TO THE EDITOR:
Chimeric antigen receptor T cells (CARTs) have now been used to treat children, adolescents, and young adults (CAYAs) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) for well over a decade. Although there is a wealth of publications on acute inflammatory toxicities related to CART,1-3 there is limited information on late effects. This gap is largely due to the relative novelty of CART and lack of systematic and longitudinal studies in long-term survivors.
In contrast, late-effects after hematopoietic cell transplant (HCT) are well-established. Although the role of post-CART HCT remains controversial due to the added toxicity4 and ability to achieve long-term cure in approximately half of patients receiving commercial tisagenlecleucel without HCT,5 it remains an important strategy.6 A consolidative HCT in those achieving post-CART remission to achieve long-term cure is often considered when experience regarding CART persistence is lacking, when there is early loss of B-cell aplasia, or minimal residual disease assays are concerning for impending relapse.7
Currently, there is no standard assessment measures of late effects following CART. However, the US Food and Drug Administration (FDA) provides limited guidance for long-term follow-up (LTFU) for gene therapy.8 This framework includes recommendations on key parameters, such as new malignancies, new incidence or exacerbation of a preexisting neurologic disorder, new incidence or exacerbation of a prior rheumatologic or other autoimmune disorder, new incidence of a hematologic disorder, and new infection (potentially product-related). Nevertheless, this approach does not comprehensively address all potential long-term issues. The study of late effects of therapies in the CAYA population has significant implications for quality-of-life especially in patients in long-term remission following CART. In the context of a patient population that largely proceeded to a consolidative HCT following treatment on a phase 1 CART trial, we sought to analyze late effects of patients who remain alive and in remission treated at our institution.
This review was an institutional review board approved retrospective evaluation to describe long-term outcomes (ClinicalTrials.gov identifier: NCT03827343) in CAYA with R/R B-ALL who received CART on 1 of our 3 phase 1 studies: CD19.28z (ClinicalTrials.gov identifier: NCT01593696), CD22.41BBz (ClinicalTrials.gov identifier: NCT02315612), or CD19/22.41BBz (ClinicalTrials.gov identifier: NCT0344839). The analysis cohort was comprised of CAYA patients who were ≥2 years following a CART-induced complete remission and had not received additional disease directed therapy with exception of a consolidative HCT. The primary objective was to describe late effects in this long-term (≥2 years after CART infusion) population. The study was conducted in accordance with the Declaration of Helsinki.
The post-CART time frame was determined by the difference between the most recent contact and CART infusion (day 0). Late effects were evaluated based on the FDA LTFU safety parameters and framework for long-term monitoring for gene therapy. These parameters facilitated gathering information on development of new primary cancers, neurologic disorders, autoimmune disorders, hematologic disorders, other illnesses, and current medications. Furthermore, providers were asked to provide recent progress notes that included current medications, treatment summaries, discharge summaries, and laboratory reports. Per FDA requirements, the study team sent these inquiries regarding patient health status at 3 months, 6 months, and yearly post-CART infusion through 15 years. Data were extracted through reviewing each patient’s LTFU note and was supplemented with a thorough chart review. All data were collated up to 1 June 2025. Demographic and assessment of late effects were captured with standard descriptive statistics.
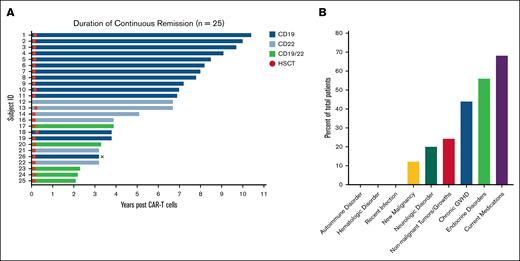
Across 25 patients in ongoing complete remission without any interval therapy, with exception for HCT, the median age was 12 years (range, 5-34) at CART infusion. Five (20%) patients were female. This cohort had previously undergone a median of 3 treatments (range, 1-9; Table 1). Furthermore, 4 (16%) patients had previously received HCT. The median follow-up time was 7.3 years (range, 3-11). At the most recent follow-up, the median age was 22 years (range, 10-38). A total of 14 (56%) patients were treated with CD19-directed CART, 6 (24%) received CD22-directed CART, and 5 (20%) received CD19/22-directed CART (Figure 1A). Subsequently, 24 (96%) patients received a post-CART HCT, with a median time of 1.8 months (range, 1.4-3.2) between CART infusion and HCT day 0. Details of the HCT course is provided in supplemental Table 1.
