Visual Abstract
Providing access to breakthrough cancer treatments and eradicating survival disparities from cancer in low- and middle-income countries poses a crucial public health challenge. It requires sustainable, targeted approaches that bolster health system capacity to provide the latest treatments while supporting patients in navigating socioeconomic barriers to care. The Max Foundation launched Max Access Solutions in 2017 to meet this public health need. Max Access Solutions is an end-to-end platform that ensures therapeutic and diagnostic products reach patients in need, while providing accompaniment services to patients and physicians through a global team of local program coordinators who understand the local health care context. Max Access Solutions and its predecessor, the Glivec International Patient Assistance Program, have successfully provided access to novel cancer treatments to >100 000 patients with cancer in over 75 countries. In this article, we describe the development, structure, and potential future directions of the Max Access Solutions model.
Introduction
Creating access to breakthrough cancer medicines in low- and middle-income countries (LMICs) is a critical public health need. Global cancer incidence is predicted to rise from ∼20 million new cases in 2022 to 35 million by 2050, with the most dramatic increases (99%-142%) in LMICs.1 Although LMICs bear a disproportionate burden of mortality from cancer, the bulk of global resources dedicated to fighting cancer are concentrated in high-income countries.2 Despite major advances in new cancer therapies in recent decades, breakthrough cancer medicines are often unavailable or unaffordable in LMICs.3-5 Less than 10% of low- and lower middle–income countries provide basic cancer management as part of universal health coverage plans,6 and cancer medicines, even those on the World Health Organization (WHO) essential medicines list, are often either unavailable or only available at full cost to patients.3,4
Breakthrough cancer medicines are defined here as new treatments emerging from the pipeline that have the potential to dramatically transform clinical outcomes, reduce mortality, and improve patients’ quality of life in a low infrastructure health care setting. However, these medicines are often expensive and only commercially available in high-income countries. Multiple interrelated barriers severely limit access to breakthrough cancer medicines in LMICs, especially for the most vulnerable populations. LMICs face critical challenges in health system infrastructure, with limited facilities (mostly concentrated in large cities) capable of providing necessary services such as screening and imaging so that patients can initiate treatment.7 Additionally, many countries face shortages of health care workers with the necessary tools and training to provide cancer treatment.2,7 Costs for cancer medicines are often prohibitive, and gaps in the supply chain result in stockouts that threaten patients’ continuity of care.8 Further, travel distances between patients’ communities and facilities offering treatment pose another key challenge, adding to the potentially impoverishing out-of-pocket costs borne by people living with cancer.9-11 Fear, anxiety, stigma, and other psychosocial impacts of cancer are additional challenges patients must navigate to access and adhere to treatment.12
Overcoming these challenges requires innovative strategies, collaboration, dedication, perseverance, and a long-term commitment. In response to the urgent need to improve access to breakthrough cancer medicines in LMICs, The Max Foundation developed Max Access Solutions, a platform dedicated to overcoming barriers to care and delivering access to medicines through local institutions and health care professionals (HCPs). This unique model, based on over 20 years of experience, creates a pathway in LMICs to make certain medicines available to patients via their treating physicians at no cost.
Evolution of Max Access Solutions
In 2001, The Max Foundation (Max) established a partnership with Novartis Pharma AG to implement the Glivec International Patient Assistance Program (GIPAP) to provide Glivec (imatinib) at no cost to eligible patients diagnosed with chronic myeloid leukemia (CML) or gastrointestinal stromal tumor (GIST). The organization developed the systems necessary to receive and assess physician requests on behalf of individual patients, verify that all requests fulfilled program criteria, request the amount of medicine prescribed for each patient, and monitor each patient’s individual treatment protocol, enabling the tracking of thousands of patients, their treating physicians, and the volume of medicine needed to supply them.13 Initial results from GIPAP showed that the model was successful in providing sustainable access to Glivec (imatinib) to treat CML and GIST in LMICs. Patients with CML in the GIPAP program had a 5-year survival rate of 89%, comparable to survival rates in high-income countries.14 This experience demonstrated that it is possible to provide sustainable and European Union Good Distribution Practice–compliant humanitarian access to cancer medicines in LMICs.
In 2017, following more than a decade of experience administering the GIPAP program, Max launched Max Access Solutions in response to growing requests by health care providers and patients in need of access to other breakthrough oncology medicines, driving the evolution of the single-drug program into a multidrug/multipartner approach. Max Access Solutions provides access to a portfolio of breakthrough medicines that are US Food and Drug Administration– or European Medicines Agency–approved but not locally registered in LMICs. The portfolio is available to physicians working at cancer-treating institutions, for patients diagnosed with an approved indication of the product but who have no other way to access that treatment.
