Key Points
CD3+ T cells from patients taken after SEL-based regimen treatment showed upregulated activation and proliferation markers.
CD138+ cells from patients with acquired SEL resistance showed enhanced poly(ADP-ribose) polymerase 1 (PARP1) expression but no induction of immune checkpoints.
Visual Abstract
Many currently approved therapies for multiple myeloma (MM) use the endogenous immune system. The indirect effects of cytotoxic antimyeloma agents on the immune microenvironment can impact on the success of subsequent or combined immune-directed therapies. In this study, we investigated the effects of the exportin 1 inhibitor selinexor (SEL) on the bone marrow (BM) microenvironment and BM myeloma cells, using serial samples from patients treated with SEL-based regimens in the STOMP trial. Digital spatial profiling of selected CD138+ myeloma cells and CD3+ T cells was performed to assess expressions of 79 proteins involved in immuno-oncology. In selected CD138+ myeloma cells from pre-SEL samples, increased expression of proliferation markers (phosphorylated MEK1 [S217/S221] and phosphorylated AKT serine/threonine kinase 1 [AKT1] [S473]) was associated with poor progression-free survival and thus might be candidate predictive biomarkers of response. In posttreatment CD3+ T cells from samples at response, upregulation of epidermal growth factor receptor (EGFR), CD127, granzyme B, and phosphorylated mitogen-activated protein kinases 1 and 3 (ERK1/2) (T202/Y204) was observed, consistent with T-cell activation. No induction of the immune inhibitory markers lymphocyte activating 3 (LAG3), programmed cell death 1 (PD-1), or CTLA4 was seen in the CD3+ T cells, either at response or in samples taken after disease progression, indicating T-cell exhaustion is likely not a mechanism of acquired SEL resistance. The ability of SEL to exhibit antimyeloma activity without inducing T-cell immune checkpoints may have clinical implications, and future studies are needed to determine whether SEL complements chimeric antigen receptor T-cell therapy and bispecific antibodies for the treatment of MM. The STOMP trial was registered at www.ClinicalTrials.gov as #NCT02343042.
Introduction
Multiple myeloma (MM), a plasma cell malignancy, is the second most common blood cancer in the United States, accounting for an estimated 35 730 new diagnoses and 12 590 deaths in 2023 alone.1,2 The treatment response and survival of patients with MM have significantly improved over the last 2 to 3 decades, in large part due to the use of targeted or biological agents (eg, proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies), the incorporation of autologous hematopoietic stem cell transplant, and the recent advent of T-cell–targeted therapies such as chimeric antigen receptor (CAR) T-cell therapy3,4 and bispecific antibodies.5-7 Unfortunately, despite this progress, nearly all patients with MM will eventually relapse and become resistant to currently available agents.8-10 There are unmet needs for better understanding the mechanisms of action and drug resistance to current agents, to enable the combination or sequencing of available treatment modalities for sustained and prolonged benefits.
The MM bone marrow (BM) microenvironment (BMM) is complex and composed of cellular and noncellular components, including infiltrating lymphocytes, extracellular matrix proteins, cytokines/chemokines, stromal cells, osteoblasts and osteoclasts, cancer-associated fibroblasts, and immune suppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, among others.11,12 The interaction between the BMM and myeloma cells can facilitate MM cell survival by allowing escape from immune surveillance, driving clonal evolution through selective pressures, and influencing the development of drug resistance, especially to immune cell engagers/immunomodulatory agents. Of particular interest in the BMM is T-cell function and fitness. In MM, the BMM creates an immunosuppressive state that hinders T-cell activation and cytotoxicity. Many currently approved therapies for MM impact T-cell function directly or indirectly use T cells for anticancer effects and, as such, maintaining or augmenting T-cell fitness and competency are crucial for the successful treatment of MM.13,14 The increasing importance of T-cell–directed therapies in the clinical management of MM further elevates the importance of understanding factors that affect both T-cell fitness and the immune microenvironment, including the therapies being sequenced or combined with them. Effective agents are needed for use as bridging therapies for disease control while patients are waiting for CAR T-cell manufacturing, as combinatory partners to CAR T-cell therapy or bispecific antibody treatments to enhance efficacy, or as maintenance therapy after treatment for sustained response after a T-cell–dependent therapy is given.
