Key Points
In patients with multiple myeloma, it is necessary to have high clinical suspicion to rule out the presence of AL amyloidosis.
Early recognition of AL amyloidosis will improve the prognosis of these patients.
Visual Abstract
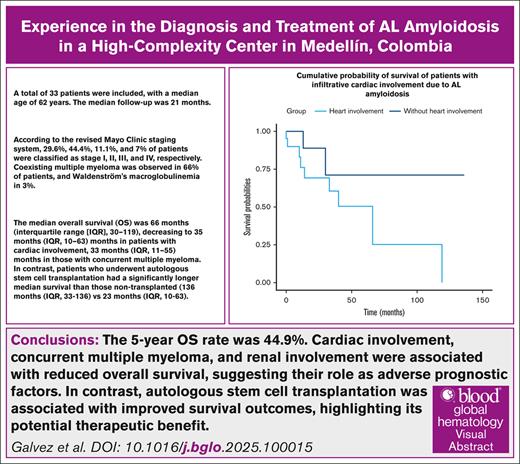
This study describes the demographic, clinical, paraclinical, imaging, therapeutic, and prognostic characteristics in patients with immunoglobulin light chain (AL) amyloidosis in a high-complexity institution. A retrospective cohort of patients diagnosed with AL amyloidosis was assessed at the Pablo Tobón Uribe Hospital in Medellín, Colombia, from January 2010 to May 2023. A total of 33 patients were included, of whom 20 (60.6%) were women, and the median age was 62 (range, 48-77) years. According to the revised Mayo Clinic staging system, disease stratification was 29.6%, 44.4%, 11.1%, and 7% for stages I, II, III, and IV, respectively. Multiple myeloma and Waldenström’s macroglobulinemia were present in 22 (66%) and 1 (3%) patient, respectively. The median overall survival was 66 months (interquartile range [IQR], 30-119), which decreased to 35 months (IQR, 10-63) with cardiac involvement and to 33 months (IQR, 11-55) with concurrent multiple myeloma. In contrast, patients who underwent hematopoietic transplantation had a longer survival of 136 months (IQR, 33-136). The 5-year overall survival was 44.9% (95% confidence interval, 26.8-63). Our findings are consistent with the existing literature, with some distinctions such as a female predominance and high frequency of concurrent multiple myeloma. Cardiac involvement, multiple myeloma, and renal involvement were associated with reduced overall survival, suggesting their role as adverse prognostic factors.
Introduction
Amyloidosis is a group of diseases characterized by the deposition of misfolded proteins in tissues. Protein aggregates are composed of organized fibrils that are insoluble and resistant to degradation, which leads to their accumulation with cell dysfunction and death in a process known as proteotoxicity.1 Production and deposition can occur in a localized manner, as occurs with Alzheimer's disease, or with involvement in multiple organs, as occurs in the systemic form.2,3 About 36 different types of proteins have been identified with the capacity to form amyloid fibrils, at least 17 of them are associated with the systemic form.1,2 The 2 most common types of systemic amyloidosis are amyloidosis related to the monoclonal production of immunoglobulin light chains (AL) and transthyretin amyloidosis in its hereditary and nonhereditary variants.1
AL amyloidosis is the most common type of disease and is usually associated with plasma cell dyscrasias. In countries like the United States, the estimated incidence of AL amyloidosis ranged from 9.7 to 14.0 cases per million person-years between 2008 and 2015.4 It is estimated that about 10% to 15% of patients with AL amyloidosis may coexist with multiple myeloma.1-3 Amyloid fibrils are formed because of the monoclonal production of κ or λ light chains and can be deposited in any tissue except for the brain.5 The clinical manifestations of the disease depend on the affected organ, with the heart and kidney being the most frequently described organs.2
Treatment of AL amyloidosis focuses on reducing the production of light chains with the use of chemotherapy that targets clonal plasma cells. Most of these treatments are derived and adapted from treatment strategies used for patients with multiple myeloma.6 Standard treatment includes the use in combination of bortezomib, cyclophosphamide and dexamethasone,7 however, hematological response rates remain suboptimal with high early mortality, for this reason the addition of daratumumab, a human monoclonal antibody against the CD38 receptor of plasma cells with a higher rate of complete hematological response and survival free of organ deterioration.8
Materials and methods
This was a retrospective cohort study which aimed to describe the demographic, clinical, paraclinical, imaging, therapeutic and prognostic characteristics in patients with AL amyloidosis in a high-complexity institution.