This evolution allowed the organization to scale up its impact and address barriers to cancer care by developing and leveraging strategic partnerships while working with stakeholders to strengthen local health systems. This model continues to transform oncology care in LMICs, setting a new standard for equitable access to cancer treatment.
Program overview: a patient-centered approach based on 4 fundamental pillars
The Max Access Solutions platform helps people living with cancer and other critical illnesses to overcome obstacles to treatment access by understanding and prioritizing each person’s needs, while leveraging a deep understanding of each country’s unique health care and cultural environment. This patient-centered approach goes beyond medicine provision to address the broader socioeconomic and logistical barriers that often hinder access to cancer care. Local program coordinators, stationed across the globe and fluent in local languages, maintain close contact with patients to understand their needs and help them navigate complex health care systems. These team members are often from the same countries as the patients they serve, providing culturally relevant support that fosters trust and effective communication. This local presence enables swift responses to challenges and ensures that patients remain on treatment, even amidst economic or political disruptions.
Max Access Solutions also prioritizes collaboration with patient advocacy organizations and other partners to address the emotional, informational, and practical needs of patients and deliver complementary programs to support patients and their families with transportation for medical appointments, continuation of schooling for children, and other areas impacted by patients’ cancer care. A limitation of many existing programs is that medicine donations often take the form of one-time excess product or emergency response donations that may not align with local patients’ priorities.15 Some patients require lifelong access to medication, creating the need for long-term commitments from programs. A key differentiator of the Max Access Solutions approach is its commitment to long-term continuity of care. Hence, donor partners agree to continue providing medicines to patients for as long as the treating physician recommends continuation of treatment and while no commercial or other access solution is in place. The platform verifies treatment continuation for each patient at least once every 12 months through patient contact, a physician reapproval request, or by obtaining a pharmacy medication dispensation record. By tracking medicine to the patient’s hand, as well as accompanying individual patients throughout their treatment journey, Max Access Solutions ensures sustainable access.
The operating model of Max Access Solutions places the patient at the center, supported by 4 key pillars (visual abstract): therapeutic and diagnostic products, a network of physicians in LMICs, a local team of coordinators, and international shipping and logistical pathways, all supported by a broader set of partnerships including patient organizations and global expert networks. These 4 pillars are further enhanced by a comprehensive data management system and the recognition that health system strengthening, in conjunction with multiple stakeholders, is key to achieving sustainable impact. These elements are described in more detail in the following sections.
Making therapeutic and diagnostic products available in LMICs
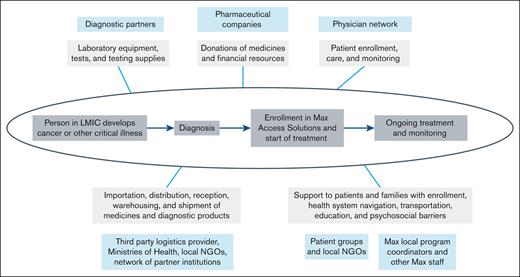
Diagnostic and therapeutic products form the first fundamental pillar of the Max Access Solutions model. Multistakeholder partnerships are critical to assuring the availability of breakthrough medicines and diagnostic products in LMICs (Figure 1). Through partnerships, pharmaceutical and diagnostic companies who support the implementation and operation of Max Access Solutions provide breakthrough medicines and diagnostic products that would otherwise be unavailable within LMICs.
The different roles of Max Access Solutions partners along the patient journey to care in assuring access to diagnosis and treatment for people living with cancer and other critical illnesses.
The different roles of Max Access Solutions partners along the patient journey to care in assuring access to diagnosis and treatment for people living with cancer and other critical illnesses.
The program selects medicines based on clinical benefits to patients, suitability for settings with limited health care infrastructure, logistical considerations, and adequate shelf life. All medicines address cancer or other critical illnesses, while diagnostic products are essential for treatment initiation and monitoring of these diseases. The breakthrough medicines selected are patent-protected and the manufacturer is typically the sole global supplier. Pharmaceutical and diagnostic companies provide medicines and diagnostic products free of charge for use in eligible countries. Some companies provide unique packaging to differentiate products provided to the program from commercial products.