Selinexor (SEL) is a first-in-class oral selective, exportin 1 (XPO1) inhibitor that is US Food and Drug Administration approved in MM and diffuse large B-cell lymphoma indications. XPO1 (also known as chromosomal maintenance 1) is a eukaryotic protein that mediates nuclear export of leucine-rich nuclear export signal–bearing proteins and their bound RNAs, which is often overexpressed in malignant cells, including MM.15 The XPO1 nuclear export pathway drives the functional and expression-based fate of many molecules, including tumor suppressor proteins (TSPs), the glucocorticoid receptor, eIF4E-bound oncoprotein messenger RNAs (eg, MYC, BCL2L1, MDM2, cyclins),16,17 as well as MAPK, histone deacetylase 5, among others.18 SEL reactivates multiple TSPs relevant to MM, reduces c-MYC levels, and reactivates glucocorticoid receptor signaling in combination with dexamethasone (Dex),19-21 resulting in selective killing of myeloma cells.22
In addition to activating TSPs and killing myeloma cells, nonclinical studies have shown SEL enhances host antitumor immunity,23,24 and exerts superior antitumor activity when given before or combined with CD19 CAR T cells25,26 or T-cell checkpoint inhibitors.27 Preclinical studies have suggested that XPO1 inhibitors have the potential to maintain T-cell fitness and to mitigate T-cell exhaustion. For instance, administration of SEL at an optimized dosing schedule in a B16 mouse model of melanoma increased the fitness of both endogenous and transplanted CD8+ T cells, confirmed by the reduced expression of exhaustive markers such as programmed cell death 1 (PD-1) and increased granzyme B secretion, and no impact on the levels of endogenous tumor-infiltrating lymphocytes.24
The STOMP trial is an ongoing multiarm, open-label, phase 1b/2 study evaluating SEL in combination with different backbone therapies (ClinicalTrials.gov identifier: NCT02343042), including pomalidomide + Dex (SPd; arm 1),28,29 bortezomib + Dex (SVd; arm 2),30 lenalidomide + Dex (SRd; arm 3),31 daratumumab + Dex (SDd; arm 5),32,33 and carfilzomib + Dex (SKd; arm 6),34,35 as well as additional arms. The median previous lines of therapy in patients enrolled in the STOMP trial was 3 (range, 1-11).36 The overall objective response rate was 63%, and the median progression-free survival (PFS) was 9.0 months, with median PFS for proteasome inhibitor (PI)-nonrefractory patients at 17.8 months and for PI-refractory patients at 6.1 months.36 STOMP trial results confirmed the effectiveness of SEL-based regimens in the treatment of relapsed/refractory MM (RRMM), but biomarkers predictive of SEL treatment response and the effects of SEL on the BMM have not been well characterized.
We therefore sought to use samples from patients enrolled in the STOMP trial at our institute to identify biomarkers predictive of treatment response to SEL-based regimens, and determine the impact of SEL with different combinations on the activation and fitness of T cells in the BMM. Our study has important implications on how a clinician can combine or sequence SEL-based regimens with T-cell–directed therapies, including CAR T-cell therapy or bispecific antibody treatment.
Methods
Patients
From January 2017 to May 2022, a total of 47 patients with RRMM were enrolled in the STOMP trial at our institute, among whom patients with paired, archived BM biopsy samples before and after SEL treatment were screened for participation in this study (supplemental Figure 1). The study was approved by the institutional review board at Duke University Medical Center (Duke institutional review board protocol identifier: pro00113077), and was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act guidelines of 1996.
DSP
NanoString GeoMx (NanoString/Burker) digital spatial profiling (DSP) was performed in the BioRepository & Precision Pathology Center at Duke University. BM biopsy samples were identified and collected from clinical archives. All BM biopsies had been performed during routine clinical workup, and were processed according to clinical laboratory protocols. BM core biopsies and clot sections were fixed in acetic acid zinc formalin, and core biopsies were decalcified using an HCl-EDTA solution. Core biopsies were preferentially used for DSP experiments, but clot sections were used if the core biopsy was inadequate (eg, inadequate sampling, excessive aspiration artifact, crush, etc). Tissue recuts were sectioned in the BioRepository & Precision Pathology Center at 5 μm thickness using molecular precautions, and placed onto Superfrost glass slides. Slide preparation and staining was performed per manufacturer’s instructions. Briefly, tissue sections were first stained for DNA (Syto13; Thermofisher), and with fluorescently labeled antibodies specific for human CD138 (clone MI15; Alexa Fluor 594 [AF-594]; BioLegend), CD3 (clone C3e/1308; AF-532; Novus), and CD45 (clone 2B11+PD7/26; AF-647; Novus) to identify myeloma, T-cell, and total leukocyte cell populations. Slides were subsequently probed with the NanoString GeoMx DSP Immuno-oncology multiplex antibody panel labeled with UV-photocleavable indexing oligonucleotides for quantitative protein expression of 79 targets and 3 control proteins with spatial resolution (supplemental Table 1). Specific regions of interest (ROIs) for proteomic profiling were annotated using fluorescence microscopy to select areas containing the cells of interest. Segmentation of each ROI was performed by hierarchically selecting first with CD138, then CD3, then CD45, and finally all other nucleated cells. ROI and segment sizes were adjusted to ensure adequate cell numbers within each lineage segment across all samples. UV light was focused on the sequentially selected segments of each ROI, and indexing oligos were released and aspirated for processing and quantification by nCounter analysis system (NanoString/Bruker) to provide direct, spatially resolved protein expression. Data from the nCounter were normalized per cell group using the geometric mean of histone H3 and ribosomal protein S6 housekeeper proteins in accordance with NanoString guidelines.37 Negative probes were used to quantify background noise and for signal correction, probes with counts <5 above the noise were imputed as 1. For each sample, at least 3 ROIs of each cell type were probed for protein expression and treated as separate data points for subsequent analyses.
