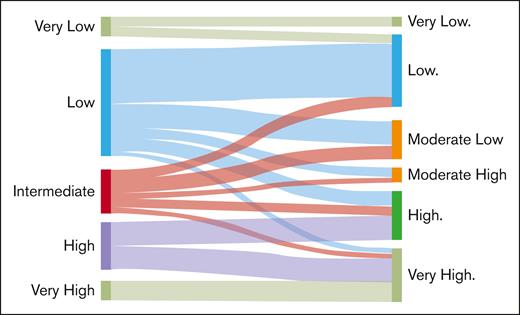
Restratification of patients from IPSS-R to IPSS-M
Comparison between IPSS-R and IPSS-M resulted in the restratification of 22 patients (42.3%), with 20 patients (38.4%) being upstaged and 2 patients (3.8%) downstaged. When stratifying scores into risk categories, IPSS-R (cutoff of 3.5 points) and IPSS-M (very low to moderate-low groups and moderate-high to very high groups), 6 patients (11.5%) were upstaged, and 1 patient (1.9%) was downstaged based on the IPSS-M, which has implications for changes in therapeutic management (Figure 3).
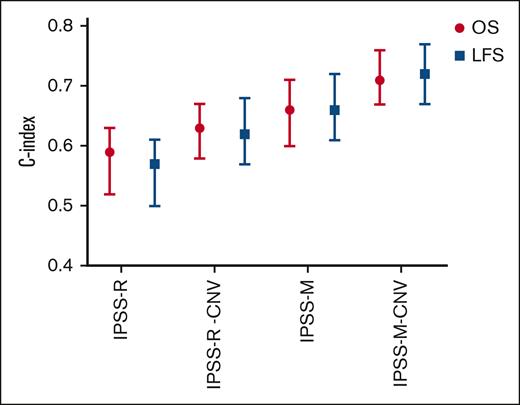
Although both IPSS-R and IPSS-M scores showed strong correlations with OS and LFS in our cohort, IPSS-M score provided superior discrimination for predicting these outcomes (Figure 2). Comparing the C-index of the 2 scoring systems, IPSS-M categories demonstrated improved performance. The C-index for OS was 0.59 (95% CI, 0.52-0.63) with IPSS-R and 0.66 (95% CI, 0.60-0.71) with IPSS-M, whereas for LFS, it was 0.57 (95% CI, 0.50-0.61) with IPSS-R and 0.72 (95% CI, 0.67-0.77) with IPSS-M (Figure 4).
Prognostic performance of various scoring models for OS and LFS. The Harrell C-index values with their 95% CIs are shown for the IPSS-R, IPSS-R-CNV, IPSS-M, and IPSS-M-CNV models, highlighting their predictive accuracy. The IPSS-R-CNV and IPSS-M-CNV models incorporate CNV data obtained through NGS to complement karyotyping and refine risk stratification. The red lines represent the prognostic performance for OS, whereas the blue lines depict the performance for LFS.
Prognostic performance of various scoring models for OS and LFS. The Harrell C-index values with their 95% CIs are shown for the IPSS-R, IPSS-R-CNV, IPSS-M, and IPSS-M-CNV models, highlighting their predictive accuracy. The IPSS-R-CNV and IPSS-M-CNV models incorporate CNV data obtained through NGS to complement karyotyping and refine risk stratification. The red lines represent the prognostic performance for OS, whereas the blue lines depict the performance for LFS.