Global health care provider network
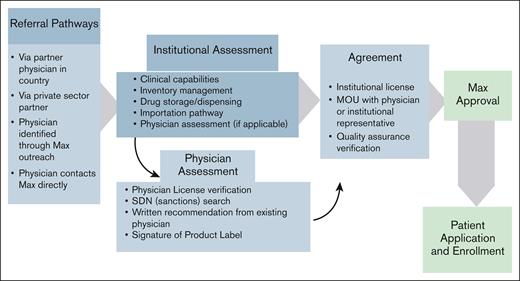
One of the 4 pillars of the model is the network of institutions and HCPs in LMICs who provide the access point through which patients enter the program. Collaboration with local institutions with capacity to diagnose and treat patients with an approved indication of the medicines available through the portfolio is essential. Potential institutions (ie, clinics and hospitals) and physicians undergo an assessment before being validated and approved (Figure 2). Treating institutions are assessed based on multiple criteria including: capacity to diagnose and treat the type of cancer or rare disease for which a product is indicated, ability to handle importation and dispensation of each eligible product, possession of a license to handle pharmaceutical products in accordance with local regulations as per European Union Good Distribution Practices, existence of appropriate quality controls, and referral from a current partner institution.
Steps in the onboarding process for physicians and institutions entering into partnership with The Max Foundation. MOU, memorandum of understanding; SDN, specially designated nationals.
Steps in the onboarding process for physicians and institutions entering into partnership with The Max Foundation. MOU, memorandum of understanding; SDN, specially designated nationals.
As part of this onboarding process, virtual or in-person site visits at each institution are performed, building relationships with HCPs and hospital leadership to better understand the local environment and challenges to diagnosing and treating patients. Outreach to patient organizations that are active in a given setting helps ensure a deep understanding of the local patient journey including barriers to diagnosing, initiating, and maintaining treatment. Additionally, building strong relationships and creating open dialogue with the local health care team is critical to the effective delivery of treatment and enables constructive support to HCPs in delivering care to their patients.
HCP participation is voluntary. HCPs are approved as authorized prescribers for each individual treatment depending on the availability of medicines. Training is provided to partner physicians on software navigation to enroll patients into the program (Figure 3), compliance with program data requirements such as managing inventory, and maintenance of dispensing logs. The program facilitates disease educational resources and training for participating physicians to support their efforts. Partner HCPs are responsible for prescribing the medications available through Max Access Solutions and driving all aspects of patient care, including diagnosis and treatment management. Once a case is approved and medication is provided for an on-label indication, HCPs manage the treatment of their patients independently based on their medical training and experience. The program requires HCPs to update the prescription record if a dose is changed for planning and forecasting purposes, notify instances of treatment discontinuation for enrolled patients, and follow local country pharmacovigilance regulations. HCPs also play a key role in obtaining local health authority approval for import permits and, if needed, tax waivers on imported medicine.
Drug distribution pathway
Creating compliant and secure drug distribution pathways, the third pillar in the model is recognized as a key challenge to implementing access programs in LMICs, although they are critical for ensuring that patients receive the right medicines in the correct doses and at the right time while maintaining the highest standards of safety and regulatory compliance. In Max Access Solutions, products are requested from manufacturers on a monthly or quarterly schedule based on known patient needs. This approach prevents stockpiling, obsolescence, and improper disposal of medicines. Upon receipt of the medicines, which are warehoused in the United States and Europe, a third-party supply chain partner performs inventory management and quality control. From there, medicines are typically shipped directly to institutions or in some cases distributed through Ministry of Health supply chains or locally based nongovernmental organizations (NGOs) (Figure 4).
Core steps in the supply chain pathway, which is adapted to the context of each country.
Core steps in the supply chain pathway, which is adapted to the context of each country.
The distribution of humanitarian donations of medicines is a highly regulated process that varies between countries, depending on health care systems, regulatory frameworks, and infrastructure. For example, regulatory frameworks within a country govern the importation, storage, and distribution of medicines, which impacts shelf life of medicines, import permits, taxes, and customs clearance. Similarly, varied health care infrastructure shapes local distribution channels and thus, the average time between order placement and delivery at an institution. In response to the unique situation of each country, the program adapts distribution pathways and strategies to the specific context and needs of the country and partners closely with local HCP teams during the drug distribution process. Coordination and collaboration among implementing partners and Ministries of Health and health authorities are also critical for successful distribution of medicines globally.
