Abstract
The treatment options for chronic myelogenous leukemia (CML) continue to evolve rapidly. Imatinib mesylate (Gleevec, Glivec, formerly STI571) has continued to show remarkable clinical benefits and the updated results with this agent are reviewed. As relapses using single agent imatinib have occurred, particularly in advanced phase patients, the issue of whether combinations of other antileukemic agents with imatinib may yield improved results is addressed. In addition, data on new agents that have potential in the treatment of CML are reviewed. These agents are presented in the context of their molecular mechanism of action. The most recent data for stem cell transplantation, along with advances in nonmyeloablative transplants, are also reviewed.
In Section I, Drs. Stephen O’Brien and Brian Druker update the current status of clinical trials with imatinib and review ongoing investigations into mechanisms of resistance and combinations of imatinib with other agents. They also present their views on integration of imatinib with other therapies.
In Section II, Dr. Jorge Cortes describes the most recent data on novel therapies for CML, including farnesyl transferase inhibitors, arsenic trioxide, decitabine, and troxatyl, among others. These agents are discussed in the context of their molecular mechanism of action and rationale for use.
In Section III, Dr. Jerald Radich updates the results of stem cell transplants for CML, including emerging data on nonmyeloablative transplants. He also presents data on using microarrays to stratify patients into molecularly defined risk groups.
I. Current Status of Trials of Imatinib Mesylate (STI571, Gleevec) Alone and in Combination
Stephen G. O’Brien, MD, PhD*
University of Newcastle, Royal Victoria Infirmary, Newcastle Upon Tyne NE1 4LP, United Kingdom
Oregon Health Sciences University, Cancer Institute, 3181 SW Sam Jackson Park Rd., L592, Portland, OR 97201-3011
Dr. Druker is supported by grants from the National Cancer Institute, The Leukemia and Lymphoma Society, Burroughs Wellcome Fund, T.J. Martell Foundation, and the Doris Duke Charitable Foundation.
Dr. O’Brien has received research support from and has been a consultant to Novartis and Schering Plough.
Acknowledgments: The authors are most grateful to Jorge Cortes, Francois Guilhot, Andreas Hochhaus, and Francois Xavier Mahon for sharing their data.
Imatinib (Gleevec, Glivec, formerly STI571) is an inhibitor of the Bcr-Abl tyrosine kinase that is central to the pathogenesis of chronic myelogenous leukemia (CML). In preclinical studies, imatinib selectivity inhibited the proliferation of cells expressing Bcr-Abl in vitro and in vivo.1 The remarkable results of imatinib in clinical trials led to rapid Food and Drug Administration (FDA) approval of this drug for chronic phase CML patients who had failed interferon and for accelerated phase and blast crisis patients. These clinical trials will be summarized along with a randomized comparison of imatinib to interferon plus cytarabine in newly diagnosed chronic phase patients. Despite the impressive responses seen in chronic phase patients, numerous questions remain. How durable will the responses to imatinib be? Will cytogenetic or molecular responses be useful surrogate markers for survival in patients treated with imatinib? As good as imatinib seems, is it possible to improve upon these results? What are the mechanisms of resistance or relapse to imatinib and what treatments are available for relapsed patients? How should allogeneic transplantation and imatinib be integrated? These issues will be addressed in this and subsequent articles.
Summary
Formerly known as CGP57148 or STI571, imatinib mesylate (Gleevec, Glivec) is a 2-phenylamino-pyrimidine derivative (C30H35N7SO4, MW 589.7) that specifically inhibits the tyrosine kinase activity of Abl proteins, c-Abl and Bcr-Abl. Imatinib is known to also inhibit the tyrosine kinase activity of KIT, ARG (Abl-related gene), and the platelet-derived growth factor receptor (PDGF-R).
Since June 1998, when the first patient was treated, at least 15,000 patients with chronic myeloid leukemia (CML) worldwide have now been treated with imatinib. Imatinib was licensed by the Food and Drug Administration (FDA) for use in the United States in May 2001. The European Medicines Evaluation Agency (EMEA) granted a license covering the UK in November 2001. The licensed indications include chronic phase CML in patients who have failed interferon therapy (lack of response or intolerance), accelerated phase CML, and blast crisis of CML. A license for newly diagnosed patients is currently being sought.
In the treatment of chronic phase CML, imatinib produces much better hematological and cytogenetic responses than interferon-α (IFN-α) with most patients sustaining these responses. In newly diagnosed CML the major cytogenetic response on imatinib therapy is 83%, with 68% complete responses (compared with 20% and 7%, respectively, with interferon + Ara-C). However, there are as yet no data to conclusively demonstrate that imatinib improves long-term survival for CML patients when compared with an interferon-containing regimen.
In accelerated phase CML, response rates are inferior to those seen in chronic phase, but many of these responses are sustained.
In blast crisis of CML (both myeloid and lymphoid), response rates compare favorably with those achieved with chemotherapy, but only a minority of patients have durable responses. The use of imatinib may be justified in order to achieve transient control of disease with minimal side effects mostly, and it may be used as a bridge to transplant. Combinations of imatinib with chemotherapy are being explored.
Initial data indicate that higher doses of imatinib may produce better responses, but these data require confirmation in further, longer-term studies.
Phase I/II trials with imatinib in combination with interferon and Ara-C, which demonstrate that combination therapy can be safely delivered with excellent response rates, have been conducted. Other combination studies are in progress. Whether combination therapy, or higher dose imatinib monotherapy, is superior to imatinib 400 mg daily in chronic phase is the subject of a forthcoming Phase III study.
Background and Mechanism of Action
Since the first description of the Philadelphia (Ph) chromosome in 1960,2 biomedical scientists have developed an impressively detailed understanding of the biology of CML.3–,7 The subsequent development of a highly successful therapeutic agent based on this knowledge is confirmation of the value of basic biomedical research in both an academic and industry setting. Bcr-Abl encodes a protein, p210BCR-ABL, with dysregulated tyrosine kinase activity,8 which is necessary and sufficient for leukemogenesis.9–,12 Imatinib mesylate is a potent inhibitor of the tyrosine kinases activity of ABL,13,14 KIT,15,16 PDGF-R,13,14,17 and ARG.18 Imatinib competitively inhibits the interaction of adenosine triphosphate (ATP) with these proteins,19 thereby inhibiting their ability to phosphorylate and activate downstream target proteins.
Published Studies to Date with Imatinib
Phase I studies
A standard dose-escalation Phase I study of imatinib began in June 1998 at 3 centers in the United States. The study population consisted of CML patients in chronic phase, refractory or resistant to interferon-based therapy or intolerant of this drug.20 At later stages of the study, patients with CML in blast crisis and patients with Ph-chromosome positive acute lymphoblastic leukemia (ALL) were also enrolled.21 Imatinib was well tolerated with minimal side effects. Despite dose escalation from 25 mg to 1000 mg in 14 cohorts of patients, a maximally tolerated dose could not be defined. Imatinib was administered once daily and pharmacokinetics showed a half-life of 13-16 hours. Significant clinical benefits were observed at daily doses above 300 mg. In chronic phase patients who had failed therapy with interferon, 53 of 54 (98%) patients treated with ≥ 300 mg per day achieved a complete hematologic response, and 96% of these responses lasted beyond 1 year.20 In myeloid blast crisis patients, 21/38 (55%) patients treated at doses ≥ 300 mg per day responded, with 18% having responses lasting beyond 1 year.21
Phase II studies of imatinib
The success of the Phase I studies prompted Phase II studies. Single agent imatinib was tested further in interferon refractory and interferon intolerant patients as well as in accelerated phase patients and patients with CML in myeloid blast crisis and Ph-chromosome positive ALL. These studies accrued over 1000 patients, at 30 centers in 6 countries, in 6 to 9 months. Results from these studies, with 18 months of follow-up, have been published and are summarized in Table 1 .22– 25
Five hundred thirty-two chronic phase patients who were refractory to or intolerant of interferon-α were treated with an imatinib dose of 400 mg daily. Eligibility criteria in this study allowed inclusion of patients with up to 15% blasts and 15% basophils in the marrow or peripheral blood. Median duration of disease was 34 months and median duration of previous interferon therapy was 14 months. Ninety-five percent of patients achieved a complete hematologic response (CHR), with the median time to CHR being less than 1 month. Imatinib induced major cytogenetic responses (≤ 35% Ph-positive metaphases) in 60% of patients, with a complete cytogenetic response rate of 41%. With a median follow-up of 18 months, the estimated progression-free survival was 89%. Only 2% of patients discontinued therapy because of adverse events.22 A major cytogenetic response at 3 months was associated with a higher rate of progression-free survival. Baseline features that independently predicted a high rate of major cytogenetic responses were the absence of blasts in the peripheral blood, a hemoglobin >12 g/dL, < 5% blasts in the marrow, CML disease duration of less than 1 year, and a prior cytogenetic response to interferon.
