Key Points
REMPs have reduced access to CART-BCMA therapies for MM.
Clinical outcomes of both approved CART-BCMA products are comparable between REMP and non-REMP patients, despite disparities in access.
Visual Abstract
Chimeric antigen receptor T-cell (CART) therapy targeting B-cell maturation antigen (CART-BCMA) is a transformative treatment for multiple myeloma (MM). However, its high cost and the need for specialized centers may limit access for racial and ethnic minority populations (REMPs), who are more affected by MM. This retrospective cohort study evaluated access and outcomes according to REMP status for 140 patients treated with CART-BCMA for MM (June 2021 to February 2024) at the Abramson Cancer Center (ACC) of the University of Pennsylvania. These patients were compared to 3 control cohorts: 3298 patients with MM from the ACC catchment area; 288 patients with MM treated at the ACC (ACC MM cohort); and 115 patients with MM who would have been eligible for CART (ACC MM CART-eligible cohort). The proportion of REMPs declined across cohorts (catchment area, 33.9%; ACC MM cohort, 28.1%; ACC MM CART-eligible cohort, 26.1%; CART cohort, 17.1%; P < .05). REMP patients receiving CART-BCMA were more likely to live closer to the ACC and were less likely to be married compared to non-REMP patients (P < .05). Nevertheless, clinical outcomes were similar, with comparable rates of very good partial response (REMP, 75%; non-REMP, 72%; P = .47), progression-free survival (P = .30), and overall survival (P = .73), both in the whole cohort and in subgroup analyses based on the product used. Similarly, safety profiles showed no significant differences in cytokine release syndrome, neurotoxicity, and long-term hematologic toxicities. In conclusion, REMPs have reduced access to CART-BCMA but show similar clinical outcomes with both approved CART-BCMA products, highlighting the need to improve equity in access.
Introduction
Chimeric antigen receptor T-cell (CART) therapy targeting B-cell maturation antigen (CART-BCMA) has emerged as a pivotal treatment option for patients with relapsed/refractory multiple myeloma (MM) after first-line therapy.1 Idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) are the 2 CART-BCMA products currently approved for clinical use.2,3 However, access to this complex therapy remains a significant challenge that could hinder the widespread and equitable adoption of CART immunotherapy.4 Several demographic and clinical factors, such as age, disease severity, and other existing comorbidities, can limit the eligibility for CART immunotherapy.5 In addition, the high cost of this treatment, uneven distribution of CART-specialized centers, and socioeconomic inequalities are significant barriers that particularly affect racial and ethnic minority populations (REMPs).6-11 These barriers are especially concerning in MM, a disease that disproportionately affects REMPs.12-15 Notably, >25% of newly diagnosed patients with MM in the United States are Black/African American; yet this group is underrepresented in clinical trials, accounting for only 1.3% of participants.12,16 Access to novel MM treatment regimens, including triplet or quadruplet therapies and autologous transplantation, is also limited for REMPs.13,17
In the ide-cel and cilta-cel registration trials, REMPs represented 6% to 18% of the study populations,13 despite constituting 25% to 30% of the overall MM population.12,16 This underrepresentation raises concerns about the generalizability of clinical trial findings to real-world populations, particularly regarding the efficacy and safety of CART-BCMA in REMPs. To address this gap, the US Multiple Myeloma Immunotherapy Consortium recently analyzed the impact of race and ethnicity on clinical outcomes in patients treated with ide-cel. Their findings indicated a higher incidence of cytokine release syndrome (CRS) among non-Hispanic Black patients and lower overall response rates in Hispanic patients. However, no significant differences were observed in progression-free survival (PFS), overall survival (OS), or the incidence of severe toxicities across racial and ethnic groups, suggesting that these therapies should be offered equitably to all patients.18
However, real-world data on cilta-cel outcomes in REMPs remain scarce, and existing studies have largely failed to account for the demographic characteristics of surrounding catchment areas when assessing equitable access. Indeed, our prior work demonstrated that, when accounting for local demographics, REMPs had reduced access to CD19-directed CART therapies for lymphoma.6
Building on these findings, we evaluated whether REMP patients have equitable access to commercial CART-BCMA therapies and comparable clinical outcomes to non-REMP patients in a real-world setting.
Methods
Study design
This is a retrospective cohort study that evaluated access and clinical outcomes of all patients with MM who received CART-BCMA between 1 June 2021 and 28 February 2024 at the Abramson Cancer Center (ACC) of the University of Pennsylvania. The study was conducted in accordance with the Declaration of Helsinki. Retrospective clinical data evaluation and analysis were approved by the internal review board.
Patient population and data source
To determine whether access to CART-BCMA was restricted in REMP patients, we evaluated 3 cohorts of patients with MM.
ACC MM catchment area cohort
This cohort comprised patients diagnosed with MM between January 2016 and December 2020 within the ACC catchment area. The ACC catchment area includes the 12 counties of residence for >80% of the patients evaluated at the cancer center. These counties are Bucks, Chester, Delaware, Montgomery, and Philadelphia in the Commonwealth of Pennsylvania; Atlantic, Burlington, Camden, Gloucester, Mercer, and Ocean in the state of New Jersey; and New Castle in the state of Delaware. To identify patients with MM and their REMP status, we analyzed the latest New Jersey, Pennsylvania, and Delaware state cancer registry data (2016 to 2020).16,19-21 Data from New Castle County were inferred by combining census data (percentage of REMPs in the county) with MM incidence for the same territory extracted from the cancer registry report.19-22 Detailed race information, such as for Asian or Pacific Islander patients, was not available in 3 county registries (Atlantic, Gloucester, and Ocean counties of New Jersey). This cohort consisted of 3298 patients with known race (supplemental Table 1).