Treatment response
Patient demographic and clinical data were collected by review of patients’ electronic medical records. The treatment response was characterized using the International Myeloma Working Group treatment response criteria, and classified as complete remission, very good partial response (PR; VGPR), PR, stable disease (SD), or progressive disease (PD).38,39 PFS was defined as the duration from the initiation of treatment to first progression or death, whichever was earlier.
Statistical analysis
Expression of detectable proteins was compared between treatment sample groups using Kruskal-Wallis and Dunn post hoc tests. False discovery rates were controlled using the Benjamini-Hochberg correction using the ggstats and rstatix packages in R (R version 4.4.1).40,41 Hazard ratios between patient-level median marker expression and PFS were calculated using the Cox proportional hazards model (survival and survminer R packages).42,43 When high and low expression groups are compared, the cut-off point was determined using a maximally selected log-rank statistic for PFS (maxstat R package). The associations between continuous variables were assessed using Pearson and Spearman rank correlation coefficients for parametric and nonparametric distributions, respectively. As this was an exploratory post hoc analysis, no statistical methods were used to predetermine sample size (all available samples were assessed), and investigators were not blinded.
Results
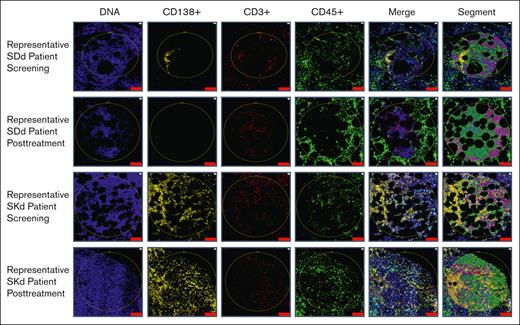
Twenty patients treated in the STOMP trial at our institution who had archived pretreatment and posttreatment BM biopsy samples were identified for this post hoc analysis. Three patients were excluded due to not having sufficient cellular elements in biopsy samples. A total of 34 BM samples from the remaining 17 patients were assessed for cell type–specific protein expression using digital spatial proteomic profiling, including 3 patients in arm 1 (SPd, [60 or 80 mg SEL by mouth once weekly, pomalidomide 3 or 4 mg by mouth once daily days 1-21, and 20 mg Dex by mouth twice weekly or 40 mg by mouth weekly]), 9 in arm 5 (SDd, 100 mg SEL by mouth weekly, 16 mg/kg daratumumab IV weekly and Dex 40 mg IV or by mouth weekly]), and 5 in arm 6 (SKd, [100 mg Sel by mouth weekly, 56 mg/m2 carfilzomib IV weekly days 1, 8 and 15, and Dex 40 mg IV or by mouth weekly]); supplemental Figure 1; supplemental Tables 2 and 3). Fifteen patients achieved a best overall response of PR or better (responders), and 2 had SD. Posttreatment SEL samples were collected from 13 patients who had not yet begun a subsequent line of therapy: 5 taken from patients anytime during an active response, 7 taken from patients at relapse after achieving PR or better, and 1 from patients at relapse who had only SD. Four posttreatment biopsy samples were excluded from the analyses due to being taken while the patient was already on the next line of therapy (supplemental Figure 1; supplemental Table 3). BM sections were labeled with CD138, CD3, and CD45 antibodies for lineage identification and segmentation (supplemental Figure 2). DSP allowed for spatially resolved characterization of BMM biopsied from patients with MM during different phases of treatment. Specific ROIs of each cell type were identified in all samples and selected for analysis via segmentation (Figure 1; supplemental Table 4). Samples taken during response showed low or absent staining for CD138+ cells (Figure 1). We first performed principal component analysis on all assessed ROIs across all samples, and found the ROIs showed strong clustering by cell type, rather than clustering by patient or time point (supplemental Figure 3).
Representative ROI selection and segmentation process. Using fluorescently labeled morphology probes, ROIs were selected to capture target cells while avoiding areas of histologic and staining artifacts. Segments containing specific cell types were hierarchically defined and are shown in the last column as: orange (CD138+ plasma cells), teal (CD3+ T cells), pink (CD45+ cells other than CD138+ plasma cells and CD3+ T cells), and green (all other cells not previously selected) zones. The top 2 rows show representative ROIs from pretreatment and posttreatment biopsy samples of a patient from arm 5 (SDd). The pretreatment sample shows plasma cell aggregates, whereas the posttreatment sample, taken during response, did not have any evidence of residual myeloma. The bottom 2 rows show representative ROIs from pretreatment and posttreatment biopsy samples of a patient from arm 6 (SKd). Unlike most patients who showed rare to no plasma cell involvement at the end of treatment, this patient showed persistently high disease burden with sheets of plasma cells at both time points. Scale bar, 100 μm.