Results of the Phase II study in accelerated phase patients were equally impressive.23 Accelerated phase was defined as 15-30% blasts or > 30% blasts plus promyelocytes in the peripheral blood or marrow, > 20% peripheral basophils, or a platelet count less than 100 × 109/L, unrelated to therapy. Two hundred thirty-five patients were enrolled in this study. Overall, 82% of patients showed some form of hematologic response, with 34% of patients achieving a CHR. Twenty-four percent of patients achieved a major cytogenetic response, with 17% complete responses. Estimated 12-month progression-free and overall survival rates were 59% and 74%, respectively. Again, these results were achieved without substantial toxicity.23
Results of the Phase II study treating 260 myeloid blast crisis patients with imatinib showed an overall response rate of 52%, with sustained hematologic responses lasting at least 4 weeks in 31% of patients. Eight percent of patients achieved a complete remission (CR = < 5% blasts) with peripheral blood recovery.24 Another 4% of patients cleared their marrows to less than 5% blasts but did not meet the criteria for CR because of persistent cytopenias. Finally, 18% of patients either returned to chronic phase or had partial responses. Major cytogenetic responses were seen in 16% of patients, with 7% having complete responses. Median survival was 6.9 months. Twenty percent of patients were still alive at 18 months with a suggestion of a plateau on the survival curve. These results compare favorably with historical controls treated with chemotherapy for myeloid blast crisis in which the median survival is approximately 3 months. In patients with Ph-positive ALL, 29/48 (60%) responded to single agent imatinib. However, the duration of response was relatively short, with a median estimated time to disease progression of only 2.2 months.25
Phase III study: comparison of imatinib with IFN-α in newly diagnosed patients
A Phase III randomized study, comparing imatinib at 400 mg per day with interferon plus cytarabine in newly diagnosed chronic phase CML patients, enrolled 1106 patients from June 2000 to January 2001. Five hundred fifty-three patients were randomized to each treatment. Baseline characteristics were well balanced for all features evaluated, including age, WBC, Sokal and Euro score, and time from diagnosis. With a median follow-up of 14 months, patients randomized to imatinib had statistically significant better results than patients treated with interferon plus cytarabine in all parameters measured (Table 2 ), including rates of CHR, major and complete cytogenetic responses, tolerance of therapy, and freedom from disease progression.26 Given the significant difference in the percentage of patients with disease progression to accelerated phase or blast crisis, 7% with interferon versus 1.5% of patients randomized to imatinib, it seems likely that this will translate into a survival benefit. A remaining question is the durability of the responses to imatinib.
Side Effects of Therapy
Imatinib has generally been well tolerated, with grade 3 or 4 nonhematologic toxicities being uncommon. The toxicities observed in newly diagnosed chronic phase patients are summarized in Table 3 . The common grade 1 or 2 toxicities include fluid retention, nausea, muscle cramps, skin rashes, fatigue, and diarrhea. Practical aspects of management of these symptoms have been reviewed elsewhere.27 Myelosuppression is more common in advanced phase patients than in the chronic phase patients, as shown in Table 4 , and imatinib induced prolonged aplasia in 1% of blast crisis patients.24 In contrast, patients with gastrointestinal stromal tumor treated with 400 or 600 mg per day of imatinib had rates of grade 3/4 neutropenia and thrombocytopenia of 5% and less than 1%, respectively, demonstrating the specificity of this side effect to leukemia patients.28 Given that 300 mg seems to be a threshold dose for optimal therapeutic responses, it is recommended that imatinib should rarely, if ever, be used at doses of less than 300 mg.20,27
Rationale for Trying to Improve upon Imatinib Monotherapy at 400 mg Daily
Despite the lack of long-term follow-up data, imatinib at 400 mg daily has emerged as the preferred therapy for newly diagnosed CML patients who do not undergo allogeneic stem cell transplant. However, only a minority (5-10%) of imatinib-treated patients achieve a molecular remission, that is, negative by RT-PCR for Bcr-Abl transcripts (B. Druker, unpublished data). Relapses in patients with more advanced disease have been common, and it is likely that resistance to imatinib will emerge in chronic phase patients. Combinations of imatinib with chemotherapy are an obvious choice for patients with advanced phase disease. If one accepts that chronic phase patients who do not achieve durable molecular remissions are at risk of relapse, then it is clear that there is much room for improvement. Options that have shown promise thus far include higher doses of imatinib and combinations of imatinib with IFN-α or cytarabine. The results of these treatments as compared with 400 mg/d of imatinib are summarized in Table 5 .
Are Higher Doses of Imatinib More Effective?
The dose of 400 mg per day of imatinib for the chronic phase studies was selected based on responses seen in the Phase I study and a lack of sufficient safety data for higher doses. As additional safety data from the Phase I study emerged, advanced phase patients were treated with 600 mg per day. Thus, in the Phase II study of accelerated phase patients, 77 patients were treated with 400 mg and 158 patients with 600 mg of imatinib.23 In the Phase II study of myeloid blast crisis patients, 36 patients were treated with 400 mg and 223 patients with 600 mg.24 Retrospective analysis of prognostic factors in the accelerated phase patients showed that the 400 and 600 mg cohorts were well matched. In both studies, there was a trend toward higher hematologic and major cytogenetic response rates in patients treated with 600 mg of imatinib (Table 6 ). In the accelerated phase study, patients treated with 600 mg had a statistically significant improvement in time to disease progression and survival.23
In a subsequent study of accelerated phase patients, patients were also treated with 600 mg daily of imatinib. As opposed to the accelerated protocol cited above, in this study, patients who otherwise had chronic phase features, but who had cytogenetic abnormalities besides a single Ph-chromosome, were defined as accelerated. Fifteen patients with this definition of accelerated phase were enrolled at Oregon Health & Science University and had a median disease duration of 45 months. With a median follow-up of 12 months, the major cytogenetic response rate was 80% (12/15), with a complete cytogenetic response of 67% (10/15). None of these patients has relapsed.29 Although a small study, the results for these relatively poor prognosis patients compare favorably with the 12-month results in newly diagnosed CML patients treated with 400 mg of imatinib.
The experience with doses higher than 600 mg per day is limited. In chronic phase patients in the Phase II study who failed to achieve a cytogenetic response following 1 year of imatinib therapy, dose escalation to 800 mg per day has been allowed. Limited experience suggests that up to one third of patients will achieve a major cytogenetic response with dose escalation (B. Druker, unpublished data). Investigators at M.D. Anderson have recently reported the results of newly diagnosed chronic phase CML patients treated daily with 400 mg versus 800 mg of imatinib.30 As seen in Table 5, there is a trend toward higher cytogenetic responses in the 800-mg dose cohort. Their experience with toxicity was similar to that reported in the Phase I study, which demonstrated that daily doses of 800 mg and higher were less well tolerated than 600 mg.20 In particular, there was a higher incidence of fluid retention, skin rashes, and muscle cramps. Daily dosing above 800 mg has generally not been used, as this was defined as the maximally tolerated dose in an EORTC study of patients with gastrointestinal stromal tumors.31 At 1000 mg per day, the dose-limiting toxicities were nausea, vomiting, edema, and skin rashes.
These data suggest that doses higher than 400 mg per day may yield improved responses. However, in the accelerated and blast crisis studies, the main impact of higher doses was on time to progression and survival. As the response rates for newly diagnosed chronic phase CML patients are already quite high, this finding implies that comparative studies of higher dose imatinib therapy in this patient population will require time to progression or survival as endpoints. Alternatively, rates of molecular remissions, if they correlate with improved survival, may be a useful early endpoint.
Combination Therapy
Imatinib in combination with interferon-α
As imatinib and interferon are the two most active forms of nontransplant therapy available for patients with CML, this combination is an obvious choice for testing. In vitro data demonstrate additive or synergistic antiproliferative effects of the combination of imatinib with interferon using Bcr-Abl-positive cell lines and in colony-forming assays using CML patient samples.32– 34 Based on these data, several Phase I/II pilot studies with this combination are in progress.
In a Phase I dose-escalation study using imatinib with regular interferon, 14 chronic phase CML patients were enrolled.35 In this study, imatinib was administered as a single agent at 400 mg daily for 2 weeks prior to adding a specified dose of interferon. In the dose-escalation, no patient was able to maintain a dose of 400 mg/d of imatinib plus 5 MU of interferon daily, primarily because of hematologic toxicity. Therefore, the current starting dose in a Phase II study for newly diagnosed patients is 400 mg/d of imatinib with 3 MU of interferon daily. Side effects and responses were similar to those in a larger study described below.
