TO THE EDITOR:
Myeloma-related bone disease (MMBD) affects up to 80% of patients with multiple myeloma (MM), frequently resulting in skeletal-related events (SREs) such as pathological fractures, spinal cord compression, and hypercalcemia.1,2 These complications not only increase morbidity and mortality but also substantially impair patients' quality of life by causing pain, decreased mobility, and loss of independence.3,4 Fractures are associated with prolonged hospitalization, increased use of health care resources, and elevated costs to health systems globally, challenges amplified in resource-limited settings such as Latin America (LATAM).5
To characterize current practices for diagnosing and managing MMBD in LATAM, we conducted an anonymous online survey targeting hematologists treating MM. The survey was piloted in 1 institution and subsequently distributed between April 29 and May 20, 2024, through social media platforms and the Latin American Myeloma Study Group (GELAMM) network. It included 25 multiple-choice questions concerning health care settings, imaging modalities, and the use of antiresorptive drugs (ARDs) (supplemetal Data).
We received responses from 204 physicians across 168 centers in 15 countries (Figure 1).
Among all the respondents, 57% worked in public institutions. At diagnosis, 26% reported using positron emission tomography-computed tomography (PET-CT) as the first imaging option, 18% whole-body low-dose computed tomography (WBLDCT), 17% X-ray, and 16% whole-body magnetic resonance imaging (WB-MRI). Regarding management, 2.5% responded that they don't use any ARDs for MMBD. Among those who reported using ARDs, 69% employ them in all patients with MM at diagnosis, whereas the rest claimed using ARDs only if patients have bone disease. Eighty-three percent of physicians reported reinitiating ARDs at relapse. Zoledronic acid was used by 93% of the respondents. Denosumab was accessible to 42% of participants, 60% for private practice, and 25.5% for public systems. As for the duration of treatment, 73% claimed to have used ARDs for 2 years, 14% for 1 year, and 6% until progression. Regarding schedule, 50% reported using ARD monthly, 10% every 3 months, and 31% monthly the first year and then every 3 months.
For patients with creatinine clearance <30 mL/min, 41% would employ denosumab (56% in private centers and 28% in public hospitals), and 40% would not use any ARDs.
Eighty-eight percent of the physicians claimed to do prior dental examinations, and 58% reported that this entails a delay in starting treatment. Twenty percent of the participants reported not using calcium and vitamin D supplementation, and 57% measured vitamin D plasma levels before starting treatment.
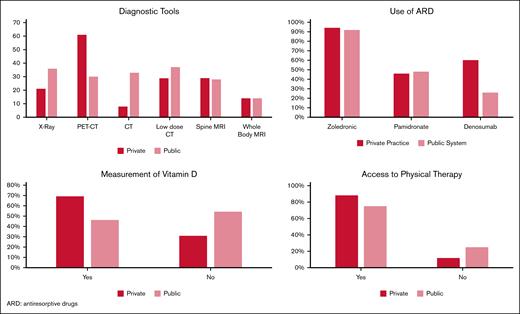
Figure 2 shows differences between answers from physicians from private and public centers.
International guidelines from the International Myeloma Working Group emphasize the use of WBLDCT, PET-CT, or WB-MRI for initial MMBD assessment because of their superior sensitivity and earlier detection of lytic lesions compared with conventional skeletal surveys.3,4 WBLDCT and PET-CT detect bone involvement in 80% to 90% of patients, whereas skeletal X-rays require ≥30% cortical bone destruction to identify lesions, delaying diagnosis.5,6,7
Despite this, only 18% and 26% of respondents reported routinely using WBLDCT and PET-CT, respectively, whereas 17% still relied on conventional X-rays. Limited access to advanced imaging in LATAM because of infrastructure constraints, reimbursement policies, and geographical disparities, especially in public health care, likely accounts for these findings. Similar challenges have been described in other low- and middle-income countries.8
Pathologic fractures severely impact patients with MM by causing debilitating pain, functional impairment, and increased risk of morbidity and mortality.9 Vertebral fractures, common in MM, can result in spinal cord compression, neurological deficits, and significant disability. Patients experiencing fractures report poorer health-related quality of life, such as increased fatigue, depression, and loss of autonomy.10 Fracture-related hospitalizations also lead to increased health care utilization and costs.5 Prevention of fractures through early diagnosis and appropriate use of ARDs is therefore crucial.
In this survey, 2.5% of physicians reported not prescribing ARDs for MMBD. Among those who did, 69% administered them to all patients at diagnosis, whereas 31% limit ARD use to patients with confirmed bone lesions, contrary to International Myeloma Working Group recommendations advocating ARD use for all patients receiving antimyeloma therapy, regardless of imaging findings.3 Zoledronic acid remains the most frequently used agent (93%), whereas access to denosumab is limited to 42% overall—60% in private centers compared with 25.5% in public institutions. Notably, denosumab was preferable in patients with renal impairment because of its favorable safety profile,11 but 40% of physicians would not prescribe any ARDs in patients with creatinine clearance <30 mL/min, reflecting gaps in optimal care.
Duration of ARD therapy varied: 73% prescribed treatment for 2 years, 14% for 1 year, and 6% until disease progression. Dosing schedules were heterogeneous, with 50% using monthly infusions, 10% every 3 months, and 31% monthly for 15 years, followed by quarterly dosing. Real-world evidence suggests that tailored de-escalation may reduce toxicity without compromising efficacy, although consensus is lacking.12
Eighty-eight percent of physicians performed dental assessments prior to ARD initiation; however, 58% reported that these evaluations delayed therapy onset. Twenty percent did not routinely prescribe calcium and vitamin D supplementation, and only 57% measured vitamin D levels prior to treatment, highlighting areas for clinical practice improvement.