ACC MM cohort
This cohort included alive patients with MM who received proteasome inhibitor, immunomodulatory drug, and anti-CD38 monoclonal antibody treatment between 1 June 2021 and 28 February 2024. It included 288 patients with known races (299 with known ethnicity). MM diagnosis was searched in the electronic medical record using the following International Classification of Diseases, Tenth Revision, Clinical Modification definition: multiple myeloma and malignant plasma cell neoplasms (C90.0-C90.3). Patients were classified as having received a proteasome inhibitor, immunomodulatory drug, and monoclonal antibody if they had either an infusion visit or an outpatient order for antineoplastic medications of all 3 classes of drugs during the above-mentioned time frame. Demographic information about REMP status, sex, ZIP code, marital status, and county of residence were electronically extracted (supplemental Table 2).
ACC MM CART-eligible cohort
This cohort included living patients with MM who progressed to a proteasome inhibitor, immunomodulatory drug, and anti-CD38 monoclonal antibody treatment and met the eligibility criteria for CART treatment (at least 4 previous lines of treatment) during the time frame from 1 June 2021 to 28 February 2024. It included 115 patients. Cytogenetic risk, extramedullary disease, age, sex, and number of previous lines of therapy were manually extracted from the patient clinical charts (supplemental Tables 3-5).
ACC MM CART-BCMA cohort
This cohort comprised patients with MM treated with commercial CART-BCMA at the ACC during the same period as the ACC MM cohort and ACC MM CART-eligible cohort. It included 140 patients with known races (141 with known ethnicity). The data collection cutoff for clinical follow-up was 31 May 2025.
Definition of REMPs
Race and ethnicity were self-reported and collected into separate fields in the electronic medical record, in accordance with the Office of Management and Budget Statistical Policy Directive No. 15 standards for maintaining, collecting, and presenting federal data on race and ethnicity, as well as our institutional policies. Racial minority population (RMP) included American Indian or Alaska Native, Asian, Black/African American, or Native Hawaiian or other Pacific Islander. Ethnicity was defined as Hispanic/Latino or non-Hispanic.23 Ethnicity was analyzed separately from RMP status because some patients identify as both Hispanic/Latino and either RMP or non-RMP. REMP status was extracted from the electronic medical record based on the patient's self-reported race and ethnicity. Patients with unreported race or ethnicity were excluded. In the ACC MM catchment area cohort, all patients were included. In the ACC MM cohort, 11 (3.6%) and 0 patients (0%) were excluded from race and ethnicity analyses, respectively. In the ACC MM CART-eligible cohort, all patients were included in the analysis. In the CART-BCMA cohort, 2 of 142 (1.4%) were excluded for race and 1 (0.7%) for ethnicity.
Patient characteristics and outcomes
For the ACC MM CART-BCMA cohort, we extracted the following demographic and clinical information: age, sex, race, ethnicity, insurance type, employment, marital status, county and ZIP code of residence, previous treatments, cytogenetics, extramedullary disease, performance status according to Eastern Cooperative Oncology Group, CART-BCMA received, clinical response, survival outcomes, and toxicities. Travel time was determined by calculating the distance between the patient’s ZIP code of residence and the ZIP code of the ACC.10 Insurance type, employment, marital status, and travel time were used to test social determinants of health that could have limited access to CART-BCMA.
High-risk cytogenetic abnormalities included del(17p), t(4;14), t(14;20), t(14;16), and gain/amplification (1q).24 Triple-class–refractory disease was defined as a disease refractory to: an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody. Penta-refractory disease was defined as a disease refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab.3
Patients were evaluated for the best overall response according to the International Myeloma Working Group modified criteria. Patients’ responses were grouped as follows: very good partial response (≥VGPR) or better, partial response (PR), and stable disease/progressive disease. PFS was calculated as the time between the date of CART-BCMA infusion and the event date (progression or death) or was censored at the last follow-up visit or at the start of maintenance therapy. OS was calculated as the time between the date of CART-BCMA infusion and the event date (death) or was censored at the last follow-up visit. CRS and immune effector cell–associated neurotoxicity syndrome (ICANS) were classified by the American Society for Transplantation and Cellular Therapy or Common Terminology Criteria for Adverse Events (version 5.0) criteria. Cytopenia was graded according to both the Common Terminology Criteria for Adverse Events (version 5.0) criteria and the immune effector cell–associated hematotoxicity grading.25
Statistical analysis
Continuous variables were summarized as medians with interquartile ranges (IQRs) and compared using t tests or the Wilcoxon test, whereas categorical variables were presented as proportions and analyzed with Fisher exact test. Survival analyses were conducted using the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate analyses for progression were conducted using logistic regression. Statistical significance was set at P value <.05. Statistical analyses were conducted using R version 4.4.3, and all resulting graphs were generated with GraphPad Prism version 10.0 or R version 4.4.3.