Local program coordinators
Locally based program coordinators represent the fourth pillar in the model and play a key role in bridging the gap between global strategies and local implementation, while focusing on supporting patients and partner physicians. Local program coordinators provide practical, emotional, and psychosocial support to patients and their caregivers in the form of transportation assistance, diagnostic support, patient education, and treatment adherence validation. Program coordinators routinely contact patients and caregivers via phone and in-person meetings and mail to ensure that patients’ needs are handled individually and holistically (Figure 5). They work closely with partner HCPs to submit program data, track individual contact with patients, obtain and enter dispensing logs, and facilitate communication among all local stakeholders. Ultimately, the local program coordinator serves as an advocate for patients in their region, helping them navigate the health system and address any challenges that arise. Program coordinators have a deep understanding of the needs of both patients and partner HCPs and can bridge communication gaps in challenging environments in a culturally sensitive way.
Typical requests from physicians in the network and actions carried out by local program coordinators.
Typical requests from physicians in the network and actions carried out by local program coordinators.
In addition, program coordinators have knowledge of the local regulatory and cultural context and are instrumental in navigating the importation process. They facilitate collaboration among various stakeholders, including local governments, advocacy groups, academic institutions, and NGOs, and are trained on the treatments and diseases within the Max Access Solutions portfolio, as well as policies and processes needed to implement and operate the program locally. The implementing team members undergo pharmacovigilance training and are required to report any adverse events identified during program activities to the relevant pharmaceutical company within 24 hours.
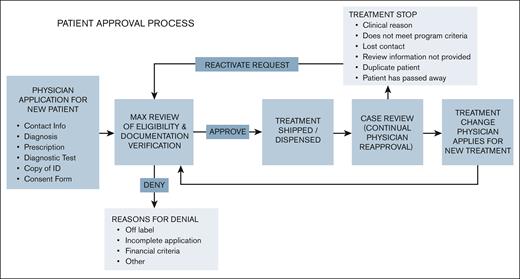
Patient Access Tracking System
Complementing the 4 pillars described above, the Patient Access Tracking System (PATS) is a cloud-based virtual environment that links all implementing partners and key data points for the program in real time. Each HCP is given their own password protected login. HCPs submit individual case requests for medication and enter basic patient data, such as age, diagnosis, and prescription. Cases are assigned a unique identification number and patients sign a consent form to participate in the program. Outputs for the system include treatment adherence, disease patterns, clinical outcomes, and drug supply data. The PATS system is also instrumental in relaying pharmacovigilance information to the partner members. All suspected adverse events, special scenarios, and/or product complaints are reported to the respective pharmaceutical company within 24 hours to ensure compliance.
By connecting each partner, PATS can provide detailed planning and forecasting capabilities by the treating institution and identify and prevent spillage and drug diversion out of the supply chain. Importantly, PATS enables users to identify pain points in the treatment continuum that impact patient compliance, such as language barriers, lack of internet, or reliance on paper prescriptions. Through these outputs, PATS supports patients’ adherence to treatment. Both physicians and Max’s local program coordinators use PATS to identify those who are not taking medications and to determine how to overcome the challenges that patients face, such as travel or work-related issues.
The primary purpose of the program data collected is to verify that individual case requests fulfill program criteria, strengthen planning and forecasting, and assess needs for patient support. This ensures that partner HCPs in LMICs, who have limited time and high caseloads, are not overburdened by extensive amounts of data entry. The program is not a clinical trial and does not collect data for research purposes.
Health systems strengthening
Another key to the success of the model is working with multiple stakeholders from the global to local levels to enhance health system capacity to provide the tests and treatments available through Max Access Solutions. Through strategic partnerships with in-country physician networks and ministries of health, the program accelerates strengthening of local health systems’ capacity to diagnose, treat, and monitor the diseases in its portfolio.
A critical barrier to treatment access in LMICs is the lack of diagnostic infrastructure, which often delays or prevents patients from starting therapy. To address this gap, strategic partnerships with diagnostic manufacturers enable access to tests, tools, and equipment, along with training for pathologists and laboratory personnel. These partnerships enable local facilities to perform critical testing, thereby enhancing diagnostic capabilities.
To ensure high-quality care delivery in LMICs, HCP training, site visits, and capacity assessments are facilitated. These initiatives are designed to build local expertise, enhance clinical skills, and improve patient outcomes. Training includes physicians, pathologists, laboratory personnel, pharmacists and other HCPs that focus on the safe administration of treatments and best practices in patient management. Additionally, site visits and capacity assessments are carried out in collaboration with local partners to evaluate health care infrastructure, identify system gaps, and develop actionable improvement plans. These assessments support the optimization of patient support, streamline medicine distribution, and enhance diagnostic capabilities. The insights gathered help prioritize investments in infrastructure and workforce development, ensuring that local health systems are equipped to manage complex oncology care.