Representative ROI selection and segmentation process. Using fluorescently labeled morphology probes, ROIs were selected to capture target cells while avoiding areas of histologic and staining artifacts. Segments containing specific cell types were hierarchically defined and are shown in the last column as: orange (CD138+ plasma cells), teal (CD3+ T cells), pink (CD45+ cells other than CD138+ plasma cells and CD3+ T cells), and green (all other cells not previously selected) zones. The top 2 rows show representative ROIs from pretreatment and posttreatment biopsy samples of a patient from arm 5 (SDd). The pretreatment sample shows plasma cell aggregates, whereas the posttreatment sample, taken during response, did not have any evidence of residual myeloma. The bottom 2 rows show representative ROIs from pretreatment and posttreatment biopsy samples of a patient from arm 6 (SKd). Unlike most patients who showed rare to no plasma cell involvement at the end of treatment, this patient showed persistently high disease burden with sheets of plasma cells at both time points. Scale bar, 100 μm.
Baseline levels of specific proteins in MM cell and T-cell subsets at screening were associated with response to subsequent SEL treatment
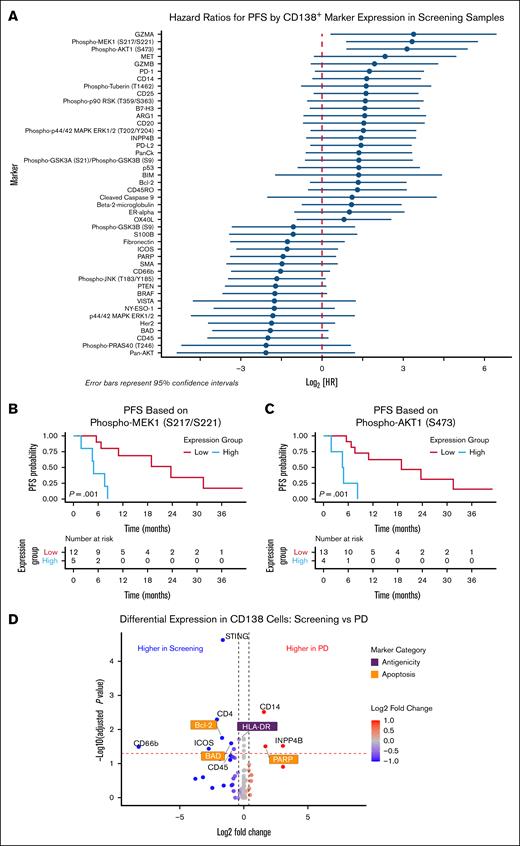
We sought to identify putative predictive markers of response to SEL treatment. Associations between protein levels at screening (before SEL treatment) in each cell type and response to SEL-based regimes were assessed. For CD138+ cells, increased baseline expression of proliferation markers (phosphorylated MEK1 [pMEK1] and pAKT1) and granzyme A was associated with poor PFS, whereas upregulation of the proapoptotic BAD protein tended toward improved PFS (not significant; Figure 2A-C). Furthermore, assessment of the CD138+ cells showed that, compared with screening samples, the PD samples had downregulation of antiapoptotic B-cell CLL/lymphoma 2 (BCL-2) and its heterodimeric, proapoptotic partner BCL2 associated agonist of cell death (BAD), as well as downregulation of immunogenic HLA-DR and upregulation of poly(ADP-ribose) polymerase 1 (PARP1) (Figure 2D), indicating these pathways might be related to mechanisms of acquired resistance/shorter duration of response. For CD3+ cells, higher baseline expression of CD45, pan-AKT serine/threonine kinases (AKT), and STING were associated with improved PFS, whereas higher levels of BAD, Ki-67, cleaved caspase 9, and phosphorylated-p90 ribosomal protein S6 kinase A1 (RSK) were associated with poor PFS (Figure 3).
Expression levels of pMEK1 and pAKT1 in CD138+ myeloma cells were associated with PFS in patients treated with SEL-based regimens. (A) Forest plot of PFS HRs for indicated marker expression in CD138+ cells from samples taken before SEL treatment at trial screening. HRs are not corrected for multiple testing. Kaplan-Meier curves showing PFS stratified by expression of pMEK1 S217/S221 (B) and pAKT1 S473 (C). (D) Volcano plot shows the differential expression of all assessed proteins for CD138+ MM cells between samples taken at screening and at time of PD after response. BRAF, B-Raf proto-oncogene, serine/threonine kinase; GZMB, granzyme B; HR, hazard ratio; ICOS, inducible T-cell costimulator; MET, MET proto-oncogene, receptor tyrosine kinase; PD-L2, programmed cell death 1 ligand 2; phospho-p90 RSK,phosphorylated p90 ribosomal protein S6 kinase A1; SMA, spinal muscular atrophy; PTEN, phosphatase and tensin homolog.