Results
REMPs had reduced access to CART-BCMA
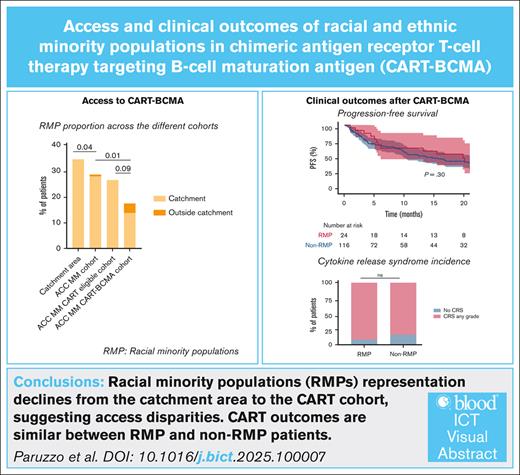
We first analyzed the proportions of RMP patients (excluding Hispanic/Latino patients) in the ACC catchment area. The ACC is located in the Philadelphia metropolitan area, which has an above-average RMP prevalence (48.2% [Philadelphia area] vs 21.7% [US average]).16,26 Publicly available cancer registry data were analyzed for the ACC catchment area. A total of 3298 people had a new diagnosis of MM: 1117 of 3298 (33.9%) were RMPs (1049 patients [31.8%] identified as Black/African American, and 67 patients [2.1%] identified as Asian); and 2181 of 3298 (66.1%) were non-RMP (Figure 1A).
RMP (excluding Hispanic/Latino patients) distribution at the ACC. (A) The distribution of racial groups among all patients with MM residing within the geographic region served by the ACC. (B) The distribution of racial groups among all patients with MM receiving treatment at the ACC. RMP percentage in the ACC MM cohort is restricted to patients originating from the ACC catchment area (top right) and outside the catchment area (bottom right). (C) The distribution of racial groups in patients with MM eligible for CART in the ACC MM cohort (left). RMP percentage in the ACC MM CART-eligible cohorts for patients from the catchment area (top right) and outside the catchment area (bottom right). (D) The distribution of racial groups in patients with MM receiving CART-BCMA (left). RMP percentage in the ACC MM CART-BCMA cohorts for patients from the catchment area (top right) and outside the catchment area (bottom right).
RMP (excluding Hispanic/Latino patients) distribution at the ACC. (A) The distribution of racial groups among all patients with MM residing within the geographic region served by the ACC. (B) The distribution of racial groups among all patients with MM receiving treatment at the ACC. RMP percentage in the ACC MM cohort is restricted to patients originating from the ACC catchment area (top right) and outside the catchment area (bottom right). (C) The distribution of racial groups in patients with MM eligible for CART in the ACC MM cohort (left). RMP percentage in the ACC MM CART-eligible cohorts for patients from the catchment area (top right) and outside the catchment area (bottom right). (D) The distribution of racial groups in patients with MM receiving CART-BCMA (left). RMP percentage in the ACC MM CART-BCMA cohorts for patients from the catchment area (top right) and outside the catchment area (bottom right).
We then investigated the number of patients who received a proteasome inhibitor, immunomodulatory drug, and monoclonal antibody treatment for MM at the ACC. The ACC MM cohort included 288 patients with MM. They included 75 Black/African Americans (26.1%), 5 Asians (1.7%), and 1 individual (0.3%) of another race. Combined RMP prevalence was 28.1% (81/288), whereas 207 patients (71.9%) were non-RMPs. To determine whether patients with MM treated at the ACC were from the ACC catchment area or outside, we analyzed patient residences. A total of 253 patients (87.8%) lived in the ACC catchment area, and 35 (12.2%) lived outside it (supplemental Figure 1A). Among the 253 patients living in the catchment area, 79 (31.2%) were RMPs, and 174 (68.8%) were non-RMPs; whereas among the 35 patients living outside it, 2 (5.7%) were RMPs, and 33 (94.3%) non-RMPs (Figure 1B).
To further validate our findings, we analyze a subgroup of patients of the ACC MM cohort who would have been clinically eligible for CART treatment (ACC MM CART-eligible cohort patient characteristics are provided in supplemental Table 4). The ACC MM CART-eligible cohort included 115 patients with MM. They included 28 Black/African American (24.3%) and 2 Asian (1.7%). Combined RMP prevalence was 26.1% (30/115), whereas 85 patients (73.9%) were non-RMPs. To determine whether MM CART-eligible patients treated at the ACC are from the ACC catchment area or outside it, we analyzed patient residences. A total of 101 patients (87.8%) lived in the ACC catchment area, and 14 (12.2%) lived outside it (supplemental Figure 1A-B). Among the 101 patients living in the catchment area, 30 (29.7%) were RMPs, and 71 (70.3%) non-RMPs, whereas for patients living outside it, all were non-RMPs (Figure 1C).
Finally, we analyzed the ACC MM CART-BCMA cohort, which included patients with MM treated with CART-BCMA (both ide-cel and cilta-cel) at the ACC between 2021 and 2024 (CART-BCMA's first ACC patient was June 2021). Of 140 patients with MM receiving CART-BCMA, 24 (17.1%) were RMPs. Among them, 22 (91.7%) were Black/African American, and 2 (8.3%) were Asian. A total of 84 patients (60%) lived in the ACC catchment area, and 56 (40.0%) lived outside of it (supplemental Figure 1A-B). In this cohort, the population in the catchment area had a higher percentage of RMP patients than the population living outside the catchment area (22.6% vs 8.1%; P = .04; Figure 1D).