Long-term follow-up. (A) Duration of continuous remission in patients who are at ≥2 years after CART infusion. Each bar represents a patient, identified by a subject ID. The length of each bar represents number of years in remission post-CART therapy. Patients are color categorized by the CART for which they received. Fourteen (56%) received CD19 CARTs, 6 (24%) received CD22 CARTs, and 5 (20%) received CD19/22 CARTs. The circles along the bars indicate when patients received HCT (median time, 1.8 months; range 1.4-3.2). No patients experienced a relapse of B-ALL. X indicates death related to cholangiocarcinoma. (B) Late-effects of CART therapy. This bar graph depicts the prevalent late-effects of CART therapy for patients in the primary cohort (n = 25). The graph represents the FDA parameters for gene therapy LTFU and the percentage of patients who developed each disorder, along with the prevalence of conditions such as GVHD, nonmalignant tumors/growths, and endocrine disorders. GVHD, graft-versus-host disease.
Long-term follow-up. (A) Duration of continuous remission in patients who are at ≥2 years after CART infusion. Each bar represents a patient, identified by a subject ID. The length of each bar represents number of years in remission post-CART therapy. Patients are color categorized by the CART for which they received. Fourteen (56%) received CD19 CARTs, 6 (24%) received CD22 CARTs, and 5 (20%) received CD19/22 CARTs. The circles along the bars indicate when patients received HCT (median time, 1.8 months; range 1.4-3.2). No patients experienced a relapse of B-ALL. X indicates death related to cholangiocarcinoma. (B) Late-effects of CART therapy. This bar graph depicts the prevalent late-effects of CART therapy for patients in the primary cohort (n = 25). The graph represents the FDA parameters for gene therapy LTFU and the percentage of patients who developed each disorder, along with the prevalence of conditions such as GVHD, nonmalignant tumors/growths, and endocrine disorders. GVHD, graft-versus-host disease.
Regarding late effects, 3 (12%) patients were diagnosed with new primary cancers: 1 with a fatal cholangiocarcinoma (which has been previously described)9 and 2 patients with thyroid cancer (Figure 1B; supplemental Table 2). Given the distance from CART alongside post-CART HCT in 2 patients and 2 prior HCTs in the other patient and lack of detectable CART—this was not attributed to CART although diagnostic material was either not available or was insufficient to assess for the CAR transgene. There were no cases of T-cell malignancies. Five (20%) patients reported new neurological symptoms including difficulty focusing, peripheral neuropathy, and poor memory (n = 2) (supplemental Table 3) identified at routine clinical visits. One (4%) patient with Down syndrome had a history of severe infection due to parainfluenza type 2 and respiratory syncytial virus requiring prolonged hospitalization and intubation in follow-up, but no concerns at the most recent time point. Seventeen (68%) patients experienced concurrent illnesses or disorders. The most prevalent were nonmalignant tumors (6 patients, 24%), endocrinopathies (14 patients, 56%), and chronic graft-versus-host disease (11 patients, 44%). At the last follow-up, 17 (68%) patients were on medications (supplemental Table 1). The most common medications included vitamin D3/cholecalciferol (11 patients, 44%), trimethoprim/sulfamethoxazole (3 patients, 12%), levothyroxine (5 patients, 20%), metformin (3 patients, 12%), and testosterone supplements (3 patients, 12%). No patients developed a new autoimmune disorder.