The study population included patients aged >18 years with a diagnosis of amyloidosis or related (ICD-10: E 85; E85.1; E85.2; E85.3; E85.4, E85.8; E85.9) evaluated in the emergency service or outpatient consultation of the Pablo Tobón Uribe Hospital in Medellín, Colombia, from January 2010 to May 2023.
Only patients who had confirmed histopathological diagnosis of AL-type amyloidosis at the institution in the period described were included.
In our setting, mass spectrometry for amyloid typing is unavailable. Therefore, the diagnosis of AL amyloidosis was established based solely on biopsy findings and immunohistochemical analysis.
Study variables included demographic, clinical, imaging and therapeutic variables: sex, age at diagnosis; type of first, second and third line chemotherapy with its response; date and status of last assessment, time of relapse to first, second and third line; comorbidities; hemoglobin, platelets, serum creatinine, glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration, proteinuria in 24 hours, beta-2 microglobulin, alkaline phosphatase, protein electrophoresis and immunofixation in serum and urine, κ free light chains and serum lambda, N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin; disease stratification according to the revised Mayo Clinic staging system; findings in echocardiogram, cardio resonance, 24-hour Holter, electromyograFphy; and organs affected by amyloid deposition. Organ involvement was established based on the 10th International Amyloidosis Symposium.9
Categorical variables were presented as absolute and relative frequencies; continuous variables, according to their distribution by the Shapiro-Wilk test, are reported as means and standard deviations or medians and ranges. Survival analysis was performed using Kaplan-Meier analysis with Stata version 11 (StataCorp, College Station, TX).
According to resolution number 8430 of 1993, this project is classified as risk-free research, hence it did not require obtaining informed consent. Medical secrecy was maintained with respect to the information contained in the medical records and other sensitive information for patients. The ethics committee of the Pablo Tobón Uribe Hospital approved this project.
Results
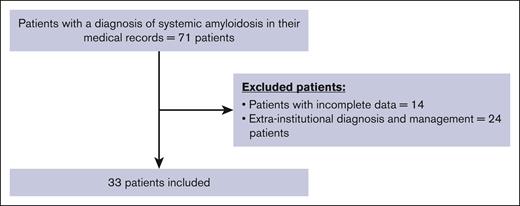
A total of 71 medical records were evaluated. Fourteen patients were excluded due to incomplete data, and 24 patients were excluded because of diagnosis or management of their AL in another institution, finally 33 patients were included (Figure 1). Of the included patient cohort, 20 (60.6%) were women, the median age was 62 (range, 48-77) years, and the median time between diagnosis and start of treatment was 11 (range, 0-323) days. The follow-up time in our cohort ranged from 0 to 136 months, with a median follow-up of 21 months, disease stratification according to the revised Mayo Clinic staging system was 29.6%, 44.4%, 11.1% and 7% for stages I, II, III, and IV respectively. Multiple myeloma and Waldenström's macroglobulinemia were reported to coexist in 22 (66%) and 1 patient (3%), respectively (Table 1).