Through these efforts, the initiative not only addresses immediate barriers to treatment but also contributes to the long-term resilience and self-sufficiency of health systems in LMICs. This holistic approach to health systems strengthening empowers local institutions to provide consistent, high-quality care, even in resource-constrained settings.
Impact
Since its launch in 2017, Max Access Solutions has expanded to 79 countries, 25 of which currently have locally based team members. As of 31 December 2024, the program provides access to 15 medicines to treat 14 diseases including CML, GIST, renal cell carcinoma, lung cancer, Philadelphia chromosome-positive acute lymphocytic leukemia, chronic lymphocytic leukemia, and breast cancer. Since 2017, the program has provided ∼36 million daily drug doses to >43 000 patients through a network of over 350 institutions, nearly 500 physicians, and 7 pharmaceutical manufacturers. Importantly, patient data is only collected for program management purposes and not for evaluation of treatment. Despite data limitations, outputs show that it is possible to operate a humanitarian access program for oncology and rare diseases in LMICs through a patient-centered approach. This model has contributed to reducing global disparities in cancer survival rates, proving that equitable access to life-saving treatments is achievable even in resource-limited settings.
Future directions
Estimates indicate the global burden of cancer will grow by 77% by 2050, with the highest increases in incidence (99%-142%) occurring in LMICs, fueled by rising rates of risk factors such as smoking, unhealthy diets, and physical inactivity.1 The health care infrastructure in many LMICs remains under-resourced, with insufficient diagnostic capacity, fragmented supply chains, and economic barriers that prevent patients from accessing life-saving therapies. An increasing number of pharmaceutical and biotechnology companies recognize the need to bolster global access to breakthrough medicines as soon as they emerge from the pipeline. Companies have undertaken various approaches to expand the availability of medicines in LMICs, including differential pricing strategies, voluntary licensing agreements, patient access and assistance programs, and donation programs.15 However, given the complex, multidimensional barriers in place, availability of medicines does not equate to accessibility. NGOs working in close partnership with HCPs, companies, patient groups, ministries of health, and other stakeholders, can play a pivotal role in bridging the gap between availability and access. Several organizations have recently announced the establishment of partnerships to further enhance global access to essential cancer medicines in LMICs. For example, the WHO and St Jude’s Children’s Research Hospital have launched the Global Platform for Access to Childhood Cancer Medicines to assure access to critical treatments for pediatric cancer in LMICs.16 In 2022, the Union for International Cancer Control launched the Access to Oncology Medicines Coalition, a network of over 40 organizations from both the private sector and civil society which, in addition to advocating at the global level for more equitable access to cancer medicines, works with governments and local partners to facilitate access in various countries.17
With over 20 years of experience in implementing a patient-centered approach to treatment and offering wrap-around patient support, Max Access Solutions provides a model that could be expanded or replicated to ensure equitable access to all breakthrough medicines and accelerate health equity across the world. Ultimately, universal access to breakthrough medicines globally is not only a critical public health need but also a fundamental human right.
Acknowledgments
Partnerships made this work possible. The Max Foundation would like to acknowledge all the individuals and organizations that have helped provide access to diagnostics, medicine, treatment, and care to patients throughout the world. This includes patient groups, physicians, health care professionals, pharmacists, businesses, nongovernmental organizations, and many others. Together, we are working to strengthen systems and eliminate health care disparities.
The authors acknowledge Alicia Annamalay, Kelsey Bray, Bryan Murphy-Eustis, and Colin Forsyth from The Max Foundation who assisted with drafting, reviewing, and preparing the manuscript. The authors' deepest thanks go to the treating physicians and health care teams, and all The Max Foundation team members around the world, whose daily efforts have been an essential driving force shaping the model and impact described in this article.
Authorship
Contribution: G.C., J.M.W., and P.G.-G. developed the overall concept and drafted, reviewed, and edited the manuscript.
Conflict-of-interest disclosure: G.C., J.M.W., and P.G.-G. represent The Max Foundation, which receives in-kind donations of medicines and diagnostics for programs, as well as financial support from Novartis, Takeda, Bristol Myers Squibb, BeOne, Pfizer, Eli Lilly, Incyte, Cepheid, and BD (Becton, Dickinson and Company). G.C. has also served as a consultant to GlycoMimetics, Inc, and Pierre Fabre Pharmaceuticals, Inc.
Correspondence: Pat Garcia-Gonzalez, The Max Foundation, 1107 NE 45th St, Ste 230, Seattle, WA 98105; email: Pato@themaxfoundation.org.