In a second Phase I/II study, imatinib has been combined with pegylated interferon. As of December 2001, 49 patients with chronic phase CML had been entered into PISCES (PEGIntron and Imatinib Combination Evaluation Study); median age 52.5 years, 32 newly diagnosed (< 6 months since diagnosis).36 Imatinib was administered for 2 weeks prior to adding a specified dose of interferon. After 6 months of treatment, the major cytogenetic response rate was 73.3% (n = 22) overall and 82.4% (n = 14) in the newly diagnosed patients (complete response; 36.7% and 41.2%, respectively) (Table 5). Of the 22 patients achieving a major cytogenetic response, 55% were taking either 200 mg/d of imatinib plus 0.25 μg/kg/wk PEGIntron (n = 8) or 200 mg/d imatinib plus 0.5 μg/kg/wk PEGIntron (n = 4). In the Phase I study, only 1/7 (14%) patients treated with 200 mg/d of imatinib had a cytogenetic response, suggesting that the combination of imatinib with interferon has improved activity over imatinib alone. Myelosuppression was common, with 30/49 patients experiencing grade 3/4 neutropenia within the first month on study while taking 400 mg/d imatinib plus 0.5 μg/kg/wk PEGIntron. Based on this result, the recommended dose for Phase II/III studies is 400 mg/d of imatinib with 0.25 μg/kg/wk PEGIntron. Grade 1/2 adverse events were common and included flu-like symptoms/fatigue (45%), increased liver transaminases (transient) (55%), musculoskeletal pain (39%), edema (33%), headache (33%), and nausea (33%). Grade 3/4 adverse events were rare, with febrile neutropenia and joint/muscle pain experienced by 6% of patients. These data indicate that the combination of imatinib plus interferon can be administered safely, but whether improved results can be obtained will require a large, prospective, randomized study.
Imatinib in combination with Ara-C
In vitro data have also demonstrated additive or synergistic antiproliferative effects for the combination of imatinib with Ara-C using Bcr-Abl-positive cell lines and in colony-forming assays using CML patient samples.32,37,38 Based on these data, Phase I/II studies of this combination are in progress.
A Phase I study of the combination of imatinib plus low-dose Ara-C was initiated in CML chronic phase patients who failed interferon.39 Twenty-two patients were enrolled in this study comprising 4 cohorts. Imatinib was given daily at 400 or 600 mg and Ara-C was administered on days 14 through 28, with cycles repeated every 28 days. In this study, the maximally tolerated dose was 400 mg of imatinib daily, with 20 mg/m2 of Ara-C given for 2 out of every 4 weeks. Myelosuppression at the highest dose level necessitated dose reductions in all patients such that no patient remained on treatment with 600 mg plus 20 mg/m2 of Ara-C. With a median duration of follow-up of 300 days, the complete hematologic response rate was 86%, and the major cytogenetic response rate was 32%. This compared favorably with the results reported in Phase I and II studies of imatinib alone and served as the impetus for a Phase II study of this combination in newly diagnosed patients.
A Phase II study was initiated in France to assess the tolerability and the efficacy of imatinib in combination with Ara-C. Imatinib was administered at a daily fixed dose of 400 mg in combination with Ara-C at 20 mg/m2 on days 14 through 28, with cycles repeated every 28 days. From June to August 2001, 30 previously untreated CML chronic phase patients within 6 months of diagnosis were recruited. Median age was 48 years (range, 22-81 years). After 6 months of treatment, 24/30 (80%) patients had achieved a major cytogenetic response; 17 (57%) achieved a complete cytogenetic response (Table 5; F. Guilhot, personal communication). The dose of Ara-C was reduced to a median of 77% to 91% of the expected dose (range, 48%-108%), and the median dose of imatinib was 400 mg/d, demonstrating that the dose of 400 mg was not affected by the addition of low dose Ara-C. Grade 3/4 nonhematologic toxicities in this study included nausea (1.1%), skin rashes (2.1%), abdominal pain (1.5%), and weight increase (1.6%). Common grade 1/2 adverse events included nausea (51%), muscle cramps (38.7%), periorbital edema (27.6%), vomiting (22%), diarrhea (20.3%), skin rashes (17%), weight increase (16.7%), myalgias (13.9%), arthralgias (12.6%), and dyspepsia (13.5%). Grade 3/4 neutropenia and thrombocytopenia occurred in 35% and 20% of patients but was rarely associated with infectious or bleeding complications.
Based on the Phase II data with higher doses of imatinib and the combinations of imatinib with interferon and cytarabine, a prospective, randomized Phase III trial has been designed (SPIRIT). This study will compare standard therapy for newly diagnosed chronic phase CML patients treated with 400 mg/d of imatinib to higher dose imatinib therapy versus imatinib at 400 mg/d with interferon versus 400 mg/d of imatinib with low-dose cytarabine. This study is expected to be activated by the end of 2002.
Other Combinations
In vitro combinations of imatinib with various antileukemic agents have been investigated and are summarized in Table 7 . These studies used various Bcr-Abl-positive cell lines, and some studies also reported on colony-forming assays using primary patient cells. A consistent observation from these studies is that increased synergy is observed at higher levels of Bcr/Abl kinase inhibition.38,40 The implication of this observation is that full doses of imatinib might be required to achieve optimal therapeutic responses in combination regimens.
In addition to the compounds listed in Table 7, many novel agents have shown activity against Bcr-Abl-expressing cell lines, and their activity in combination with imatinib is summarized in Table 8 . More details on the activity of these agents are contained in Section II and in a review by La Rosee et al.41
Resistance to Imatinib
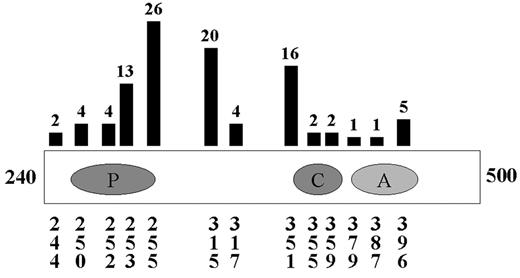
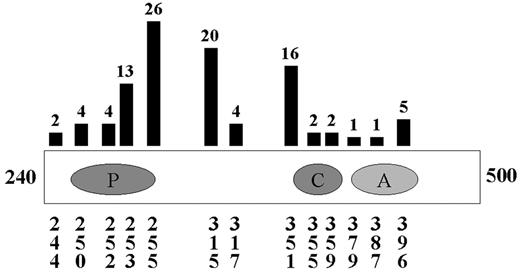
Although many of the above combinations will be tested in clinical trials based on single agent activity, more rational combinations would be based on known mechanisms of resistance. In the largest studies of resistance or relapse, several consistent themes emerge. In patients with primary resistance, that is, patients who do not respond to imatinib therapy, Bcr-Abl-independent mechanisms are most common.42 In contrast, the majority of patients who relapse on therapy with imatinib reactivate the Bcr-Abl kinase. In these studies, greater than 50% and perhaps as many as 90% of patients with hematologic relapse have Bcr-Abl point mutations in at least 13 different amino acids scattered throughout the Abl kinase domain (Figure 1 ).42–,47 Other patients have amplification of Bcr-Abl at the genomic or transcript level.42
It should be noted that the studies described above represent a minority of CML patients. Most of the patients described had advanced phase disease. The majority of patients diagnosed with CML will be in the chronic phase, most will obtain a complete cytogenetic response with imatinib, and very few have relapsed. However, only a minority attain a molecular remission. It is assumed that if these patients relapse, similar mechanisms will be operative. However, the more pressing question involves the mechanism of molecular resistance; that is, why do residual leukemia cells persist? One postulated mechanism is that quiescent stem cells may be insensitive to imatinib,48,49 suggesting that management of this group of patients will differ substantially from patients with relapses.
Regardless, in relapsed patients, the Bcr-Abl kinase remains a good target. Abl kinase inhibitors with specificity that differs from imatinib have already been synthesized.50–,52 Whether or not these compounds will inhibit some or all of the Bcr-Abl mutations that have been described needs to be determined. It is conceivable that several inhibitors, analogous to cocktails of protease inhibitors for human immunodeficiency virus (HIV), would be necessary and that the appropriate inhibitors would be chosen based on the molecular profile of mutations present in individual patients. Given that Bcr-Abl kinase activity has been reactivated in relapsed patients, it might also be useful to target downstream signaling pathways, such as Raf/MEK/ERK, PI-3 kinase, AKT, or ras. For example, two groups recently reported in vitro sensitivity of imatinib-resistant Bcr-Abl-positive cell lines to a farnesyl transferase inhibitor.53,54 Moreover, Hoover et al observed that this compound sensitized cells to imatinib, even imatinib-resistant cell lines.54 Alternatively, strategies to decrease Bcr-Abl protein levels by using agents such as geldanamycin, 17-AAG, or arsenic trioxide might be useful.53,55,56
What Are the Goals of Imatinib Therapy and How Should Treatment Failure Be Defined?
The simplistic aim of all leukemia treatment is to cure the patient without undue toxicity. Whether imatinib can achieve this or indeed improve survival is, at the time of this writing, still uncertain. Hemopoietic stem cell transplantation is the only proven curative treatment for CML, but the procedure is only available to one third of patients and carries significant risks.57–,59 Given the unprecedented rates of cytogenetic response that have been observed with imatinib, it seems reasonable to expect that improvements in survival will be seen, but only time will tell. The outcome of patients achieving major cytogenetic responses on interferon therapy is certainly impressive, but even in patients who achieve a complete cytogenetic response, long-term leukemia-free survival is not guaranteed, and responses can be lost. Data from the combined European groups60 suggest that other prognostic factors are important in predicting outcome in complete cytogenetic responders, and presumably this reflects the biological heterogeneity of the disease.