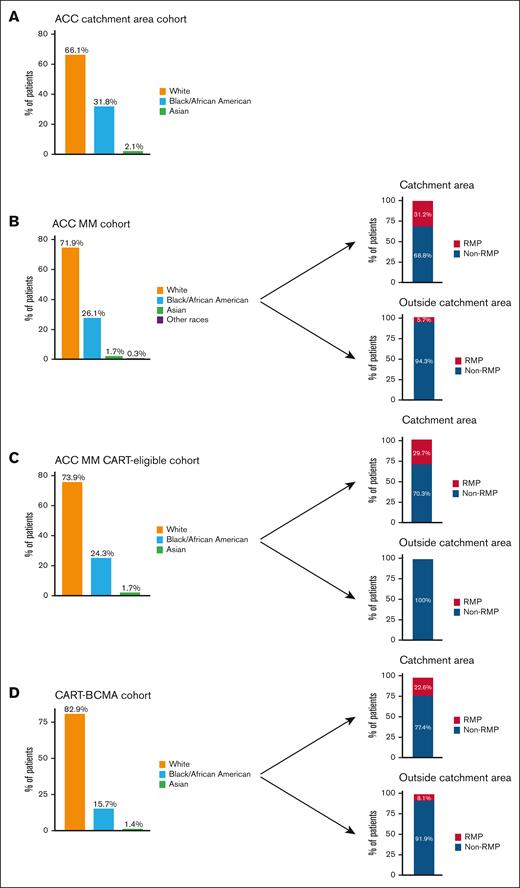
Comparing the RMP proportion in the 3 cohorts, we observed the following: a significant reduction in the proportion of patients with MM who were RMP from the ACC catchment area cohort to the ACC MM cohort (33.9% vs 28.1%; P = .04); no differences between the ACC MM and ACC MM CART-eligible cohorts (28.1% vs 26.1%; P = .71); and a further significant reduction from the ACC MM cohort (28.1%) and ACC CART-eligible cohort (26.1%) to the CART-BCMA cohort (17.1%; P = .01 for the ACC MM; P = .09 for the ACC CART-eligible cohort; Figure 2A). Furthermore, to eliminate confounding factors due to patient referrals from outside the catchment area, we analyzed only patients within the ACC catchment area. In this subcohort, the RMP proportion was numerically similar between the ACC catchment area, ACC MM, and ACC MM CART-eligible cohorts (33.9%, 31.2%, and 29.7, respectively; P > .05 [for all comparisons]) but appeared significantly reduced in the ACC MM CART-BCMA cohort (22.6%; P = .03 [compared to the catchment area]; Figure 2B).
RMPs have limited access to CART-BCMA. (A) Proportion of RMPs across the different cohorts based on whether they come from or outside the catchment area. (B) Proportion of RMPs across the different cohorts only within the catchment area. Comparisons between proportions were conducted using Fisher exact test.
RMPs have limited access to CART-BCMA. (A) Proportion of RMPs across the different cohorts based on whether they come from or outside the catchment area. (B) Proportion of RMPs across the different cohorts only within the catchment area. Comparisons between proportions were conducted using Fisher exact test.
Regarding ethnicity, the representation of patients who self-reported as Hispanic/Latino significantly declined from the ACC catchment area to the ACC MM cohorts (ACC catchment, 165/3298 [5%]; ACC MM, 5/288 [1.7%]; P = .01) but remain stable between the ACC MM (1.7%), ACC MM CART-eligible (1.7%), and ACC MM CART-BCMA cohorts (2.1%).
Social determinants of health affect RMPs' access to CART-BCMA
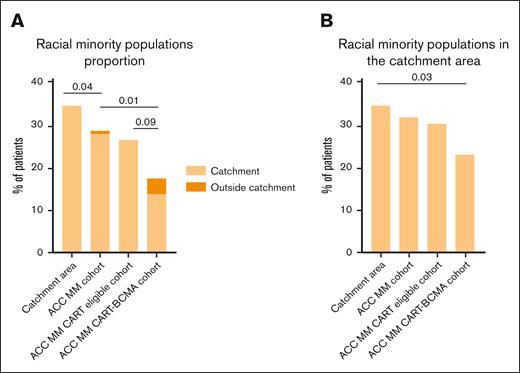
We examined potential social determinants of health that could limit RMP patients' access to CART-BCMA. Travel time from the residence ZIP code to the CART center was higher for non-RMP patients (ACC MM CART-BCMA cohort: median time [IQR], 30 [20-52] minutes for RMP vs 53 minutes [IQR, 33-83] for non-RMP [P < .01]; catchment area ACC MM CART-BCMA cohort: median time, 28 minutes [IQR, 18-42] for RMP vs 43 minutes [IQR, 31-79] for non-RMP [P = .002]; Figures 3A-B). In addition, more non-RMP patients treated with CART were married (RMP, 50.0%; non-RMP, 77.6%; P = .01; Figure 3C). To confirm the latter association, we investigated the proportion of married patients in the ACC MM cohort (RMP, 43.2%; non-RMP, 73.9%; P < .001) and in the ACC MM CART-eligible cohort (RMP, 43.3%; non-RMP, 72.9%; P = .006), showing that in this cohort also non-RMP patients were more commonly married. Finally, employment status (percentage of employed, 37.5% [RMP] vs 33.6% [non-RMP]) and insurance type (commercial, Medicare, Medicaid, and other insurance, 27.5%, 63.7%, 6.1%, and 2.5% for RMPs and 29.1%, 54.1%, 12.5%, and 4.1% for non-RMPs, respectively) were not statistically different by RMP status in the ACC MM CART-BCMA cohort.