Demographic characteristics of the population
| Demographic characteristics of the cohort . | |
|---|---|
| Characteristic . | N = 33 patients . |
| Age, y | |
| Median (range) | 62 (48-72) |
| Distribution, n (%) | |
| <65 years | 22 (66.6) |
| ≥65 years | 11 (33.3) |
| Sex, n (%) | |
| Male | 13 (39.3) |
| Female | 20 (60.6) |
| Light chain type, n (%) | |
| Lambda | 25 (75.7) |
| Kappa | 8 (24.3) |
| Associated hematological malignancy, n (%) | |
| Multiple myeloma | 22 (66.6) |
| Waldenström macroglobulinemia | 1 (3) |
| Organ involvement, n (%)∗ | |
| Heart | 21 (63) |
| Kidney | 20 (61) |
| Gastrointestinal | 11 (33) |
| Nerve | 11 (33) |
| Skin and soft tissues | 8 (24) |
| Lung | 6 (18) |
| Liver | 5 (15) |
| Number of organs involved, n (%) | |
| 1 | 5 (15) |
| 2 | 13 (39) |
| ≥3 | 15 (45) |
| Demographic characteristics of the cohort . | |
|---|---|
| Characteristic . | N = 33 patients . |
| Age, y | |
| Median (range) | 62 (48-72) |
| Distribution, n (%) | |
| <65 years | 22 (66.6) |
| ≥65 years | 11 (33.3) |
| Sex, n (%) | |
| Male | 13 (39.3) |
| Female | 20 (60.6) |
| Light chain type, n (%) | |
| Lambda | 25 (75.7) |
| Kappa | 8 (24.3) |
| Associated hematological malignancy, n (%) | |
| Multiple myeloma | 22 (66.6) |
| Waldenström macroglobulinemia | 1 (3) |
| Organ involvement, n (%)∗ | |
| Heart | 21 (63) |
| Kidney | 20 (61) |
| Gastrointestinal | 11 (33) |
| Nerve | 11 (33) |
| Skin and soft tissues | 8 (24) |
| Lung | 6 (18) |
| Liver | 5 (15) |
| Number of organs involved, n (%) | |
| 1 | 5 (15) |
| 2 | 13 (39) |
| ≥3 | 15 (45) |
Organ involvement was defined based on 8.
Multiorgan involvement (affecting ≥3 organs) was observed in 14 patients (42%), with renal (46%) and cardiac (40%) involvement being the most frequently reported disease onset. The most common initial clinical presentations were edematous syndrome and nephrotic-range proteinuria in patients with renal infiltration, whereas heart failure with restrictive cardiomyopathy predominated in those with cardiac involvement.
During follow-up, the prevalence of organ involvement increased, with cardiac involvement identified in 21 patients (63%), renal involvement in 20 patients (60%), and gastrointestinal and nervous system involvement in 11 patients (33%) each. Table 1 provides detailed data.
We identified a monoclonal peak in serum electrophoresis in 48% of patients, and an M protein was positive on 77% and 73% of the serum and urine immunofixation, respectively. The monoclonal peak measurement in the gamma region was 1.39 g/dL (range, 0.4-5.9). The most frequently altered light chain subtype was the lambda chain in 25 (75.7%) patients, with a median difference between involved and uninvolved free chains of 151 mg/L (range, 0.88-5957.25; Table 2).
Paraclinical characteristics of the entire cohort of patients with AL systemic amyloidosis
| Paraclinical characteristics of the cohort . | |
|---|---|
| Hemoglobin, mg/dL, median (range) | 12.3 (7.3-15.2) |
| Serum creatinine, mg/dL, median (range) | 1.01 (0.5-16.2) |
| Serum creatinine >1.5, n (%) | 6 (18) |
| Platelets, /mm3, median (range) | 302 500 (110 000-985 000) |
| eGFR, mL/min per 1.73 m2, median (range) | 69.5 (3-112) |
| Protein in urine 24 hours, median (range), g/d | 3 (0-15.2) |
| Protein in urine >3500, mg/d, n (%) | 13 (48) |
| Beta-2 microglobulin, mg/dL, median (range) | 3.6 (0.6-1347) |
| Alkaline phosphatase, medium (range) | 109 (52-365) |
| Monoclonal peak in gamma region, g/dL, median (range) | 0.85 (0.2-5.9) |