Another issue is whether molecular remissions are an appropriate goal. In patients undergoing allogeneic stem cell transplantation, the majority attain this landmark. In some patients who have low but stable levels of residual detectable Bcr-Abl transcripts, it is possible that allo-immunity prevents relapse. However, for patients treated with imatinib to minimal residual disease, there is no allo-immunity. Unless imatinib can maintain patients in this state, relapse is inevitable. Again, predictions as to the likelihood of this occurring are impossible, but it seems likely that if patients have residual leukemia, they are destined to relapse if imatinib is discontinued. Thus, if nontransplant therapy is to compete with allotransplant, then molecular remissions will have to be the goal of therapy, unless imatinib is able to prolong the chronic phase of the disease indefinitely. As the data from the initial studies mature, the answers to these questions should be forthcoming.
So, should we be making efforts to define “failure” of imatinib in order to tailor disease management for the individual? This decision is particularly relevant in young patients with viable allografting options who have chosen a trial period of imatinib to see what happens. Should such a patient be directed toward allografting if there has been no complete cytogenetic response after 6 or perhaps 12 months, or is it reasonable to continue regardless? It is evident that rates of allografting in CML have dropped off recently,61 but we must be very cautious about abandoning a known curative therapy that, in young patients with a low-risk sibling option, arguably has an acceptable toxicity, given the prospect of cure.
There is no consensus definition of “failure” or perhaps more precisely “inadequate treatment response” to imatinib therapy. As few newly diagnosed patients have relapsed, it is perhaps too soon to develop algorithms with parameters that consider failure of imatinib therapy. These parameters could include failure to achieve a cytogenetic response or molecular remission after a specified period of time. In examining relapses in later stage chronic phase patients who failed therapy with interferon, one group has suggested that failure to achieve a major cytogenetic response at 6 months is a poor prognostic factor.62 A second group has demonstrated that patients with a major cytogenetic response are less likely to relapse and that most patients who will achieve a major cytogenetic response will do so within the first 6 months of therapy.63 Specifically, if patients were greater than 65% Ph positive at 6 months, they had a 10% chance of obtaining a major cytogenetic response at 1 year (95% confidence interval 0-30%). However, some patients did respond at later times, presumably because of dose escalation of imatinib. In both studies, there were large numbers of patients with ongoing complete hematologic responses, but no cytogenetic response. As noted, these patients have a higher risk of relapse, but they are still a minority. Further, 84% of newly diagnosed patients can be expected to achieve a major cytogenetic response at 1 year. Thus, for the majority of patients, other parameters may need to be investigated.
Attempts have been made to suggest treatment algorithms for newly diagnosed patients,59 but these are often difficult to utilize in the clinic, as increasingly patients have strong views of their own that may be contrary to an ideal algorithm. In our practice, we have adopted somewhat of a compromise position, especially for younger patients who otherwise would have been considered good candidates for allo-transplants but who wish to avoid transplantation. Patients are offered imatinib as a single agent or are enrolled in a clinical trial of a combination of imatinib with either interferon or cytarabine. Regardless, if a patient might be a transplant candidate, we recommend HLA typing of the patient and appropriate family members. If no sibling matches are found, we recommend a preliminary donor registry search to determine if the patient will be easy or difficult to match. This is done so that patients know their options in advance, as the availability or lack of availability of a donor may factor into decision-making. If patients fail to achieve a complete hematologic response at 3 months or are > 65% Ph positive at 6 months, they are encouraged to reconsider transplantation. However, the majority of patients are expected to achieve a complete cytogenetic response. When a complete cytogenetic response is achieved, we switch to monitoring Bcr-Abl transcript levels with quantitative RT-PCR. As long as this value is stable or improving, we are comfortable continuing therapy. However, if this value rises significantly, patients are again encouraged to reconsider allo-transplantation. Our hope is that by monitoring for early relapses, we can transplant patients with minimal residual disease. Further, we hope that transplanting patients with minimal residual disease will result in a superior outcome compared with patients transplanted with active disease and that this will offset any delay in performing the transplantation. Unfortunately, there are no data to support this seemingly common sense approach, and close, careful monitoring of these patients will be necessary to determine whether this approach is advisable.
II. Novel Therapies for Chronic Myelogenous Leukemia
Jorge Cortes, MD*
M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Box 428, Houston, TX 77030
Dr. Cortes has received research support from HGS, Janssen, Schering Plough, ChemGenex, and Novartis.
The introduction of imatinib mesylate to the therapeutic armamentarium has changed the approach to CML. As we continue to gather more information from the ongoing trials with imatinib, it has become the building block in the treatment of CML. It is also evident that there is a need to continue to investigate new therapies for CML as we discover patients who become resistant to therapy with imatinib. Molecular remissions are achieved in only a minority of patients treated with imatinib alone. The focus now is to investigate therapeutic modalities with specific targets that could eventually lead to a sequential blockade of intracellular pathways and lead to increased rates of complete molecular remissions and, eventually, cure from CML. This section will cover novel approaches currently being investigated in CML; an outline of these options is presented in Table 9 .
Troxacitabine
Troxacitabine is a novel nucleoside analogue that differs from other dideoxycytidine analogs by being in the L-configuration. L-enantiomers of nucleosides were originally not considered antineoplastic agents, as they were thought to be poor substrates for activating enzymes. Some of these L-enantiomers (e.g., lamivudine) were found to be potent antiviral agents, and others were then investigated as antineoplastic agents. Troxacitabine has shown antineoplastic activity against several solid tumor cell lines and animal models.1 It has also shown significant activity in patients with relapsed acute myeloid leukemia (AML). A Phase I study was recently conducted in patients with acute leukemias and CML in blast phase (BP). Evidence of activity was found in AML and myelodysplastic syndrome (MDS), and the one patient with CML in BP treated on this study achieved a second chronic phase (CP).2 In a recent Phase II study, 17 patients with CML in myeloid BP were treated with the dose identified in the previous study.3 Six of these patients had failed prior therapy with imatinib, and 9 were in the second or later relapse. Six of 16 evaluable patients (37%) returned to a CP.3 One patient relapsed after 20 months, 1 died in second CP from sepsis, and 4 continue in second CP after a follow-up of 2 to 11 months. Further investigation of troxacitabine in CML BP is warranted.
Homoharringtone
Homoharringtone (HHT) is a plant alkaloid derived from an evergreen tree. The antileukemia activity of HHT in AML has been known for several years, and more recently there has been suggestion of activity also in acute promyelocytic leukemia (APL) and MDS. Significant activity has been reported in CML. Patients in late CP treated with this agent after having failed IFN-α-based therapy and with a median time from diagnosis of 3 years had a CHR rate of 67% and a cytogenetic response (CGR) rate of 33% (major cytogenetic response [MCR] in 15%) (Table 10 ).4 Similar results were obtained using a combination of HHT and low-dose cytarabine (CHR 72%, CGR 32%, MCR 15%) with a suggestion of improved survival with the latter combination compared to single-agent HHT.5 Visani et al investigated the in vitro effect of HHT in leukemia cells from patients with CML in different stages.6 A dose-dependent growth inhibition and apoptosis were documented in CP samples. Synergistic cytotoxicity was also documented with HHT in combination with IFN-α, Ara-C, and IFN-α + Ara-C.6 On the basis of this synergy, HHT has been combined with IFN-α, and with IFN-α and ara-C in treating patients with early CP CML. The combination of HHT and IFN-α in patients in early CP CML resulted in an MCR rate of 27% with a significantly shorter median time to achievement of an MCR compared with IFN-α alone (27% at 6 months with HHT + IFN-α vs 11% with IFN-α).7 In addition, the median daily dose of IFN-α in the first 12 months of therapy with the combination was 2.4 MU/m2 compared to 5 MU/m2 with IFN-α alone. The use of a lower dose resulted in a significant reduction in IFN-α-related toxicities.7 Available now are new formulations of HHT that allow for subcutaneous administration of this agent. HHT is also synergistic with imatinib in vitro.8 Therefore, HHT remains an attractive therapeutic option for patients with CML after imatinib failure or in combination with imatinib. Such clinical studies are being developed.
Decitabine
DNA methylation is an epigenetic phenomenon restricted in mammalian DNA to CpG islands. CpG methylation results in altered chromatin organization, leading to repression of gene transcription. Hypermethylation of regulatory genes involved in cell proliferation and differentiation is a common phenomenon in cancer.9 The availability of agents that inhibit DNA methyltransferase activity have made methylation an active area of translational research. Hypermethylation of the Pa promoter of ABL1 has been reported, with increasing levels of methylation in advanced stages of CML,10,11 and increased methylation may have adverse prognostic implications.12 Although the biologic consequences of this methylation are not well understood, hypomethylating agents have been investigated in CML. Both 5-azacytidine (AZA) and 5-aza-2′-deoxycytidine (decitabine, DAC) are potent DNA methylation inhibitors and have shown significant antileukemic activity in myeloid malignancies, including AML and MDS.9 In CML, these agents have been mostly used in the accelerated phase (AP) and BP. AZA in combination with etoposide or mitoxantrone has resulted in responses in 25% to 60% of patients treated in several studies. DAC has been used mostly as a single agent in CML. In 31 patients with myeloid BP, 2 achieved a CR and 6 had objective responses (hematologic improvement or back to chronic phase), for an overall response rate of 26%.13 In AP, 9 of 17 (53%) patients treated had an objective response.14 These studies used a dose of DAC of 50 to 100 mg/m2 over 6 hours every 12 hours for 5 days. At these high doses, DAC induced DNA synthesis arrest with a cytotoxic effect. At lower doses, cytotoxicity has been minimal, and gene-specific hypomethylation is observed. A study investigating the minimal effective dose of DAC was recently reported. Significant activity with minimal toxicity was reported in patients with AML, MDS, and CML, with the most effective dose being 15 mg/m2 daily for 10 days.15 This schedule is being investigated in patients with imatinib-resistant CML in CP, AP, and BP. The potential synergy of DAC with imatinib will also be studied.