When we compare responses between myeloma-doctors from private and public centers, we see differences in the availability of PET-CT, denosumab, measurement of vitamin D, and somewhat more access to physical rehabilitation. This could indicate inequity between both systems, as we have seen in other studies published by GELAMM.13,14
SREs, particularly fractures, significantly increase direct and indirect costs because of surgical interventions, rehabilitation, and prolonged hospitalization.15 In resource-limited settings such as Latin America, the economic burden underscores the importance of cost-effective strategies to prevent MMBD complications.
Early detection with sensitive imaging modalities can facilitate the timely initiation of ARDs, potentially preventing costly fractures and hospitalizations. Although denosumab is more expensive than bisphosphonates upfront, its renal safety profile and potential to reduce SRE rates may yield cost savings in the long term. Nevertheless, limited access to advanced diagnostics and treatments in public health sectors remains a significant barrier.
This survey represents the most extensive assessment of MMBD diagnosis and management practices in LATAM to date. It reveals significant heterogeneity and a gap between clinical guidelines and real-world practices, particularly regarding imaging use and ARD accessibility. The high prevalence and profound impact of fractures on patients’ quality of life and health care systems call for urgent policy changes, such as investment in imaging infrastructure, improved access to bone-modifying agents, and standardization of care protocols.
Our findings should inform regional strategies to improve MMBD outcomes through equitable, guideline-concordant care, ultimately reducing fracture incidence and its associated clinical and economic burden.
Acknowledgment: The authors thank the International Myeloma Society (IMS) for recognizing C.R. with the young investigator award in 2024.
Contribution: C.R. and C.P. wrote the project and developed the survey; and all the other authors analyzed the results and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GELAMM appears in “Appendix.”
Correspondence: Camila Peña, Hospital del Salvador, Avenida Salvador 364, Providencia, Región Metropolitana, Chile; email: camipena@gmail.com.
Appendix
Members of the GELAMM study group include:
Agnerys López Sacerio, Virginia Abello Polo, Cristina Acon, María Adela Aguirre, Milagros Anahais Altamirano Molina, José Luis Álvarez Vera, Lidiane Katherine Andino Neves, Maria Isabel Arbelaez Monroy, Carlos Bermudez, Erika Barbara Brulc, Pamela Bustamante, Maria Lorena Cardozo Villagra, Antonio Alfredo Carrasco Yalan, Juan Antonio Choque Pacheco, Yusleidy Concepción Fernández, Carmen Noelia Corrales Alfaro, Mario Ernesto Correa Correa, Ariel Alfonso Corzo, Daniel Ruben Del Carpio Jayo, Javiera Paz Donoso Pineda, Alicia Enrico, Jose Ramiro Espinoza Zamora, Dorotea Fantl, Julio Dámaso Fernández Aguila, Yrving Ernesto Figueredo Peguero, Jair Figueroa Emiliani, Maria Lucia Fonseca Bolaños, Cristobal Augusto Frutos Ortiz, David Garrido, Virginia Gilli, David Gómez Almaguer, Reynaldo Gonzalez, Manuel Antonio Granja Moran, Elizabeth Hernandez, Marcos Hernández Jimenez, Elizabeth Guadalupe Hernández Infante, Carlos Ramon Hernández Padrón, José Carlos Huaylinos Rodriguez, Henry Idrobo Quintero, Paola E. Leone, Elena Lisott, Agnerys López Sacerio, Hernan Lopez Vidal, Sergio Gustavo Lopresti, Humberto Martinez-Cordero, Claudia Ruth Mendoza Barrenechea, Maribel Milagros Molina Almanza, Yanina Mucha Sánchez, Juan Ramón Navarro Cabrera, Andrea Noboa, Paola Ochoa, Sergio Orlando, Juan Alejandro Ospina Idárraga, Diana Margarita Otero De La Hoz, Andrés Parrado Rey, Bonell Patiño Escobar, Camila Peña, Ines Peña, Judith Ivon Pineda Palma, Maria Lourdes Posadas, Guillermo Enrique Quintero Vega, Luis Dario Quiroga, Jhoanna Ramírez, Aline Guillermina Ramírez Alvarado, Yorleni Sulema Ramirez Mejia, Guillermina Remaggi, Rosa Oliday Ríos Jiménez, Eloisa Riva, Luis Fernando Rivas, Macarena Roa Salinas, Jose Ramon Roca Leyva, Yusaima Rodriguez Fraga, Gloritza Rodriguez Matos, Yainer Rubio Blanco, Guillermo José Ruiz-Argüelles, María Soledad Saez, César Samanez, Celia Carela Sandoval Villa, Michel Santos Gonzalez, Natalia Paola Schütz, Cristian Maximiliano Seehaus, Claudia Marta Shanley, Irma Slavutsky, Patricia Beatriz Sorroche, Alejandro Sosa Espinoza, Claudia Sossa Melo, Pablo Maximiliano Soto Vargas, Flavia Stella, Martha Leticia Suarez Acuña, Irma Tello Villalaz, Maria Alejandra Torres Viera, María Cristina Tripodi Fleitas, Jule Franve Vasquez Chavez, Rosa Ines Vengoa Figueroa, Verónica Isabel Verri, Fiorella Villano, Ricardo Villegas Nava, Seisha Alana Von Glasenapp Gossen, Alfredo Wong Chang, Soledad Zabaljauregui, Patricio Duarte, Patricia Graffigna.
References
Author notes
Data are available upon request. Readers may contact the corresponding author, Camila Peña (camipena@gmail.com) to request underlying data.
The full-text version of this article contains a data supplement.