Social determinants of health among CART-treated patients with myeloma by RMPs (excluding Hispanic/Latino patients). (A) Geographical distribution of RMPs in the catchment area. (B) Geographical distribution of non-RMPs in the catchment area. The color scale represents the proportion of patients in a specific county. The red star is the ACC location. (C) Proportion of married patients in the ACC MM CART-BCMA cohort. Comparisons between proportions were conducted using Fisher exact test.
Social determinants of health among CART-treated patients with myeloma by RMPs (excluding Hispanic/Latino patients). (A) Geographical distribution of RMPs in the catchment area. (B) Geographical distribution of non-RMPs in the catchment area. The color scale represents the proportion of patients in a specific county. The red star is the ACC location. (C) Proportion of married patients in the ACC MM CART-BCMA cohort. Comparisons between proportions were conducted using Fisher exact test.
RMPs have comparable clinical outcomes to non-RMPs
We then investigated whether clinical outcomes after BCMA-directed CART therapy were similar between RMPs (excluding Hispanic/Latino) and non-RMPs. The clinical characteristics of the CART-BCMA cohort were generally consistent with those of the CART-eligible cohort (supplemental Table 5), suggesting that disparities by RMP status are more likely due to differences in access to care rather than clinical ineligibility. Within the CART-BCMA cohort, baseline characteristics were well balanced between RMP and non-RMP patients, with the exception of age and number of previous lines of therapy. RMP patients were younger (median age [IQR], 62 [57-66] years vs 66 [61-70] years; P = .02) and had received fewer previous lines of therapy (median [IQR], 5 [5-7] vs 6 [5-8]; P = .04) than non-RMPs (Table 1; supplemental Table 6). The median follow-up for the CART-BCMA cohort was 25 months.
CART-BCMA cohort patient characteristics
| Characteristic . | RMP (n = 24) . | Non-RMP (n = 116) . | P value . |
|---|---|---|---|
| Patient age, median (IQR), y | 62 (57-66) | 66 (61-70) | .02 |
| Male | 11 (46) | 68 (59) | .36 |
| Product infused | |||
| cilta-cel | 13 (54) | 58 (50) | .81 |
| ide-cel | 11 (46) | 58 (50) | |
| Out-of-specification products | 1 (4.2) | 5 (4.3) | .99 |
| R-ISS at diagnosis >1 | 17/20 (85) | 58/77 (75) | .54 |
| Median previous lines of therapy (IQR) | 5 (5-7) | 6 (5-8) | .04 |
| Previous autologous SCT | 22 (92) | 100 (86) | .69 |
| Previous allogeneic SCT | 0 (0) | 5 (4.3) | .67 |
| Triple-class refractory∗ | 19 (79) | 96 (83) | .90 |
| Penta-refractory disease† | 5 (21) | 39 (34) | .31 |
| ECOG at infusion <2 | 22 (92) | 86 (74) | .11 |
| High-risk cytogenetics at diagnosis‡ | 7/17 (41) | 42/105 (40) | .99 |
| Extramedullary disease | 7 (29) | 25 (22) | .42 |
| Bridging therapy | 23 (96) | 109 (94) | .99 |
| Lymphodepletion | |||
| Flu/Cy | 23 (96) | 100 (87) | .53 |
| Cy-based | 1 (4) | 13 (11) | |
| Bendamustine | 0 | 1 (0.9) | |
| M-spike at CART, median (IQR), g/dL | 0.9 (0-1.7) | 0.25 (0-1.3) | .15 |
| Differential light chain at CART, median (IQR), mg/L | 245 (35-661) | 91 (20-425) | .20 |
| Characteristic . | RMP (n = 24) . | Non-RMP (n = 116) . | P value . |
|---|---|---|---|
| Patient age, median (IQR), y | 62 (57-66) | 66 (61-70) | .02 |
| Male | 11 (46) | 68 (59) | .36 |
| Product infused | |||
| cilta-cel | 13 (54) | 58 (50) | .81 |
| ide-cel | 11 (46) | 58 (50) | |
| Out-of-specification products | 1 (4.2) | 5 (4.3) | .99 |
| R-ISS at diagnosis >1 | 17/20 (85) | 58/77 (75) | .54 |
| Median previous lines of therapy (IQR) | 5 (5-7) | 6 (5-8) | .04 |
| Previous autologous SCT | 22 (92) | 100 (86) | .69 |
| Previous allogeneic SCT | 0 (0) | 5 (4.3) | .67 |
| Triple-class refractory∗ | 19 (79) | 96 (83) | .90 |
| Penta-refractory disease† | 5 (21) | 39 (34) | .31 |
| ECOG at infusion <2 | 22 (92) | 86 (74) | .11 |
| High-risk cytogenetics at diagnosis‡ | 7/17 (41) | 42/105 (40) | .99 |
| Extramedullary disease | 7 (29) | 25 (22) | .42 |
| Bridging therapy | 23 (96) | 109 (94) | .99 |
| Lymphodepletion | |||
| Flu/Cy | 23 (96) | 100 (87) | .53 |
| Cy-based | 1 (4) | 13 (11) | |
| Bendamustine | 0 | 1 (0.9) | |
| M-spike at CART, median (IQR), g/dL | 0.9 (0-1.7) | 0.25 (0-1.3) | .15 |
| Differential light chain at CART, median (IQR), mg/L | 245 (35-661) | 91 (20-425) | .20 |
Data are presented as n (%) unless otherwise specified. Bold values are statistically significant (P < .05).