Farnesyl Transferase Inhibitors
One of the best-recognized downstream events resulting from the tyrosine kinase activity of Bcr/Abl is the activation of Ras. Ras is synthesized as an inactive protein in the cytoplasm, which is activated through posttranslational changes consisting of a prenylation process that allows attachment to the cellular membrane. This process is mediated most prominently by farnesyl transferase (FTase), and alternatively through geranylgeranyl protein transferase.16 Thus, one approach to inhibit Ras activation is to inhibit FTase activity. Several FTase inhibitors (FTI) are under clinical development and have shown activity in some solid tumors and hematologic malignancies, including AML and MDS.17–,19 In CML, the activity of FTI has been demonstrated in a model using Bcl/Abl-transformed BaF3 cells.20 SCH66336, an FTI, induced a dose-dependent inhibition of colony formation and proliferation of transformed cells; colony formation of primary cells from patients with CML was also inhibited. Mice treated with SCH66336 after injection of Bcr/Abl-BaF3 cells survived for up to 12 months, whereas all mice treated with the vehicle only died within 1 month. Similar results have been reported in a Bcr/Abl-positive ALL mouse model.21 SCH66336 has been shown to inhibit the proliferation of imatinib-resistant Bcr/Abl positive cell lines and the colony formation of cells from imatinib-resistant CML patients.22 Furthermore, SCH66336 may sensitize imatinib-resistant cells to imatinib-induced apoptosis.22 The results of a pilot study using a different FTI, R115777, were recently reported. Twenty-two patients with CML (10 in CP, 6 in AP, and 6 in BP) were treated with R115777 at a dose of 600 mg PO BID. Ninety-one percent of the patients had received prior therapy with IFN-α, and 77% with imatinib. Seven patients (32%) (6 in CP and 1 in AP) had a CHR (n = 5) or partial hematologic response (n = 2); 4 of these patients also had a minor CGR.23 Responses were usually transient, with a median duration of 9.4 weeks. In a Phase I study with R115777, 35 patients with high-risk acute leukemias or CML in BP were treated with escalating doses of R115777. Ten of 24 evaluable patients (29%) responded (8 partial remission [PR], 2 CR). Among 3 patients treated for CML in BP, the 2 patients who had the Philadelphia chromosome responded. SCH66336 is currently being investigated in this setting. Combination studies based on the in vitro synergy of these agents with STI are also ongoing.
Arsenic Trioxide
Arsenicals were used in the treatment of CML many years ago. Newer and safer formulations of arsenicals have resurrected interest in these agents. Arsenic trioxide (As2O3) is an agent with significant clinical activity in APL. Although the mechanism of action is still being investigated, there is evidence suggesting downregulation of antiapoptotic proteins in APL cell lines after exposure to As2O3. A similar effect has been reported in many other hematologic malignancy models.24,25 Incubation of CML cell lines with concentrations of As2O3 that are clinically achievable resulted in dose-dependent growth inhibition and induction of apoptosis.26 A significant reduction in the expression of the Bcr-Abl protein was also observed but did not appear to interfere with the kinase activity of Bcr-Abl.26,27 As2O3 and imatinib mesylate are synergistic in growth inhibition and induction of apoptosis in CML cell lines.28,29 Because of this reported synergy, clinical trials investigating this combination are ongoing.
Proteasome Inhibition
The ubiquitin-proteasome pathway is the principal intracellular pathway responsible for the degradation of proteins.30 Multiple proteins with a variety of cellular functions are substrate for this pathway, including many that participate in cell cycle regulation and tumor growth. Thus, proteasome inhibitors are being investigated as a new approach for anticancer therapy. Although the specific mechanism through which these agents exert their antineoplastic activity is unclear, inactivation of NF-κB appears to play a prominent role. NF-κB is inhibited in the cytoplasm through binding to IκB, a substrate for proteasomes.31 In CML, Bcr-Abl activates NF-κB-dependent transcription, and NF-κB may be required for Bcr-Abl-mediated transformation.32,33 Proteasome inhibition with N-carbobenzoxy-L-leucyl-L-leucyl-norvalinal (LLnV) decreased the expression of the Bcr/Abl protein in K562 cells with no change in the expression of p145Abl.34 The molecular mechanisms leading to this decreased expression of Bcr/Abl protein are unclear, but the reduction led to caspase activation and apoptosis. PS-341 is a potent and selective inhibitor of proteasomes that has shown activity against a broad range of human tumor cells and is currently being investigated in various types of malignancies. Significant clinical activity has been demonstrated in multiple myeloma. In vitro, PS-341 induced significant growth inhibition and apoptosis of several Bcr/Abl positive cell lines, as well as both imatinib-sensitive and -resistant cell lines.35 Clinical studies in imatinib-resistant patients are ongoing.
Antiangiogenic Agents
There is growing evidence of the significance of angiogenesis in leukemia. Elevations of plasma vascular endothelial growth factor (VEGF) levels have been reported in patients with CML compared to controls (76.3 vs 26.7 pg/mL) and were the highest among all leukemias tested (including chronic lymphocytic leukemia [CLL], MDS, AML, ALL, and chronic myelomonocytic leukemia [CMML]). Plasma levels of bFGF, HGF, and TNFα were also elevated.36 There is also a significant increase in bone marrow vascularity as determined by the number of blood vessels and area of vascularity. Patients with CML had a median of 21.4 blood vessels (11.2 for controls, P = 0.003), and the relative vascular area was 6.2% (compared to 2.8%, P = 0.02). These values were the highest among all leukemias.36 VEGF has also been shown to suppress the function of dendritic cells,37 which have been shown to stimulate autologous antileukemia T-cell response, particularly in CML. Antibodies to VEGF may enhance the function of dendritic cells.38 Recently, increased cellular expression of VEGF has been associated with shorter survival in a multivariate analysis in 148 CML patients in CP.39 Therefore, suppression of VEGF might prove beneficial in CML, possibly through enhancing a specific anti-CML immune reaction. Specific anti-VEGF monoclonal antibodies (i.e., bevacizumab) are currently in clinical trials in patients with CML.
Peptide Vaccines
The development of a specific immune therapy has been of significant interest for some time. Evidence of the antileukemia effect from the graft-versus-leukemia effect has triggered investigations into approaches to develop specific antileukemic immunity in a more specific and less toxic way. One such approach currently in clinical trials is the use of leukemia-specific peptides to stimulate a T-cell-mediated antitumor effect. The chimeric p210bcr-abl protein generated by the fusion of the Abl and Bcr genes is a candidate peptide because it is tumor specific and it contains a sequence of amino acids not expressed in normal cells. Because T cells do not recognize intact proteins but rather peptide fragments of 8 to 12 amino acids in length presented through the major histocompatibility complex (MHC), small peptides derived from the fusion region of p210bcr-abl have been investigated. The immunogenicity of such peptides has been documented in animal models, where immunization of mice can elicit a peptide-specific CD4+ T-cell response.40 These T cells recognized and proliferated in response to the intact p210bcr-abl protein but not other proteins with a different junction sequence (e.g., p185bcr-abl) or unrelated proteins. Several peptides have been identified that can bind to class I and II HLA molecules and elicit HLA-restricted cytotoxicity in vitro.41 Using a mixture of these peptides, Pinilla-Ibarz et al vaccinated patients with Ph-positive CML in CP in PR or CR after therapy with IFN-α or hydroxyurea.42 Patients received 5 vaccinations over 10 weeks with 1 of 4 different doses. While none of the 12 patients treated had delayed-type hypersensitivity (DTH) before therapy, 2 developed significant reactivity after vaccination. Similarly, 3 patients developed peptide-specific T-cell proliferation not observed before therapy. All responses occurred at 1 of the 2 highest doses tested. No significant cytotoxic or antibody response was obtained in any patient. No significant toxicity was observed. One patient had a transient molecular response and one a transient partial cytogenetic response.42 In a Phase II trial using this approach, all 12 evaluable patients developed a DTH and CD4 proliferative response after vaccination and 6 had IFN-γ release by CD4 ELISPOT.43 Three patients (all receiving IFN-α) had a decrease in the percentage of Ph-positive metaphases, and 1 patient vaccinated for recurrence after bone marrow transplant (BMT) became transiently PCR negative.43
Another peptide being investigated is PR1. Proteinase 3 is a serine protease that is induced during differentiation, stored in azurophilic granules, and overexpressed in myeloid leukemias. PR1 is a 9-amino acid peptide derived from proteinase 3 with a binding motif for HLA-A2.1.44 PR1 was used to generate primary cytotoxic T lymphocytes (CTL) in vitro, and these CTL demonstrated significant lytic activity against fresh leukemia cells from patients with HLA-A2.1+ CML or AML.44 CTL generated through stimulation with PR1 inhibited colony-forming units from HLA-A2.1+ CML patients but not HLA-A2.1+ bone marrows or HLA-A2.1 CML, demonstrating the specificity of these lymphocytes.45 PR1-specific CTL have been identified in 11 of 12 patients who responded to IFN-α and in 6 of 8 who responded to allogeneic BMT but were undetectable in untreated patients, those treated with chemotherapy, or those who failed to respond to IFN-α.46 In a Phase I study, patients have been receiving escalating doses of PR1 together with granulocyte-macrophage colony-stimulating factor (GM-CSF). Preliminary data showed the development of a significant increase in PR1-specific CTL that correlated with a molecular response in 1 patient with APL, but no response was observed in 1 patient with CML.47 This vaccine is currently being investigated in CML and other myeloid leukemias.