Expression levels of pMEK1 and pAKT1 in CD138+ myeloma cells were associated with PFS in patients treated with SEL-based regimens. (A) Forest plot of PFS HRs for indicated marker expression in CD138+ cells from samples taken before SEL treatment at trial screening. HRs are not corrected for multiple testing. Kaplan-Meier curves showing PFS stratified by expression of pMEK1 S217/S221 (B) and pAKT1 S473 (C). (D) Volcano plot shows the differential expression of all assessed proteins for CD138+ MM cells between samples taken at screening and at time of PD after response. BRAF, B-Raf proto-oncogene, serine/threonine kinase; GZMB, granzyme B; HR, hazard ratio; ICOS, inducible T-cell costimulator; MET, MET proto-oncogene, receptor tyrosine kinase; PD-L2, programmed cell death 1 ligand 2; phospho-p90 RSK,phosphorylated p90 ribosomal protein S6 kinase A1; SMA, spinal muscular atrophy; PTEN, phosphatase and tensin homolog.
Increased expressions of T-cell activation markers at time of screening were associated with improved PFS in patients treated with SEL-based regimens. (A) Forest plot of PFS HRs for the expression of indicated markers on CD3+ T cells from screening samples. HRs are not corrected for multiple testing. Kaplan-Meier curves show PFS stratified by CD3+ T-cell expression of CD45 (B), STING (C), MET (D), and phosphorylated p90 RSK T359/S363 (E). MET, MET proto-oncogene, receptor tyrosine kinase; phospho p90 RSK, phosphorylated p90 ribosomal protein S6 kinase A1; STING, stimulator of interferon response CGAMP interactor 1.
Increased expressions of T-cell activation markers at time of screening were associated with improved PFS in patients treated with SEL-based regimens. (A) Forest plot of PFS HRs for the expression of indicated markers on CD3+ T cells from screening samples. HRs are not corrected for multiple testing. Kaplan-Meier curves show PFS stratified by CD3+ T-cell expression of CD45 (B), STING (C), MET (D), and phosphorylated p90 RSK T359/S363 (E). MET, MET proto-oncogene, receptor tyrosine kinase; phospho p90 RSK, phosphorylated p90 ribosomal protein S6 kinase A1; STING, stimulator of interferon response CGAMP interactor 1.
Treatment with SEL-based regimens induced T-cell activation markers but not expression of inhibitory T-cell markers
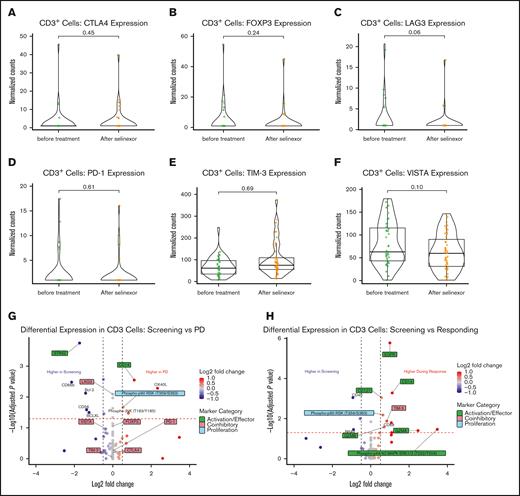
To examine the effects of SEL-based combination regimens on T-cell function markers, we analyzed CD3+ ROIs from BM biopsy samples, comparing screening samples with posttreatment samples. Importantly, no induction of immune inhibitory markers, cytotoxic T-lymphocyte associated protein 4 (CTLA4), forkhead box P3 (FOXP3), PD-1, lymphocyte activating 3 (LAG3), T-cell immunoglobulin mucin family member 3 (TIM-3, also called hepatitis A virus cellular receptor 2), or V-set immunoregulatory receptor (VISTA) were noted in the posttreatment group compared with paired screening/pretreatment samples (Figure 4A-F). To further delineate the impact of SEL in the context of T-cell functionality, posttreatment samples were subdivided, by their respective clinical context, into those taken from patients at a time of PD, and those taken during clinical response. No induction of coinhibitory molecules was evident from patients at the time of PD, indicating that progression was likely not mediated by impaired antitumor T-cell function (Figure 4G). In the CD3+ cells from patients who responded to SEL, several T-cell activation markers and proteins associated with T-cell proliferation, survival, and effector function were upregulated: epidermal growth factor receptor (EGFR), CD14, CD127, granzyme A and B, and phosphorylated mitogen-activated protein kinases 1 and 3 (pERK1/2, T202/Y204; Figure 4H). Conversely, increase of the coinhibitory molecule TIM-3 was also noted in samples from patients responding to therapy (Figure 4H).
Effects of SEL-based treatment on T-cell marker expression. Violin plots show the comparison between pre- and on-treatment CD3+ cells for normalized expression levels of CTLA4 (A), FOXP3 (B), PD-1 (C), LAG3 (D), TIM-3 (E), and VISTA (F). Volcano plots display differential expression for all assessed proteins in CD3+ T cells between pretreatment samples and on-treatment samples taken at the time of disease progression (G), or during a period of clinical response (H). FOXP3, forkhead box P3; LAG3, lymphocyte activating 3; PD-1, programmed cell death 1; VISTA, V-set immunoregulatory receptor.