Cy, cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; Flu, fludarabine; R-ISS, Revised International Staging System; SCT, stem cell transplantation.
Triple-class–refractory disease was defined as disease refractory to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody.
Penta-refractory disease was defined as a disease refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab.
High-risk cytogenetic abnormalities included del(17p), t(4;14), t(14;20), t(14;16), and gain/amplification of 1q.
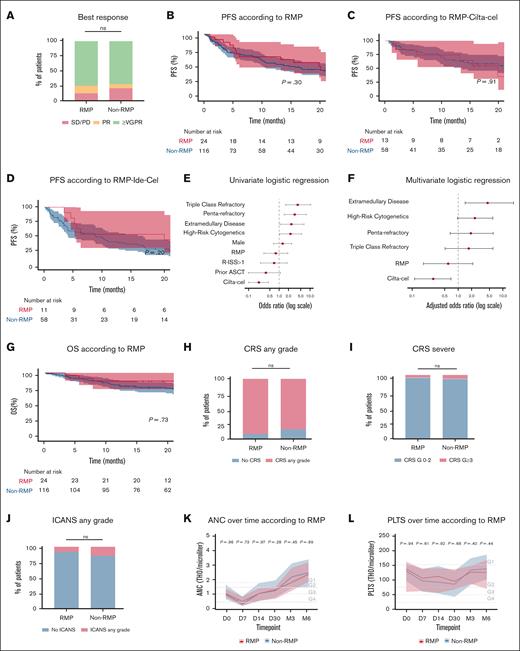
Best overall response rates were comparable between RMPs and non-RMPs in the overall population (VGPR or better, 75.0% vs 72.1%; PR, 12.5% vs 6.9%; stable disease/progressive disease, 12.5% vs 21.0%; P = .47; Figure 4A) and remained consistent in subgroup analyses stratified by CART product (ide-cel [VGPR or better, 73% (RMP) vs 61% (non-RMP)] and cilta-cel [VGPR or better, 77% (RMP) vs 83% (non-RMP)]; P > .05 [for all comparisons]; supplemental Figure 2A). PFS was also similar between groups across the full cohort (hazard ratio [HR], 0.74; 95% confidence interval, 0.41-1.31; P = .3) and product-specific subgroups (P > .05 [for all comparisons]; Figure 4B-D). In both univariate and multivariate analyses, RMP status was not associated with progression, suggesting that race and ethnicity do not affect the long-term efficacy of BCMA-directed CART therapies (Figure 4E-F). This finding was further supported by comparable OS between RMPs and non-RMPs, with median OS not reached in either group (HR, 0.87; 95% confidence interval, 0.39-1.94; P = .73; Figure 4G).
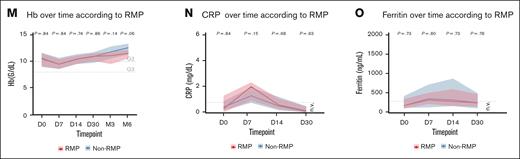
RMPs have comparable clinical outcomes to non-RMPs. (A) Best response according to RMP status. (B-D) PFS according to RMPs in the general population (B), cilta-cel (C), and ide-cel (D). (E) Univariate logistic regression for progression. (F) Multivariate logistic regression for progression of disease. (G) OS according to RMPs in the general population. (H) CRS of any grade according to RMP status. (I) CRS severity (grade ≥3) according to RMP status. (J) ICANS any grade according to RMP status. (K-M) ANC, PLTS, and Hb from day 0 to month 6 according to RMP status. (N-O) CRP and ferritin according to RMP status. Univariate and multivariate analyses for progression were performed using logistic regression. Comparisons between proportions were conducted using Fisher exact test. Longitudinal blood count values were analyzed using nonparametric tests. ANC, absolute neutrophil count; ASCT, autologous stem cell transplant; CRP, C-reactive protein; G1,grade 1; G2, grade 2; G3, grade 3; G4, grade 4; Hb, hemoglobin; n.v., normal value range; ns, not significant; PLTS, platelets; R-ISS, Revised International Staging System; SD/PD, stable disease/progressive disease.
RMPs have comparable clinical outcomes to non-RMPs. (A) Best response according to RMP status. (B-D) PFS according to RMPs in the general population (B), cilta-cel (C), and ide-cel (D). (E) Univariate logistic regression for progression. (F) Multivariate logistic regression for progression of disease. (G) OS according to RMPs in the general population. (H) CRS of any grade according to RMP status. (I) CRS severity (grade ≥3) according to RMP status. (J) ICANS any grade according to RMP status. (K-M) ANC, PLTS, and Hb from day 0 to month 6 according to RMP status. (N-O) CRP and ferritin according to RMP status. Univariate and multivariate analyses for progression were performed using logistic regression. Comparisons between proportions were conducted using Fisher exact test. Longitudinal blood count values were analyzed using nonparametric tests. ANC, absolute neutrophil count; ASCT, autologous stem cell transplant; CRP, C-reactive protein; G1,grade 1; G2, grade 2; G3, grade 3; G4, grade 4; Hb, hemoglobin; n.v., normal value range; ns, not significant; PLTS, platelets; R-ISS, Revised International Staging System; SD/PD, stable disease/progressive disease.