New Kinase Inhibitors
The major clinical success of imatinib in CML has triggered increased enthusiasm for the investigation of other agents that could inhibit tyrosine kinases. An alternative approach to tyrosine kinase inhibition is the use of molecules that prevent the binding of peptide substrates rather than ATP binding. A family of such agents, called tyrphostins, has been found to inhibit the Bcr/Abl tyrosine kinase. A member of this family, AG957, can inhibit Bcr/Abl autophosphorylation but is not specific for Bcr/Abl and is not as active as imatinib.48,49 Adaphostin is the adamantyl ester of AG957 with greater in vitro potency than AG957.48 Imatinib-resistant cell lines are sensitive to adaphostin, and a combination of these 2 agents may be synergistic.50 Inhibitors of other kinases are also being investigated. AG490 is a potent inhibitor of JAK2, another tyrosine kinase, and is synergistic with STI571. Src kinase inhibitors are also being investigated. Although these molecules are earlier in their development, they may prove valuable in the near future either in combination with imatinib or alone for patients who have developed resistance to imatinib.
IFN-α
The efficacy of IFN-α in CML is unquestionable. Patients treated in early chronic phase CML with IFN-α achieved MCR rates of 20% to 40% and complete cytogenetic response (CCR) rates of 5%-30%. Combining IFN-α with cytarabine may increase the rate of CCR to 25%-35%. The introduction of imatinib has changed the therapeutic algorithm of CML. Thus, the use of IFN-α as first-line therapy in CML is likely to disappear soon. However, it is still a valuable option and in coming years there will be an increasing number of patients whose CML may have become resistant to imatinib and who would have not been exposed to IFN-α. New formulations of IFN-α may improve tolerance and efficacy. Attachment of IFN-α to polyethylene glycol (PEG) prolongs the half-life of IFN-α, allowing a weekly administration. In a Phase I study on patients with CML that failed to respond or were intolerant to IFN-α, significant activity was observed. Seven of 19 patients with active disease achieved a CHR and 2 (11%) had a CCR. Seven of 8 patients treated in CHR improved their response to complete (n = 4) or partial cytogenetic response (n = 3). All 6 patients intolerant to IFN-α tolerated PEG-IFN, and 4 improved their cytogenetic response. A different approach at sustained release formulations of IFN-α is Albuferon, composed of recombinant human albumin fused to recombinant human IFN-α. Because the median half-life of albumin is approximately 20 days, it prolongs the half-life of IFN-α and allows for administration every 2 weeks. This drug is currently being investigated in CML.
Summary
Multiple new agents are currently being developed in CML. Most of these agents are now being investigated in early clinical trial in patients who have developed resistance to imatinib. Their mechanisms of action are diverse, and many may be synergistic with imatinib. These agents will soon be used in different combinations, most likely including imatinib, with the hope of obtaining a complete blockade of the intracellular pathways that are triggered by Bcr/Abl. If this is successful, complete eradication of disease may become a reality for the majority of patients with CML.
III. Transplant Approaches for CML
Jerald Radich, MD*
Fred Hutchinson Cancer Research Center, 1100 Fairview Ave., N., #D4-100, Seattle, WA 98104
Dr. Radich is supported by grants from the National Cancer Institute, the Friends of Jose Carreras Foundation, and contract laboratory support from Novartis.
Acknowledgments: I thank Dr. Derek Stirewalt and Reginald Clift for their careful reading and helpful comments. Because of the size constraints of this review, important work from many colleagues may have been omitted. I apologize that I could not mention all of the contributions they have made to this field.
The exciting success of imatinib has dramatically revised the way clinicians think about treating CML. Although blood or marrow transplant (BMT) is thus far the only “curative” approach in CML, the prolonged cytogenetic remissions seen with imatinib therapy in chronic phase patients make medical therapy a tempting first-line approach, even in patients who may be fine transplant candidates. Inevitably, physicians and patients must now consider the option of imatinib against the option of a transplant. In making this comparison, they must weigh what is known about the curative potential for BMT and imatinib with consideration of the potential toxicities of each therapy. Complicating the issue is that while the impressive results of imatinib have deservedly seized the headlines in CML therapy, transplantation results for CML have also improved considerably over the years. This brief review will attempt to define the current strategies and outcomes following transplantation (Table 11 ).
The widespread application of BMT has been limited by donor availability and the high toxicity of the procedure in older patients, which limits the age of eligibility to less than 55-65 years at many centers. Ongoing advances in alternative donor sources (including unrelated donors and, to a lesser degree, cord blood), more accurate molecular HLA typing, and less toxic regimens, including reduced intensity or nonmyeloablative transplants, are broadening the potential use of this treatment modality.
Matched Related Allogeneic Transplants
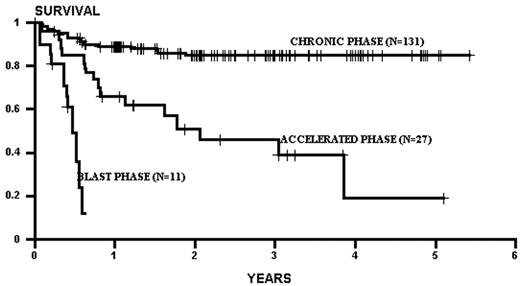
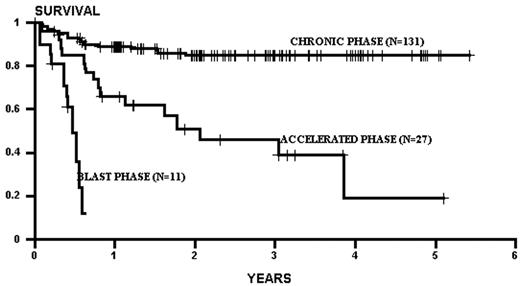
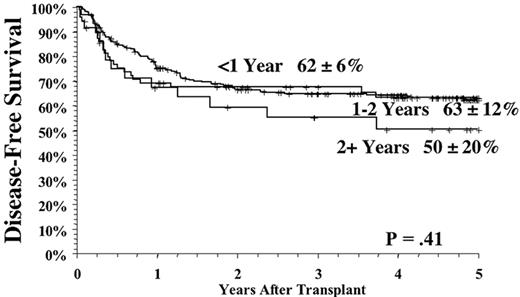
Several factors influence the outcome of allogeneic related transplants. The principal determinant of survival is the phase of disease at the time of transplantation. As a rule of thumb, survival decreases by half as one migrates from chronic phase, through accelerated phase, to blast crisis (Figure 2 ).1,2 Patients transplanted in remission after blast phase (also known as the second chronic phase) tend to have survival outcomes similar to accelerated phase cases. Survival in chronic phase is quite good, ranging from 60-80% at 5 years.1–,7 The use of chemotherapy preparative regimens (as opposed to total body irradiation–based [TBI-based] regimens) and improved supportive therapy (including cytomegalovirus and fungal prophylaxis) may have improved survival even more. For example, both an initial and a follow-up report of chronic phase CML patients randomized to a preparative regimen of TBI and cytoxan or busulfan and cytoxan (BU/CY) demonstrated similar effectiveness of both regimens.3,8 Subsequently, a pharmocologic assay for blood busulfan levels revealed that patients with levels > 900 ng/mL had better survival, and less relapse, compared to patients with a level < 900 ng/mL.9 This fact has led to subsequent targeting of busulfan in all patients to a level > 900 ng/mL (this strategy also allows correction for patients with a high busulfan level to prevent potential toxicity). This change in preparative regimens may have contributed to the apparent improvement of survival in CML chronic phase patients transplanted using targeted BU/CY. Indeed, in 131 consecutive chronic phase CML patients treated at our institution using a preparative regimen of pharmacologically targeted BU/CY, the 3-year survival was 86%, with a relapse rate of 8%. Unfortunately, advances that may have pushed progress in chronic phase patients have not been easily translated to accelerated and blast crisis transplants. Survival rates in these phases have remained relatively static over the past decade, with survival of accelerated phase approximately 40% and blast crisis approximately 10-20%. Thus, phase continues to be the driving force in any decision making, weighing the morbidity of the transplant against the risk of progression of disease with the associated poorer transplant outcomes seen with advanced disease.