Effects of SEL-based treatment on T-cell marker expression. Violin plots show the comparison between pre- and on-treatment CD3+ cells for normalized expression levels of CTLA4 (A), FOXP3 (B), PD-1 (C), LAG3 (D), TIM-3 (E), and VISTA (F). Volcano plots display differential expression for all assessed proteins in CD3+ T cells between pretreatment samples and on-treatment samples taken at the time of disease progression (G), or during a period of clinical response (H). FOXP3, forkhead box P3; LAG3, lymphocyte activating 3; PD-1, programmed cell death 1; VISTA, V-set immunoregulatory receptor.
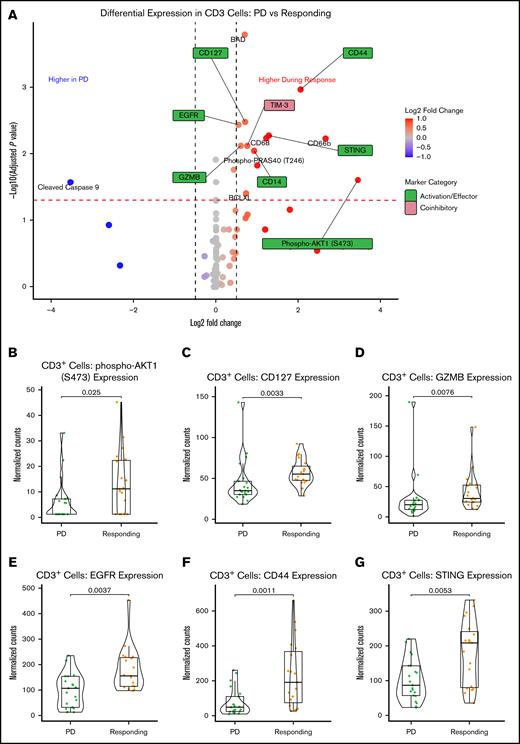
We next directly compared protein expression in CD3+ T cells from samples taken during response to those from PD. T-cell activation and effector markers, including EGFR, CD14, CD44, CD127, pAKT1 (S473), STING, and granzyme B, were significantly upregulated in patients who were responding compared with those with PD (Figure 5A-B). This was again accompanied by an upregulation of TIM-3.
Treatment with SEL-based regimens induced the expression of T-cell activation markers in responding compared with relapsed samples. (A) Volcano plot shows the differential expression of all assessed proteins in CD3+ T cells comparing post-SEL treatment samples taken at the time of PD vs a period of clinical response. Normalized expression levels of pAKT1 (B), CD127 (C), granzyme B (D), CD44 (E), EGFR (F), and STING (G) showed significant upregulation in samples from patients responding while on treatment compared with those at time of relapse (PD). GZMB, granzyme B.
Treatment with SEL-based regimens induced the expression of T-cell activation markers in responding compared with relapsed samples. (A) Volcano plot shows the differential expression of all assessed proteins in CD3+ T cells comparing post-SEL treatment samples taken at the time of PD vs a period of clinical response. Normalized expression levels of pAKT1 (B), CD127 (C), granzyme B (D), CD44 (E), EGFR (F), and STING (G) showed significant upregulation in samples from patients responding while on treatment compared with those at time of relapse (PD). GZMB, granzyme B.
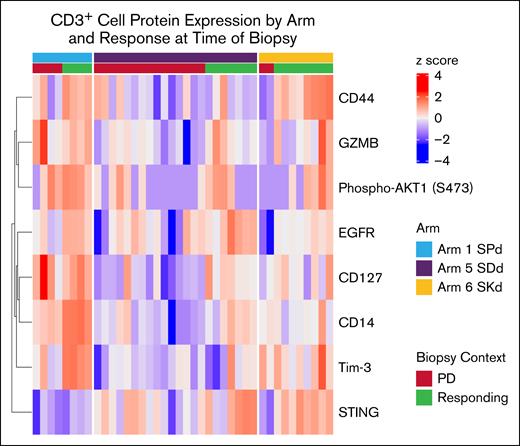
Differentially regulated T-cell proteins were additionally analyzed by study arm, and demonstrated upregulation across samples taken during response compared with samples taken during PD. Figure 6 presents a supervised clustering visualization of these results with patients ordered by arm, then response status at posttreatment biopsy. Interestingly, upregulation of STING was overall stronger in the responders from arm 5 (SDd) and arm 6 (SKd), whereas CD44 upregulation was specific to responders from arm 1 (SPd) and arm 6 (SKd). TIM-3 expression was the highest in responders from the SPd arm, and also coincided with the highest general upregulation of effector/activation proteins, and granzyme B was also highest in the SPd arm patients, especially the responder samples (Figure 6; supplemental Figure 4). These results are consistent with our hypothesis that the T-cell–activating effects of immunomodulatory drugs (IMiDs) and SEL may be additive in nature.