To evaluate the impact of CNV analysis on prognostic scoring, the IPSS-R score was initially assessed using conventional karyotyping and then reevaluated by integrating NGS-CNV data to refine the cytogenetic risk classification (IPSS-R-CNV). This approach led to the restratification of 10 patients (19.2%), all of whom were upstaged. When applying an IPSS-R threshold of 3.5 points to define risk categories, 4 patients (7.7%) were moved to higher-risk groups, a change that could significantly influence therapeutic decision-making. The comparison of C-index between the 2 versions showed improved performance with IPSS-R-CNV. The C-index for OS increased from 0.59 (95% CI, 0.52-0.63) with IPSS-R to 0.63 (95% CI, 0.58-0.67) with IPSS-R-CNV, whereas for LFS, it improved from 0.57 (95% CI, 0.50-0.61) with IPSS-R to 0.62 (95% CI, 0.57-0.68) with IPSS-R-CNV. Similarly, the IPSS-M score was evaluated using conventional karyotyping and subsequently refined with the inclusion of NGS-CNV data (IPSS-M-CNV). This resulted in the restratification of 6 patients (11.5%), all of whom were upstaged. When dividing patients into low- and high-risk groups, 2 patients (3.8%) moved to a higher-risk category. The predictive accuracy of the IPSS-M-CNV score was superior to that of the standard IPSS-M score. The C-index for OS increased from 0.66 (95% CI, 0.60-0.71) with IPSS-M to 0.71 (95% CI, 0.67-0.76) with IPSS-M-CNV, whereas for LFS, it rose from 0.66 (95% CI, 0.61-0.72) with IPSS-M to 0.72 (95% CI, 0.67-0.77) with IPSS-M-CNV (Figure 4). These findings indicate that the IPSS-M-CNV score provides the highest predictive accuracy among the evaluated scoring systems. The integration of CNV data enhances the predictive performance of both IPSS-R and IPSS-M in our setting, highlighting its potential value in refining risk stratification and guiding clinical management.
Discussion
Genetic abnormalities are crucial to the pathogenesis of MDS and play a pivotal role in predicting treatment responses and survival outcomes.24 This study highlights the importance of integrating CNV analysis and somatic mutation profiling into the management of MDS and CMML, particularly in resource-limited settings such as in Uruguay.
In Uruguay, as in many Latin American countries, routine cytogenetic testing faces technical, logistical, and financial challenges, leading to a high prevalence of cytogenetically normal cases (69% in this cohort) and a low number of metaphases obtained consistent with regional studies.25-30 This reflects sample processing limitations, such as insufficient metaphase yield, which hinder the detection of chromosomal abnormalities and risk stratification. To address these issues, the study highlights the integration of advanced molecular techniques, such as CNV analysis via NGS. NGS demonstrated a sensitivity of 76.9% and specificity of 77.8%, identifying clinically relevant abnormalities in 10 patients previously classified as cytogenetically normal, thereby increasing the overall detection rate of abnormalities to 40%. These findings are consistent with similar reports in the literature.16,31,32 Moreover, in 2 cases, NGS revealed >3 CNVs in patients with normal karyotypes, suggesting the presence of complex karyotypes undetectable by standard methods. These findings demonstrate the added value of NGS in uncovering hidden abnormalities, particularly in resource-constrained settings.
This study demonstrated that integrating molecular data with traditional clinical and cytogenetic parameters significantly improves prognostic accuracy. Incorporating NGS-CNV data into the IPSS-R and IPSS-M frameworks led to the restratification of 42.3% of patients, with 38.4% upstaged to higher-risk categories. This integration also enhanced the C-index of the scoring systems, with direct clinical implications, especially in resource-limited settings in which treatment decisions must align closely with individual risk profiles.
NGS offers a practical solution for genetic evaluation in resource-constrained environments. By enabling the simultaneous detection of somatic mutations and common CNVs within a single assay, NGS enhances conventional cytogenetics with superior sensitivity and resolution. However, these methods are complementary: karyotyping provides a genome-wide overview crucial for detecting complex karyotypes, whereas NGS is limited to the targeted regions it covers. Despite these limitations, the high-quality DNA typically available in hematological diseases supports sequencing-based approaches as effective alternatives or adjuncts to traditional cytogenetics.
In contrast, molecular profiling has significantly advanced our understanding of the biological mechanisms underlying MDS.9,33-36 Recurrent mutations are commonly identified in 40 to 50 genes associated with MDS. Using NGS technologies, 70% to 90% of patients with MDS are found to carry at least 1 genetic mutation.33,36-38 Despite the pivotal role of gene mutations in MDS, there remains a notable lack of comprehensive data on the mutation landscape of patients with MDS in Latin America, including Uruguay. The high costs and limited accessibility of advanced genetic testing technologies, such as NGS, pose substantial barriers to research efforts.17-19 Notably, our laboratory is currently the only facility in the country equipped with a myeloid panel specifically tailored for studying MDS, underscoring the scarcity of resources and the challenges involved in characterizing the genetic profile of this disease.