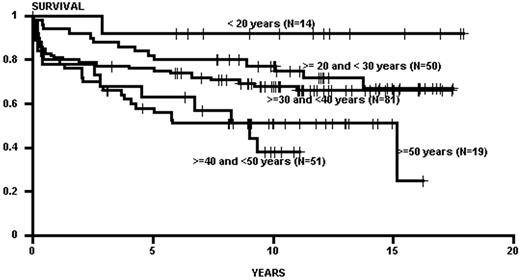
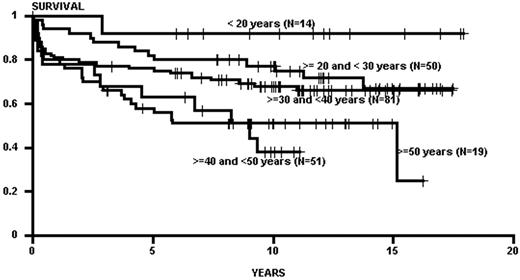
Advancing age has been the major constraint in transplants; in general, the rate of regimen-related toxicity climbs with patient age.2,4 Age limits have gradually increased over time with the introduction of new preparative regimens and improved supportive therapies. The effect of age, however, may be less now than in previous times, especially when applied to chronic phase patients. Chronic phase patients transplanted with TBI-based regimens generally demonstrated an age effect, with superior outcomes in young patients (< 21 years of age), with steady drop off in survival by decade of age.2 This age effect may have been mitigated by the use of non-TBI regimens (e.g., targeted BU/CY), as some recent data no longer show an age effect in patients up to 65 years of age (Figure 3 ). For patients in accelerated phase and blast crisis there are no similar data, but given the high regimen-related mortality seen in these patients (especially in those with blast crisis) one would suspect that increasing age would continue to have an untoward effect on survival.
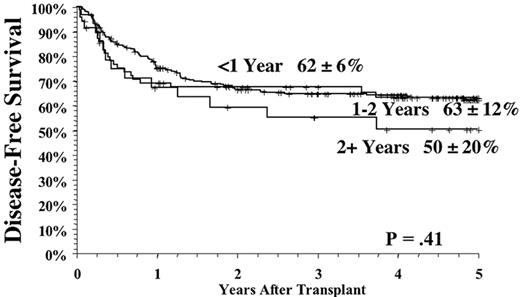
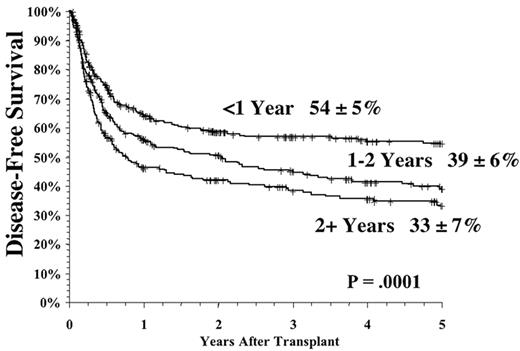
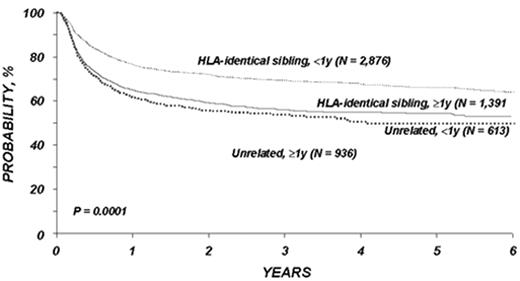
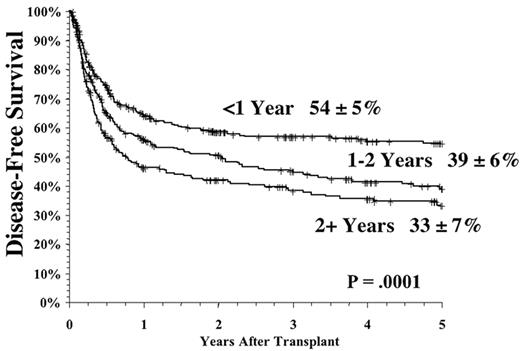
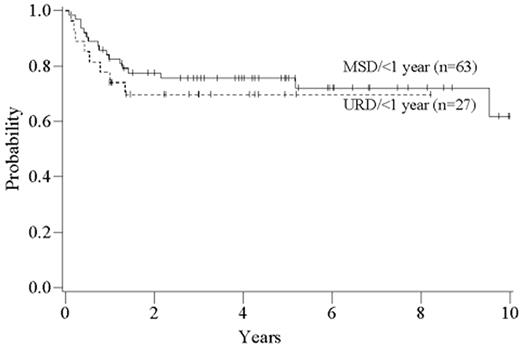
The time from first diagnosis to transplantation is a risk factor for poor outcome, with longer time intervals being associated with more toxicity and perhaps greater relapse rates.1,2,4,5 The critical cutoff time is unclear. Recent data from the International Bone Marrow Transplant Registry support a significant outcome advantage for patients transplanted < 1 year from diagnosis in both the related and unrelated setting (Figure 4 ); recent data from Seattle suggest that for matched related donor transplant the cutoff may be at 2 years postdiagnosis.2 The etiology of this effect has never been entirely clear, but it is likely mainly due to the cumulative toxicity of the conventional therapy, leading to an increase in non-leukemic mortality. It is clear that prolonged therapy with oral busulfan was associated with a poor outcome,1,10 and results have been controversial as to the effects of prolonged interferon therapy and subsequent transplant toxicity.11– 15 However, the interaction of time from diagnosis and outcomes has been difficult to study adequately in the “modern age” of transplantation since so few patients eligible for transplantation wait longer than 1-2 years to undergo the procedure. The long-term effect of imatinib on transplant outcomes is unknown but obviously must be determined in the future.
Unrelated Donor Transplants
The use of unrelated donor (URD) transplants is limited by both the availability of donors and the increased toxicity of the procedure compared to related transplants, largely owing to the effects of graft-versus-host disease (GVHD). Fully matched unrelated donors are now available for over 50% of Caucasian patients, but unfortunately, the percentage of available donors remains substantially lower for patients from other ethnic groups. The advent of molecular DNA assessment of HLA typing has made for a rigorous and stringent selection of unrelated matched donors, and this improvement in typing has translated into greatly improved transplant outcomes. Thus, in many centers, the success after URD transplantation is comparable to that of matched related donor transplants.16– 18
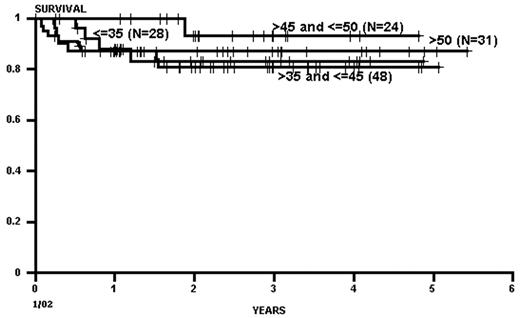
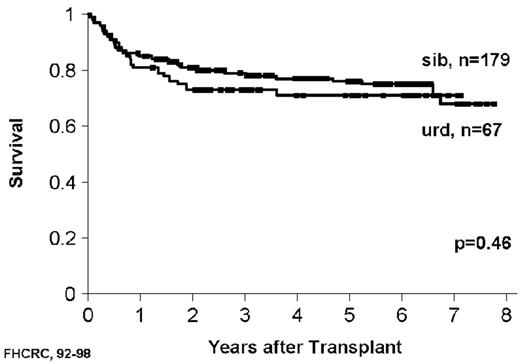
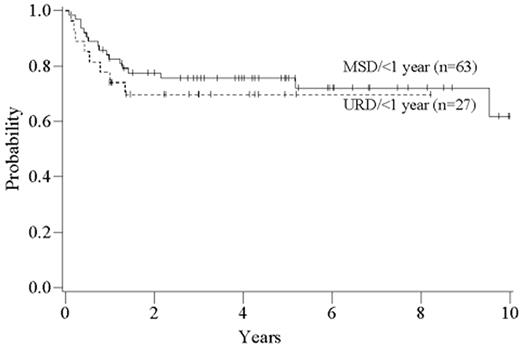
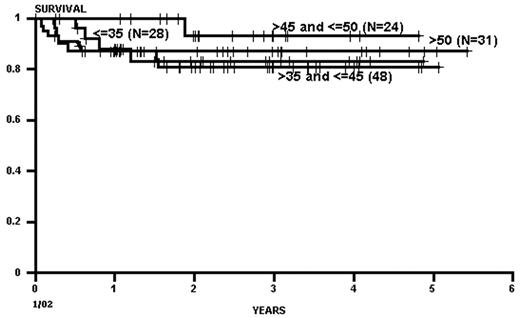
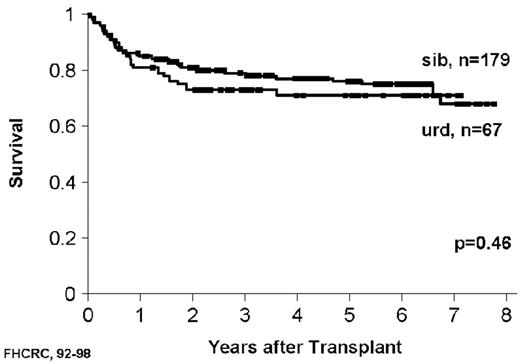
As with related donor transplants, disease phase is the major determinant in URD transplant outcome, with 3-year survival in chronic phase patients at least double that of patients transplanted in accelerated phase.18,19 Survival following a URD transplant for blast crisis is unusual, and indeed, most clinicians feel that patients in blast crisis should be re-induced into a chronic phase before a transplant is attempted, if possible. Age continues to be a major determinant in the outcome of URD transplantation, although a clear-cut breakpoint in determining “good” and “bad” risk groups has not been demonstrated (Figure 5 )16,17,19 In addition, time from diagnosis to transplant is strongly associated with outcome, with a significant increase in mortality occurring when transplant is delayed for greater than a year (Figures 5 and 6Figure 5,Figure 6). For “younger” patients, the survival results in chronic phase CML are similar for fully matched, unrelated matched, and related transplants, especially for patients in “good risk” groups.18 In a multicenter analysis of National Marrow Donor Program (NMDP) data, disease-free survival of unrelated and related transplants for CML chronic phase patients aged 30-40 years, transplanted within 1 year of diagnosis, was 67% versus 57%, respectively. In addition, 2 other studies have observed a near-equivalence of disease-free survival for chronic phase CML using either a fully matched unrelated or related donor.16,17 Estimates of disease-free survival of > 70% were found for “younger” patients (≤ 50 years of age) transplanted within a year of diagnosis (Figure 7 ).17
Bone Marrow Versus Peripheral Blood Stem Cells
Peripheral blood stem cells (PBSC) have emerged as a preferred source of stem cells in autologous transplantation, where their use has been associated with quicker engraftment and less regimen-related toxicity compared to stem cells derived from bone marrow (BMSC). In the allogeneic transplant setting; however, the use of PBSC is more controversial. A randomized trial showed improved early overall survival and disease-free survival in PBSC compared to bone marrow recipients in patients with “high risk” leukemias (which included CML in accelerated phase and blast crisis).20 It is unclear whether PBSC are superior to, equal to, or worse than BMSC in the chronic phase setting. The concern here is that given the excellent results of conventional bone marrow transplantation in chronic phase CML, any possible benefits from PBSC may be offset by potential increase in GVHD.