| Kappa light chains, mg/dL, median (range) | 41.5 (0.6-578) |
| Lambda light chains, mg/dL, median (range) | 103.365 (1.5-5999.4) |
| Difference between affected and unaffected light chain, median (range) | 151.65 (0.88-5957.2) |
| Lactate dehydrogenase, median (range) | 221 (15-424) |
| NT-proBNP, pg/mL, median (range) | 1929 (5.9-19708) |
| hs-cTnI, ng/mL, median (range) | 25 (0.0034-482) |
| Plasma cells in bone marrow, %, median (range) | 10 (1-80) |
| Plasma cells in bone marrow >30%, n (%) | 4 (14) |
| Paraclinical characteristics of the cohort . | |
|---|---|
| Hemoglobin, mg/dL, median (range) | 12.3 (7.3-15.2) |
| Serum creatinine, mg/dL, median (range) | 1.01 (0.5-16.2) |
| Serum creatinine >1.5, n (%) | 6 (18) |
| Platelets, /mm3, median (range) | 302 500 (110 000-985 000) |
| eGFR, mL/min per 1.73 m2, median (range) | 69.5 (3-112) |
| Protein in urine 24 hours, median (range), g/d | 3 (0-15.2) |
| Protein in urine >3500, mg/d, n (%) | 13 (48) |
| Beta-2 microglobulin, mg/dL, median (range) | 3.6 (0.6-1347) |
| Alkaline phosphatase, medium (range) | 109 (52-365) |
| Monoclonal peak in gamma region, g/dL, median (range) | 0.85 (0.2-5.9) |
| Kappa light chains, mg/dL, median (range) | 41.5 (0.6-578) |
| Lambda light chains, mg/dL, median (range) | 103.365 (1.5-5999.4) |
| Difference between affected and unaffected light chain, median (range) | 151.65 (0.88-5957.2) |
| Lactate dehydrogenase, median (range) | 221 (15-424) |
| NT-proBNP, pg/mL, median (range) | 1929 (5.9-19708) |
| hs-cTnI, ng/mL, median (range) | 25 (0.0034-482) |
| Plasma cells in bone marrow, %, median (range) | 10 (1-80) |
| Plasma cells in bone marrow >30%, n (%) | 4 (14) |
eGFR, estimated glomerular filtration rate; hs-cTnI, high-sensitivity assay for troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
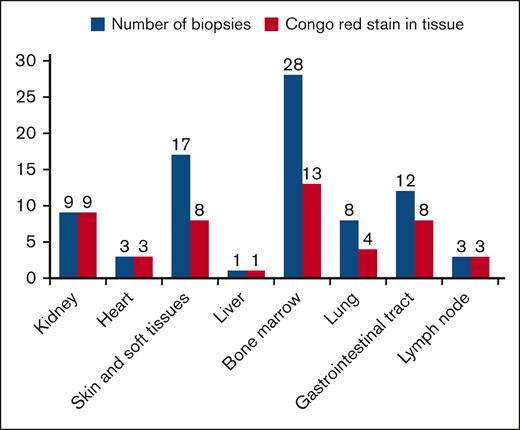
The most frequently biopsied sites were the bone marrow in 28 (84%) patients, the skin and abdominal fat in 17 (51%), the gastrointestinal tract in 12 (36%), the kidney in 9 (27%), the lung in 8 (24%), and the heart and lymph nodes in 3 (9%). Identification of amyloid material with Congo red staining was carried out mainly in the bone marrow and kidney in 39% and 27% respectively. Table 3 and Figure 2 describe the histopathological characteristics of the cohort.
Number of biopsies performed and positivity of Congo red in the stains
| Organ damage and histopathological characteristics∗ . | ||
|---|---|---|
| Organ . | Organ biopsies performed, n (%) . | Biopsies with Congo red positivity, n (%) . |
| Kidney | 9 (27) | 9 (100) |
| Heart | 3 (9) | 3 (100) |
| Skin and soft tissues | 17 (51) | 8 (47) |
| Liver | 1 (3) | 1 (100) |
| Bone marrow | 28 (84) | 13 (46) |
| Lung | 8 (24) | 4 (50) |
| Gastrointestinal tract | 12 (36) | 8 (66) |
| Lymph node | 3 (9) | 3 (100) |
| Organ damage and histopathological characteristics∗ . | ||
|---|---|---|
| Organ . | Organ biopsies performed, n (%) . | Biopsies with Congo red positivity, n (%) . |
| Kidney | 9 (27) | 9 (100) |
| Heart | 3 (9) | 3 (100) |
| Skin and soft tissues | 17 (51) | 8 (47) |
| Liver | 1 (3) | 1 (100) |
| Bone marrow | 28 (84) | 13 (46) |
| Lung | 8 (24) | 4 (50) |
| Gastrointestinal tract | 12 (36) | 8 (66) |
| Lymph node | 3 (9) | 3 (100) |
∗The histopathological studies carried out at the institution are presented, which explains why there is a greater proportion of patients with organ damage than those shown in this table.