With nearly a decade of using CART for lymphoid malignancies, there has been substantial progress that led to significant improvement in overall survival. With positive outcomes in the immediate post-CART period, it becomes even more imperative to identify and understand late effects associated with CART. Although our population represents a cohort of patients who received consolidative HCT after CART therapy, this analysis remains relevant as a substantial proportion of CAYA with B-ALL will receive consolidative HCT.10
Using the FDA’s long-term follow-up safety parameters after administration of human gene therapy products, we identified 3 patients (12%) with a new non–T-cell primary malignancy. Because these patients are heavily pretreated with a median of 3 lines of therapy, including immunotherapies and chemotherapies, their risk of secondary malignancy does not seem higher than the reported experience of CAYA who proceed to HCT without receiving prior CARTs.11 In 1 of our previous analyses on long-term outcomes of CD19, CD22, and CD19/22 CARTs as a bridge to HCT, CAYA showed improved overall survival, event-free survival, and a lower risk of post-HCT complications.6
Across this cohort, 5 patients (20%) reported a new neurologic disorder after CART therapy. Because of extensive prior therapy, attribution of cognitive impairment and neuropathy to CART vs chemotherapy alone and/or HCT is complicated, particularly because the latter are established risk factors for development of long-term neurotoxicity and neurocognitive difficulties.12,13 Although a previous study suggests an association between neurotoxicity immediately after CART infusion and cognitive difficulty,14 the data are scarce, and larger studies, particularly of patients not receiving a consolidative HCT, are needed to understand any correlation. Establishing standardized neurologic and neurocognitive evaluations in the late post-CART setting would facilitate identification of any longer-term findings.
Despite limitations inherent to the retrospective nature of the analysis and a relatively small sample size, our results support ongoing safety of CART in CAYA. Although consolidative HCT complicates the ability to attribute potential effects of CART alone, this analysis provides a comparison for those who remain HCT-naïve. Future efforts that incorporate real-time patient reported outcomes and other quality-of-life items such as fertility are needed to fully capture the patient experience in the long-term.
In conclusion, this retrospective review highlights the long-term safety profile of CART in CAYA for R/R B-ALL. Further studies are critical to elucidate and capture long-term effects of CART infusion in patients to ultimately identify patients at risk and provide early interventions using a standardized approach. In this regard, our group, in collaboration with other centers, are in active efforts to elucidate the late-effects of CART in CAYA through 2 future studies: Prospective evaluation for delayed effects of pediatric CART therapy (PROSPECT, NCT06579469) and CART long-term follow-up, quality of life, and adverse reactions (CONQUER study). By re-engaging families through interviews and standardized quality-of-life and neurocognitive assessments, future efforts in CART late-effects will provide additional information beyond the required follow-up.
Acknowledgments: The authors gratefully acknowledge the study participants and their families, the National Cancer Institute Leukemia, Lymphoma, Transplantation and Cell Therapy research team, referring medical and chimeric antigen receptor T-cell teams, the faculty and staff of the National Institutes of Health (NIH) Clinical Center, who provided their expertise in the management of the study participants, and the data managers and research nurses and patient-care coordinators involved with this work.
This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the Warren Grant Magnuson Clinical Center (ZIA BC 012187; N.N.S.).
Contribution: B.Y., C.N.H., V.M.G., and N.N.S. wrote the first version of the manuscript, performed primary data analysis and evaluated correlative studies; F.G. provided additional input with data analysis and manuscript writing during the revision process; L.L., M.E., J.B., C.M., and T.F. provided support for long-term follow-up time points; B.Y., V.M.G., H.S., and N.N.S. provided patient care; and all authors contributed to reviewing the final manuscript and agreed to be coauthors.
Conflict-of-interest disclosure: N.N.S. has participated in advisory boards for CARGO Therapeutics, Sobi, and VOR Bio and has research funding from CARGO, VOR Bio, and Lentigen. The remaining authors declare no competing financial interests.
The current affiliation for H.S. is Novartis, East Hanover, NJ.
Correspondence: Nirali N. Shah, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, NIH Building 10, Room 1W-5750, Bethesda, MD 20892; email: nirali.shah@nih.gov.
References
Author notes
Deidentified participant data that support the findings of this article will be shared upon approved written request to researchers who provide a methodologically sound proposal for the purposes of achieving specific aims outlined in that proposal. To gain access, data requesters will need to sign a data access agreement and to confirm that data will only be used for the agreed purpose for which access was granted. Requests can be directed to the corresponding author, Nirali N. Shah (nirali.shah@nih.gov).
The full-text version of this article contains a data supplement.