Similarly, no significant differences were observed in the incidence or severity of toxicities. Rates of CRS and ICANS were comparable between RMP and non-RMP patients (any-grade CRS, 87.5% vs 80.1%; CRS grade ≥3, 4.1% vs 6.0%; any-grade ICANS, 8.3% vs 14.6%; all P > .05; Figure 4H-J). These findings were consistent in subgroup analyses of both ide-cel (any-grade CRS, 91% vs 79%; CRS grade ≥3, 0% vs 7%; any-grade ICANS, 0% vs 12%; all P > .05; supplemental Figure 2B) and cilta-cel (any-grade CRS, 85% vs 81%; CRS grade ≥3, 8% vs 5%; any-grade ICANS, 15% vs 17%; all P > .05; supplemental Figure 2C). Additionally, hematologic toxicities and the incidence of grade ≥3 immune effector cell–associated hematotoxicity at 3 months (RMP, 5%; non-RMP, 4.1%; P = .47) were similar between groups (Figure 4K-M). Finally, inflammatory markers, specifically C-reactive protein and ferritin, also showed no statistically significant differences at baseline or during the first month after infusion (Figure 4N-O).
The limited number of Hispanic/Latino patients in the cohort precluded meaningful analyses of outcomes by specific ethnic subgroups.
Together, these findings indicate that BCMA-directed CART therapies yield comparable clinical responses and toxicity profiles in REMPs, highlighting the importance of promoting equitable access to CART-BCMA.
Discussion
This study evaluated the access and outcomes of REMP patients with MM treated with CART-BCMA in the metropolitan area of Philadelphia, which has a higher REMP proportion than the US average.16 To test whether REMP patients had equitable access to CART, we used 3 cohorts of patients as controls: the ACC catchment area, the ACC MM cohort, and the ACC MM CART-eligible cohort. Consistent with our previous findings on CD19-targeted CART access,6 we observed a significant decrease in REMP representation from the catchment area to the CART-BCMA cohort, with a less pronounced but still numerically significant reduction when considering only patients residing within the catchment area. These findings align with prior studies highlighting decreased CART access among REMPs across all the following indications: B-cell non-Hodgkin lymphoma, B-cell leukemia, and MM.6-10,27,28
Patient access to CART might be limited by geographic, insurance, and logistic challenges.8-10,27,29-32 Only slightly over 100 cancer centers in the United States are accredited to provide this treatment, and there are entire states without a CART center. Indeed, Snyder et al showed that CART patients have to travel for >1 hour to reach the closest CART center.31 The ability to travel for treatment is linked to the socioeconomic status of the patients, and socioeconomic status is correlated with race and ethnicity.33 According to American Community Survey estimates, the 12-month median income for non-Hispanic Black/African American households is lower than that of non-Hispanic White households ($51 286 vs $80 404).22 Socioeconomic status might also influence insurance coverage. Approximately two-thirds of US health plans restrict cell and gene therapy coverage policies, with commercial insurers providing greater reimbursement than Medicare.29 REMP patients are more likely to not be able to afford health insurance, have Medicaid, or have only Medicare insurance, which might cause these patients to not be offered, forego, or delay cancer treatment.10,34 However, in our study, no differences in insurance types were observed, whereas household income data were unavailable.
Another notable barrier is the need for CART recipients to stay within 60 minutes of the treatment center after infusion and the presence of a dedicated caregiver. In our study, RMP patients lived closer to the CART center and were less likely to be married, suggesting that referral from distant centers and the absence of a caregiver constitute barriers that may limit access to CART-BCMA.
However, this study was neither powered nor designed to capture the specific causes that led to reduced access to CART-BCMA in REMPs. Notably, its retrospective nature limited our ability to capture why patients who otherwise met medical eligibility criteria for CART-BCMA either declined the therapy or were offered alternative treatments. Indeed, barriers to accessing CART therapy can be multifaceted, encompassing structural, clinical, clinician-related, and patient-related factors. Structural barriers include the availability of CART treatment at locally accessible centers; clinical barriers involve stringent eligibility criteria for therapy; clinician-related barriers pertain to whether the treatment was offered at all and may include unconscious biases; and patient-related barriers involve factors such as knowledge, attitudes, beliefs, trust, financial burdens, insurance limitations, and broader social determinants of health, such as transportation challenges.35 To effectively address these disparities and improve access to novel cellular therapies for REMPs, a comprehensive, prospective evaluation of these socioeconomic factors is essential to pinpoint specific barriers and implement targeted interventions.