Histopathological studies carried out in different tissues and the positivity for Congo red in each of these.
Histopathological studies carried out in different tissues and the positivity for Congo red in each of these.
The first-line treatment protocols prescribed in the cohort were CyBorD (cyclophosphamide, bortezomib, dexamethasone) in 21 (63%) patients, protocols with bortezomib in conjunction with other drugs such as daratumumab, lenalidomide or thalidomide in 6 (18%) patients, other protocols with melphalan or thalidomide in 5 (15%) patients and 1 (3%) patient did not receive treatment.
AL amyloidosis and multiple myeloma
In the 22 patients who presented concomitant multiple myeloma and AL amyloidosis, anemia was identified as the most frequent defining event of the CRAB (calcium elevation, renal insufficiency, anemia, and bone lesions) component in 68% of the patients, followed by renal and bone involvement and hypercalcemia in 59%, 22%, and 9% of the series. Electrophoresis showed a monoclonal peak in 50% of this subgroup, immunofixation in serum was altered in 77% and immunofixation in urine identified the Bence Jones protein in 80% of the tests performed. The median monoclonal peak measurement in the gamma region on altered serum electrophoresis was 2.5 g/dL (range, 0.69-5.9). The median percentage of plasma cells in bone marrow was 15% (range, 10-80). The most common type of myeloma was IgG lambda type in 42%, followed by lambda light chain involvement in 35%. Infiltrative cardiac and renal involvement due to amyloid deposition were mainly described in 77% and 54% of patients with both concomitant pathologies.
The Revised International Staging System was used to assess the risk stratification of multiple myeloma in our cohort of 22 patients. The distribution was as follows: stage I was identified in 4 patients (18%), stage II in 9 patients (41%), and stage III in 4 patients (18%). The Revised International Staging System stage was not determined in 5 cases (23%) due to insufficient data.
AL amyloidosis and cardiac infiltrative involvement
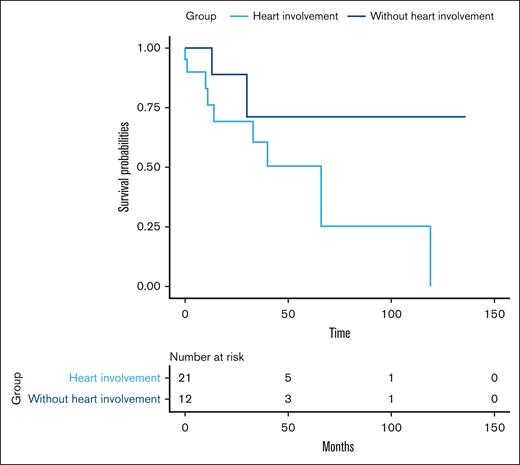
Systemic AL amyloidosis and cardiac infiltrative involvement: A total of 21 patients (63%) were presented with infiltrative cardiac involvement due to AL amyloidosis. The median age was 61 (range, 48-77) years. In 5 patients (23%), the heart was the first organ affected by the disease; in the remaining patients (77%), it was identified later during the disease. The median NT-proBNP values in this subgroup were 2562 ng/L (range, 5.9-19708), and the median high-sensitivity troponin values were 54.5 ng/mL (range, 0.04-482), with a median difference between involved and uninvolved free chains of 231.43 mg/L (range, 2-5957.25). The echocardiographic characteristics of this subgroup are described in Table 4. Additionally, there was a simultaneous diagnosis of multiple myeloma in 17 patients (80%).