Our results provide novel insights into the genetic landscape of MDS in Uruguayan patients, contributing valuable data to the global understanding of these diseases. A substantial proportion of Uruguayan patients with MDS and CMML carry genetic mutations, with 75% of patients exhibiting at least 1 mutation and a median of 2 mutations per patient. The overall mutation frequency observed in our MDS cohort is consistent with the findings reported by Papaemmanuil et al.12 However, both a study group from China, with a reported mutation frequency of 91.4%, and Haferlach et al, with a frequency of 89.5%, documented higher rates.13,39
Furthermore, patients with ≥3 mutations showed more aggressive disease features, including higher blast counts and reduced OS and LFS. This observation aligns with existing literature, which indicates that a higher mutational burden correlates with more aggressive disease and poorer outcomes.12 The most frequently mutated gene groups in our cohort were those involved in DNA methylation, followed by those associated with RNA splicing. The most affected genes were DNMT3A, TP53, TET2, ASXL1, and SF3B1. This mutational profile agrees with previous studies conducted in different populations, indicating that these genes play a critical role in the pathogenesis of MDS and CMML globally.7,13,40 We observed a slightly higher frequency of DNMT3A mutations (23%) in MDS, leading to a greater prevalence of mutations in the DNA methylation group (∼50%) than previously reported. For comparison, DNMT3A mutations were found in 12% of cases in the study by Haferlach et al13 and 8% in the study by Walter et al.41 These differences could be attributed to genetic variability between populations or differences in sample size and selection criteria across studies.
The high prevalence of TP53 mutations in our cohort (21.2%) is notably higher than the 5% to 10% typically reported at diagnosis, likely reflecting the elevated incidence of secondary MDS in our population.42-45 Among TP53 mutation, 70% exhibited multihit mutations, which are strongly associated with poor prognosis and adverse clinical outcomes.11,46 In addition, only 1 patient with TP53 mutation initially presented with a complex karyotype. However, integrating CNV-NGS findings with karyotype analysis increased the detection of complex karyotypes in TP53-mutated cases to 40%, similar to what has been reported in other studies.
Our data show that the IPSS-M, integrating molecular findings, outperforms the IPSS-R in prognostic accuracy for OS and LFS. It restratified 42.3% of patients, with 38.4% moved to higher-risk groups and improved C-index scores, supporting its use to refine risk assessment and treatment in MDS.
Our findings highlight the role of molecular profiling in guiding treatment decisions and improving outcomes. Mutations such as SF3B1, linked to favorable prognosis and response to luspatercept,47 aid in therapy selection, whereas targets such as IDH1/IDH2 mutations inform the use of drugs such as ivosidenib and enasidenib. High-risk mutations, such as multihit TP53, may warrant more aggressive treatments such as allogeneic stem cell transplantation.48
Our study offers valuable insights into the molecular landscape of MDS and CMML in Uruguay, a country with a population of ∼3 444 263, but faces limitations, including its retrospective design and small sample size. With an estimated 141 annual MDS cases in this aging population, the inclusion of 52 patients represents a significant proportion, shedding light on the disease’s molecular and clinical characteristics. Future research should expand cohort sizes, explore genetic mutations’ therapeutic implications, and prioritize collaborative efforts to improve access to advanced diagnostics and region-specific treatment guidelines in Latin America.
In conclusion, this study provides a comprehensive analysis of the genetic and clinical characteristics of MDS and CMML in Uruguayan patients. By integrating CNV analysis and somatic mutation profiling into routine diagnostics, we demonstrate the feasibility and utility of a single-run approach that enhances diagnostic precision and streamlines workflows in resource-limited settings. Our findings support the broader adoption of NGS in clinical practice and underscore the urgent need to address disparities in access to advanced molecular testing in Latin America.
Acknowledgment
This work was funded by a grant from the Fondo Sectorial de Salud, Agencia Nacional de Investigación e Innovación, Montevideo, Uruguay.
Authorship
Contribution: M.B., A.C., D.L., and S.G. conceptualized the study; M.B., S.R., and S.G. curated the data; S.G. performed formal analysis; A.I.C., C.O., S.R., M.B., and S.G. performed the study investigation; A.I.C., C.O., and S.G. developed the methodology; S.G. was responsible for project administration and writing the original manuscript draft; and A.C. and D.L. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sofía Grille, Hospital de Clínicas, Facultad de Medicina, Universidad de la República, Av Itali sn, Montevideo 11300, Uruguay; email: sofiagrille@gmail.com.
References
Author notes
A.I.C. and M.B. contributed equally to this study.
The data sets generated and analyzed during this study are available upon reasonable request from the corresponding author, Sofía Grille (sofiagrille@gmail.com).
The full-text version of this article contains a data supplement.