Nevertheless, despite the disparities in access, clinical outcomes after CART-BCMA, including response rates, toxicity, PFS, and OS, were comparable between RMP and non-RMP patients. This suggests that RMP patients derive comparable clinical benefits to non-RMP patients once access barriers to CART-BCMA therapy are overcome. These findings are consistent with a recent report from the US Multiple Myeloma Immunotherapy Consortium, which demonstrated that race or ethnicity does not influence the risk of severe toxicity or long-term outcomes in ide-cel. Notably, in our cohort, different from what was reported by Peres et al, the incidence of any-grade CRS was also similar. This may be attributed to the absence of baseline differences in inflammatory protein levels by RMP status, factors that have been previously linked to the development of CART-related toxicities.36,37 In our cohort, RMP patients are younger and have received fewer previous lines of therapy. Younger age can be linked with the peculiar MM biology in Black/African Americans, which leads to MM diagnosis 4 to 5 years earlier than the non-RMP population.38 However, when combined with fewer previous therapeutic lines, this may suggest that RMPs receiving CART-BCMA are selected for higher fitness and reduced comorbidities.28
A limitation of this analysis is the nature and clinical characterization of the control cohorts, which may be influenced by intrinsic confounding factors such as referral bias, comorbidities, unmeasured barriers to access (eg, financial insecurity), small sample size, limited availability of clinical data from the ACC catchment area, differences in registry lock dates, and the potential bias introduced by patients from the ACC catchment area receiving CART therapy at other institutions. Additionally, the findings related to the travel distance for the RMP population are limited by the fact that distance estimates based on ZIP code centroids may misrepresent actual participant residence locations, especially in large or rural areas, and do not account for road networks, traffic, or geographic barriers that influence real-world travel time. Finally, the study is limited to a single cancer center. Although ACC is a major institution, results may differ in other regions or health care systems. Future studies could consider expanding to multiple centers for broader representation. However, the strength of the study lies in the simultaneous evaluation of clinical outcomes and access to both approved CART-BCMA products, which gives a real-world snapshot of these therapies at our institution.
In conclusion, this study highlights that although the access of REMP patients with MM at our institution is substantially preserved, their access to CART therapy is reduced, suggesting that REMPs may have inequitable access to this therapeutic option.6 Nevertheless, when treated with CART-BCMA, REMP patients have comparable efficacy and toxicity to the non-REMP population. Prospective studies should be designed to investigate the causes of reduced access and ultimately develop strategies to improve equity in access to the most novel and effective therapies. Although a detailed discussion of effective strategies to address inequitable access falls outside the scope of this article, we think that achieving equitable access to cellular immunotherapies requires a collaborative approach. Patient associations, scientific societies, health care providers, government, and pharmaceutical companies must align efforts to address socioeconomic and logistical barriers.
Acknowledgments
The authors thank Berman and Maguire Funds for supporting Lymphoma Research at the University of Pennsylvania.
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authorship
Contribution: L.P. and M.R. had full access to all data in the study and took responsibility for the integrity of data and accuracy of data analysis; L.P., J.A.D., A.L.G., C.E.G., S.P.S.A., and M.R. designed research; L.P., K.E., G.G., M.H., A.D., E.F., M.P., A.C., F.S., A.I., V.B.D.S., and P.G. collected clinical data; L.P. performed statistical analysis and interpreted data; L.P., C.E.G., and M.R. wrote the manuscript; A.J.W., A.D.C., D.T.V., S. Kapur, L.M., S. Kaparis, R.B., J.A.D., D.L.P., A.L.G., E.A.S., C.E.G., S.P.S.A., and M.R. provided critical feedback on the manuscript for important intellectual content; M.R. obtained funding; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: M.R. holds patents related to chimeric antigen receptor T cells; has served as a consultant for NanoString, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), Bayer, Gerson Lehrman Group, Sana, Acera Bio, and AbClon; receives research funding from AbClon, Oxford NanoImaging, LUMICKS, NanoString, and Beckman Coulter; and is also the scientific founder and chair of the scientific advisory board of viTToria Biotherapeutics. G.G. served as a scientific consultant for viTToria Biotherapeutics. A.D.C. is a scientific advisor for Janssen and BMS; and has received research support and royalties from Novartis. D.T.V. has received research funding from Takeda and Active Biotech; and consulting fees from Takeda, Karyopharm Therapeutics, GSK, Genentech, and Sanofi. E.A.S. declares an affiliation with Oncopeptides; and consultancy fees from Amgen, BMS Celgene, GSK, Janssen, and AbbVie. D.L.P. declares research funding from Novartis and BMS; membership on the National Marrow Donor Program board of directors; on advisory committees for and receives honoraria from Novartis, Kite/Gilead, Angiocrine, Mirror Biologics, Sana Biotechnology, and Verismo; is a current equity holder at Genentech; and has patents and royalties from Novartis and Tmunity/Kite. A.L.G. declares research support from Janssen, Novartis, Tmunity, and CRISPR Therapeutics; consultancy fees/honoraria from Janssen, Novartis, BMS, GSK and Legend Biotech; and Data and Safety Monitoring Board membership for Janssen. J.A.D. reports receiving personal fees from AbbVie, Acadia, Janssen, Merck, Otsuka, and Takeda; and grants from Janssen, Merck, and Spark Therapeutics unrelated to the submitted work. C.E.G. is a principal investigator on a Genentech grant to the trustees of the University of Pennsylvania; serves on the advisory boards of Roche, Natera, and Guardant Health; and owns stock in Editas Medicine, CRISPR Therapeutics, Beam Therapeutics, and Intellia Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Marco Ruella, Lymphoma Program, Abramson Cancer Center, University of Pennsylvania, 3400 Civic Center Blvd, SPE 8-312, Philadelphia, PA 19104; email: mruella@upenn.edu.
References
Author notes
All requests for raw and analyzed data are promptly reviewed by the University of Pennsylvania and the corresponding author to determine whether they are subject to intellectual property or confidentiality obligations. Patient-related data not included in the article may be subject to patient confidentiality. The request should be directed to the corresponding author, Marco Ruella (mruella@upenn.edu). Any data that can be shared will be released via a material transfer agreement.
The full-text version of this article contains a data supplement.