Differences in effects on CD3+ protein expression by SEL cotherapy and response status. Heat map shows z score–scaled normalized expressions of the indicated proteins in each posttreatment sample (columns). Annotation tracks indicate the SEL combination therapy arm and patient response status at the time of biopsy. GZMB, granzyme B.
Differences in effects on CD3+ protein expression by SEL cotherapy and response status. Heat map shows z score–scaled normalized expressions of the indicated proteins in each posttreatment sample (columns). Annotation tracks indicate the SEL combination therapy arm and patient response status at the time of biopsy. GZMB, granzyme B.
Discussion
The clinical outcomes of patients treated in the STOMP trial at our institute were similar to what have been previously reported,28,29,32-36 demonstrating the beneficial effects of SEL-based regimens in patients with RRMM. This study focused on the interaction between the BMM and the effects of SEL-based treatment. Preclinical data have suggested that SEL has the potential to maintain T-cell homeostasis and to reduce T-cell exhaustion.23,24 Herein we found that patients with RRMM who were responsive to SEL combination therapy demonstrated upregulation of T-cell activation markers. Furthermore, no induction of inhibitory immune checkpoints was observed, even in patients who developed PD while on SEL. The potential T-cell–activating effects of SEL are of increasing importance given the recent advancement of immune-directed therapies such as CAR T-cell therapy and bispecific antibody treatments. Current unmet clinical needs in the context of these new revolutionary therapies include preserving T-cell fitness to allow successful CAR T-cell manufacturing, bridging therapies that can control the disease and do not negatively impact the immune cells during manufacturing, subsequent treatment for patients who have relapsed, and possible maintenance therapies for those who are still in response. Investigating the best currently available MM therapies to address these needs is now of substantial interest. Studies have suggested that bortezomib is harmful to T cells, and a detriment when used in bridging before CAR T-cell therapy.44,45 Prior antibody drug conjugate therapy or T-cell engager therapy has a negative impact on subsequent rechallenging and is associated with inferior outcomes of CAR T-cell therapy (Cartitude-2 cohort C).46 Given its antimyeloma effects and potential T-cell–activating activity, SEL might be a suitable choice for bridging therapy for some patients, especially in combination with immunomodulatory agents. The use of agents such as SEL as maintenance after CAR T-cell therapy or bispecific antibody treatment is also an intriguing concept.
Clinical research to optimize the use of SEL as a bridging therapy is ongoing, and retrospective studies have already reported some relevant results.47 A novel 2-step administration of SEL is under investigation, with treatment used in the first phase of bridging therapy with discontinuation 1 week before lymphodepletion, then treatment resuming during a second phase as a single-drug maintenance therapy with a lower dose of 20 or 40 mg per week after the recovery of platelets.47 In a retrospective assessment of SEL-based trials, a report identified 7 patients with PD after CAR T-cell treatment who were then treated with SEL-based regimens. Six of 7 patients achieved a PR or better, including 1 stringent complete remission and 3 VGPRs, with durations of response ranging from 1.4 to 7.4 months.48 Likewise, a retrospective study by Gill et al showed that patients with RRMM treated with a SEL-based regimen prior to apheresis for B-cell maturation antigen (BCMA) CAR T-cell therapy had objective response rate of 100% after CAR T-cell therapy, with all patients achieving VGPR or better.49 Because of the synergistic/additive effects on immune enhancement, the combination of SEL with mezigdomide is currently being investigated in a trial for patients with RRMM (ClinicalTrials.gov identifier: NCT02343042). Likewise, we are currently launching a phase 2 trial combining SEL with bispecific antibody treatment for patients with RRMM.
The effect of SEL on T-cell activation likely involves multiple pathways. Several markers were found upregulated on CD3+ cells in samples from patients responding to SEL compared with those with PD: EGFR, CD127, p44/42 MAPK, granzyme B, and CD14. Both expression of EGFR and p44/42 MAPK (ERK1/2) on T cells have been shown to enhance their proliferation and survival, and likewise CD127 activation by interleukin-7 enhances T-cell proliferation and prevents apoptosis.50-52 Granzyme B is a direct marker of T-cell functional activation, as it is secreted by effector CD8+ T cells during immune response, and triggers caspase-mediated apoptosis of targets.53,54 The observation that CD14 was upregulated is interesting, and given that the DSP is not limited to truly single-cell analysis such as microfluidics-based methods, we acknowledge that CD14 might indicate infiltrating monocytes/macrophages. We also observed that higher baseline levels of CD45 in CD3+ T cells were associated with improved PFS in patients with RRMM treated with SEL-based regimens. CD45 is a crucial receptor-type protein tyrosine phosphatase that plays a significant role in T-cell signaling and function, by acting as a gatekeeper to control the threshold at which a T cell will respond to an antigen and initiate an immune response.55-57 One of our interesting findings is the association between TIM-3 upregulation and T-cell activation. TIM-3 is expressed on various cells, including lymphocytes, myeloid cells, and nonimmune cells, and is considered a negative regulator of T-cell activation.58 However, recent findings suggest that TIM-3 may also have costimulatory functions under certain conditions.59 For example, during acute infections, TIM-3 expression can enhance effector T-cell responses, indicating that its role may vary depending on the immune context. TIM-3 is also transiently expressed during effector function, consistent with the highest concurrent upregulation of activation/effector molecules seen in patients responding on the SPd arm. We further show that TIM-3 is significantly positively correlated with effector granzyme B expression (supplemental Figure 5). Importantly, we also demonstrate that TIM-3 expression during response was not correlated with time to disease progression in patients with upregulated TIM-3, with a median time to progression exceeding 1 year (supplemental Figure 6). Taken together, these data are consistent with SEL-based treatments activating T cells in responding patients.