Echocardiographic characteristics of patients with infiltrative involvement due to cardiac amyloidosis in the series of patients
| Patients with infiltrative cardiac involvement . | |
|---|---|
| Diameter of the posterior wall (inferolateral) diastole, mm, median (range) | 1.3 (0.9-2) |
| Diameter of the interventricular septum in diastole, mm, median (range) | 1.5 (1.5-2) |
| Global longitudinal strain, %, median (range) | −11(−7-−20) |
| Relative thickness, mm, median (range) | 0.7 (0.39-114) |
| Ejection fraction, %, median (range) | 58 (20-70) |
| Patients with infiltrative cardiac involvement . | |
|---|---|
| Diameter of the posterior wall (inferolateral) diastole, mm, median (range) | 1.3 (0.9-2) |
| Diameter of the interventricular septum in diastole, mm, median (range) | 1.5 (1.5-2) |
| Global longitudinal strain, %, median (range) | −11(−7-−20) |
| Relative thickness, mm, median (range) | 0.7 (0.39-114) |
| Ejection fraction, %, median (range) | 58 (20-70) |
AL amyloidosis and ASCT
Autologous hematopoietic stem cell transplantation (ASCT) was performed in 8 (24%) patients. The median age was 60.5 (range, 56-69) years, the median time between the moment of diagnosis and the transplant was 11 (range, 6-20) months, 7 (87%) patients had compromise by lambda type light chain deposition, the disease stratification according to the revised Mayo Clinic staging system was 62% and 38% for stage I and II, there were no patients in stage III or IV. The difference between involved and uninvolved free chains presented a median of 38.955 mg/L (range, 9-5957.25).
The 8 patients who underwent transplantation had infiltrative involvement between 1 to 2 organs, the most frequently involved organ was the kidney in 6 (75%) patients followed by infiltrative involvement in skin soft tissues in 4 (50%) and the heart in 3 (37.5%). Finally, 4 (50%) patients who underwent transplantation had a concurrent diagnosis of multiple myeloma.
The first-line treatment regimens administered were CyBorD in 4 patients (50%), VRD (bortezomib, lenalidomide, and dexamethasone) in 2 patients (25%), dexamethasone/cyclophosphamide in 1 patient (12.5%), and thalidomide/cyclophosphamide/dexamethasone in 1 patient (12.5%).
Treatment response was evaluated according to the International Society of Amyloidosis criteria. Complete response was achieved in 3 patients (2 treated with CyBorD and 1 with VRD), while a partial response was observed in 3 patients (2 receiving CyBorD and 1 VRD). Response assessment was not determined in 2 cases due to insufficient data. Given the retrospective nature of the study, organ response could not be determined.
Survival
During a median follow-up of 21 months, 11 patients (33%) died. The median overall survival in the study population was 66 months (interquartile range [IQR], 30-119). Among patients who underwent autologous stem cell transplantation, the median overall survival was 136 months (IQR, 33-136), whereas for those who did not undergo transplantation, it was 23 months (IQR, 8-55). In the subgroup of patients with cardiac involvement, the median survival was 35 months (IQR, 10-63) and 52 months (IQR, 23-136) in those without cardiac involvement as detailed in Figure 3. Patients diagnosed with concurrent multiple myeloma had a median survival of 33 months (IQR, 11-55), whereas those without multiple myeloma had a median survival of 52 months (IQR, 23-136). For individuals with renal involvement, the median survival was 33 months (IQR, 11-119), compared to 54 months (IQR, 23-66) in patients without renal involvement. The 5-year overall survival of the cohort was 44.9% (95% confidence interval, 26.8-63).
Cumulative probability of survival of patients with infiltrative cardiac involvement due to AL amyloidosis.
Cumulative probability of survival of patients with infiltrative cardiac involvement due to AL amyloidosis.
The log-rank (Mantel-Cox) test did not reveal statistically significant differences in survival across the different subgroups, including cardiac involvement, renal involvement, and the presence of multiple myeloma. This lack of significance is likely attributable to the small sample size, which limits the statistical power to detect differences between groups.
Discussion
We present a series of 33 patients with a diagnosis of AL-type amyloidosis confirmed by biopsy in a high-complexity institution in the city of Medellín over a period of 13 years. We acknowledge that our study is limited by its small sample size, which may constrain broad conclusions regarding the disease. However, we emphasize that these findings represent data specific to our region, providing valuable descriptive information that may contribute to the understanding of this pathology in our setting.