Among the pre-SEL treatment CD138+ cells, we found that higher expressions of pMEK1, pAKT, and granzyme A were associated with poorer PFS. MEK1 is a kinase that is crucial for the phosphorylation of ERK. High levels of pERK along with AKT and NF-κB have been associated with Adriamycin and Dex resistance.60 Because MM cells typically do not express granzyme A, further investigation into its association with inferior PFS may be warranted as there are some reports showing expression of granzyme B (GZMB) by MM cells with immune-damaging effects in the BMM.61
The development of drug resistance to SEL is not fully understood, but several investigations have described potential mechanisms, including increased NF-κB activity,20 upregulation of the transcription factor early region 2 binding transcription factor 1 (E2F1),62 and heterozygous mutation of cysteine 528 in XPO1.63 Our assessment of CD138+ cells from samples of patients with PD after SEL treatment revealed relative upregulation of the DNA damage repair protein PARP1. High levels of PARP1 expression in myeloma cells are associated with poor prognosis, and may contribute to genomic instability and progression of the disease,64,65 indicating the results we observed may not be specific to SEL treatment. PARP1 inhibitors, such as olaparib and talazoparib, have been used in the treatment of solid tumors, and the combination of SEL and PARP1 inhibitors has shown promising benefits in preclinical models of ovarian, breast, and lung cancer.66,67 Future studies testing the combination of SEL with PARP1 inhibitors may identify a strategy to prolong the efficacy of SEL.
Limitations of this study include that this was a retrospective analysis, and no significance was allocated to these studies when calculating power for the clinical trial from which samples were obtained. The study is also limited by only using samples from patients who were available instead of mandating collections from all patients who were treated on the trial. The results described could benefit from validation with an independent sample set.
In summary, our study suggested that increased expression of proliferation markers (pMEK1 and pAKT1) in CD138+ myeloma cells correlated with shorter duration of response to SEL-based treatment. Importantly, this analysis provided evidence that SEL-based regimens do not negatively impact CD3+ immune cell function, and that response to SEL (in the context of the combinations used to treat patients in this report) may at least in part be due to immune cell antimyeloma activity. This study may provide some rationale for the clinical use of SEL-based regimens as bridging therapy (before) or maintenance therapy (after) to T-cell engaging therapies such as CAR T-cell therapy or bispecific antibodies treatment.
Acknowledgments
The authors thank the BioRepository & Precision Pathology Center (BRPC), a shared resource of the Duke University School of Medicine and Duke Cancer Institute, for providing access to the NanoString equipment and platform.
The BRPC receives support from the P30 Cancer Center Support Grant from the National Cancer Institute, National Institutes of Health (P30 CA014236).
Authorship
Contribution: Y.K. conceptualized the study, wrote the original draft, and reviewed and edited the manuscript; J.L.N. performed experiments, analyzed data, and reviewed and edited the manuscript; A.E. conceptualized the study, analyzed data, wrote the original draft, and reviewed and edited the manuscript; W.R.J. performed experiments and analyzed data; C.G., X.W., X.C., and Z.S. performed the experiments; C.J.W. conceptualized the study, analyzed data, wrote the original draft, and reviewed and edited the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: Y.K. received a research grant from Karyopharm Therapeutics. A.E. and C.J.W. are employed at Karyopharm Therapeutics. C.G. serves on the scientific advisory board for Karyopharm Therapeutics and received honorium as speaker for Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: Yubin Kang, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, DUMC 3961, 2400 Pratt St, Suite 5000, Durham, NC 27710; email: yubin.kang@duke.edu; and Christopher J. Walker, Karyopharm Therapeutics Inc, 85 Wells Ave, Suite 210, Newton, MA 02459; email: christopher.walker@karyopharm.com.
References
Author notes
Deidentified individual participant data are available from the corresponding author, Yubin Kang (yubin.kang@duke.edu), on request. Original NanoString nCounter data are available from the author, Jadee L. Neff (jade.neff@duke.edu), or the corresponding author, Christopher J. Walker (christopher.walker@karyopharm.com), on request.
The full-text version of this article contains a data supplement.