The epidemiological data of our cohort are consistent with those found in the literature; the median age of 62 years is similar to the average of 60 to 65 years reported in other series.9-12 However, there are reports of some cohorts with a higher average age,13 which is expected considering that prevalence increases with age, doubling after 65 years. Additionally, diagnostic methods have improved over time, which may make direct comparisons difficult.
The literature highlights a predominance of the disease in male patients in North American and Chinese studies10,12; however, our study reported a predominance of female patients (60%), which was also observed in a series of patients from Greece.11 Given the small sample size of our cohort, this observation should be interpreted with caution and may not be generalizable. In order of frequency, the main organs affected by amyloid deposition were the kidneys, the heart, the liver, and the peripheral nervous system, consistent with reports from other studies.10-12,14
In patients with multiple myeloma, some studies report positive staining for amyloidosis in more than 1 organ in up to 38% of patients at the time of diagnosis.15 Conversely, in a series of patients with AL amyloidosis, 10% to 15% were found to have a concurrent diagnosis of multiple myeloma.1,10 In our study, 66% of patients had simultaneous diagnoses, explained by the thorough search for malignant clonal bone marrow plasma cell disorders. This is supported by the fact that a substantial proportion of patients (84%) underwent bone marrow aspiration and biopsy.
In our series, the most frequently biopsied organs, in addition to the bone marrow for the reasons previously mentioned, were the skin and soft tissues, the kidney, and the gastrointestinal tract. Congo red staining showed a higher positivity rate in the kidney, heart, and liver, although the heart and liver were underrepresented in our cohort. The findings and diagnostic yields were similar to those reported in other studies.10,11 Notably, although the skin and subcutaneous fat are easily accessible for histopathological study, the positivity rate in the cohort was only 47%. Therefore, a negative result from these sites should not be considered sufficient to rule out the disease.
Paraproteinemia studies showed a lower identification of monoclonal peak in serum protein electrophoresis compared to reports in the literature, 48% vs 67%.11 However, the positivity of immunofixation in serum and urine, 77% and 73% respectively, was similar to that of other cohorts.11,12 All patients in our study had monoclonal protein detected in serum or urine, highlighting the importance of conducting complementary studies to assess monoclonality.
Overall survival was evaluated based on prognostic factors described in previous studies, showing similar results with reduced survival in patients with cardiac and renal involvement, multiple myeloma, or bone marrow infiltration by plasma cells.10-12 Patients who underwent ASCT had improved survival, though this may reflect selection bias, because these patients were generally younger, had fewer affected organs, and exhibited a lower rate of infiltrative cardiac involvement.
Limitations of the study
The main limitation of this study is its retrospective design, which hinders the comprehensive data collection needed for an objective assessment of disease severity stratification, treatment response, and organ response based on established criteria. Additionally, patient follow-up was incomplete. Another limitation is the absence of mass spectrometry, which would have allowed for a more precise confirmation of AL amyloidosis involvement; instead, diagnoses relied solely on immunohistochemical and histopathological studies. Lastly, the small sample size limits the ability to generalize findings, though it provides valuable descriptive insights within our settings (Colombia), which remains the primary focus of this study.
Authorship
Contribution: J.F.M.R. and V.C. participated in the data collection process; J.F.M.R. was responsible for statistical analysis and data interpretation; all authors contributed to the writing of the manuscript; and K.G. was involved in all aspects of the study including data collection, statistical analysis, data interpretation, manuscript writing, and conducted a comprehensive review of the final version.
Conflict of interest disclosure: K.G., J.F.M.R., and V. C. received research funding from Johnson & Johnson.
Correspondence: Kenny Galvez, Investigations Unit, Hospital Pablo Tobón Uribe, Calle 78B # 69-240, 9th Floor, Building B, Medellín, 050031 Colombia; email: kennygalvez@gmail.com.
References
Author notes
Deidentified participant data can be obtained on reasonable request from the corresponding author, Kenny Galvez (kennygalvez@gmail.com).