A few studies suggest an advantage of PBSC in the URD transplant setting. Two studies from the Essen group evaluating the role of PBSC in the unrelated transplant setting have demonstrated the superiority of PBSC versus BMSC. The first study showed that patients receiving PBSC had the bcr-abl fusion transcript detected less often compared to BMSC patients, suggesting a more dramatic graft-versus-leukemia effect in the PBSC patients.21 The second study compared PBSC with BMSC in chronic phase CML and found that PBSC were associated with improved survival for the PBSC group (94% vs 66%), owing mostly to a decrease in acute transplant-related mortality (5% vs 30%). Overall incidence of acute and chronic GVHD appeared similar in the two groups.22 Last, a retrospective study of PBSC transplants compared to a matched set of BMSC URD transplants suggested that PBSC and BMSC transplants had similar rates of GVHD, relapse, and survival.23 The effects appeared similar in all phases of CML.
Reduced Intensity or Nonmyeloablative Transplants
Innovative approaches using reduced intensity or non-myeloablative transplants have recently been pioneered to engender a graft-versus-leukemia effect without exposing the patient to the toxicity of the preparative regimen. The first candidates for these transplants have been patients deemed at high risk of transplant complications—that is, relatively older patients or those with compromised organ function. These studies were performed on a variety of hematological malignancies. The procedure seems best suited for patients with slow-growing tumors, such as low-grade lymphomas, chronic leukemias, or acute leukemia in remission. This is because the nonmyeloablative approach is essentially a race between the reconstitution of the immune system and the associated antileukemic effect, versus the growth of the malignancy. Major, durable responses were documented in chronic lymphocytic leukemia, myeloma, low-grade lymphomas, acute leukemia, and renal cell carcinoma.24,26 The data in CML are limited.25,26 The M.D. Anderson group reported on 27 CML patients, of which 21 were in “transformed” state.25 Overall survival for these CML cases at 1 year was 32%. A study by McSweeney et al used a less intense regimen and included 9 patients with CML.26 Of the 6 patients transplanted in chronic phase, 4 achieved not only a cytogenetic remission but also a “molecular remission” studied by RT-PCR. Not surprisingly, these molecular responses lagged behind cytogenetic remissions by several months. In the 3 accelerated phase patients, 2 had progressive disease, yet 1 achieved a molecular remission. Graft rejection has been observed more frequently in non-ablative than in ablative transplant, especially in patients with CML and MDS. This is most likely the result of a relatively active donor immune system, which in CML has been spared exposure to cytotoxic and immunosuppressive therapy. Nonetheless, the early results are promising and suggest that the nonmyeloablative approach may be quite attractive in CML, especially in chronic phase. This is not entirely surprising, as many lines of evidence suggest that CML is especially sensitive to immunologic effects (including the high relapse rates in syngeneic and T-cell-depleted transplants; the beneficial effects of donor leukocyte infusions [DLI]; and the effects of interferon). However, the question of whom to apply the nonmyeloablative approach to is problematic. Restricting the procedure to only those patients who fail imatinib or those who have a related or unrelated donor but are too old or too ill for conventional transplantation would confine the treatment to a very small niche, indeed. One possible approach might be to add non-myeloablative transplants as an immunologic adjuvant after initial “debulking” with imatinib, followed by the addition to imatinib after transplant to “mop up” any residual disease.
Autologous Transplants
Autologous transplants have the attraction of circumventing the toxicities associated with GVHD, but alas, the curative potential may be limited by both the lack of immunologic effect and potential problems of contamination of the graft with residual leukemia cells. Nonetheless, several studies have suggested a potential extension of the natural history of chronic phase CML after autologous transplantation, with long-term survival of > 40% after 5 years for those patients transplanted in chronic phase. As with allogeneic transplants, the success of autologous transplants is most influenced by the stage of disease at the time of transplantation. In a review of the experience in 8 centers, McGlave et al found the 3-year survival for patients transplanted in chronic, accelerated, and blast phase to be approximately 60%, 30%, and 0%, respectively.27 More recently, the Hammersmith group reported on the results of 56 patients who received a busulfan-only preparative regimen.28 The 3-year survival was 76% for patients in chronic phase and 30% for patients with advanced phase disease (accelerated, blast, or second chronic phase). However, only 2 of 45 patients studied 12 months post-transplant achieved a CCR.
Although it is logical to prefer a stem cell product “purged” of CML cells, is it essential? On one hand, Carella et al treated 30 patients with high-dose chemotherapy and collected PBSC; after transplantation, patients received a short course of interleukin-2 and remained on interferon.29 Only 2 patients progressed to blast crisis. Of the 16 patients who received Ph-negative PBSC, 8 remained in CCR (thus, overall 8/30 patients achieved CCR). No patient who received Ph-bearing PBSC products achieved a CCR. On the other hand, Meloni et al published results on 26 CML chronic phase patients who had unpurged PBSC collected at diagnosis after granulocyte colony-stimulating factor (G-CSF) mobilization, followed by autologous transplantation.30 All patients received interferon post-transplant. The 10-year survival of this cohort was 55%, and remarkably, 8 of 26 patients achieved a CCR at some time during their posttransplant course. Thus, the role of purging stem cells is still unclear, although the concept is appealing. The common denominator of post-transplant immunotherapy seems important.
Given that imatinib can often produce cytogenetic remissions, the question of harvesting PBSC at the time of cytogenetic remission, with potential use at the time of progression, has come to the forefront. Anecdotal reports suggest that stem cells collected after imatinib therapy can engraft, but the issue of engraftment is still unanswered. After engraftment comes the issue of relapse. Given that interferon post-transplant may prevent or delay relapse, the addition of imatinib after autologous transplant may be a logical next step for study.
Minimal Residual Disease
Relapse continues to be a major obstacle to cure in transplantation, increasing in frequency with advancing phase of disease. As such, relapse occurs in ∼10-20% of chronic phase transplants and up to 50% for patients transplanted in blast crisis. Risk increases with the use of T-cell depletion and in twin and autologous transplants, presumably from a lessening of the graft-versus-leukemia effect. It is now well established that the detection of the chimeric bcr-abl mRNA transcript by RT-PCR is a powerful predictor of subsequent relapse.31–,44 In general, the qualitative test (yes versus no) is predictive of relapse “early” (6-12 months) posttransplant, and quantitative assays increase the precision of predicting relapse.33,35,37,40,43,44 With effective monitoring techniques and intervention strategies, one can envision a day when grafts are manipulated to decrease the risk of GVHD, and then an intervention (e.g., T cells, interferon) is added at the point of detectable residual disease. Such a strategy decreases the toxicity of the transplant (by decreasing GVHD) while preserving the anti-leukemic effect of the transplant.
The early detection of minimal residual disease (MRD) allows for treatment to be initiated when the disease burden is far less than at frank cytogenetic or pathological relapse. The underlying assumption is that such early treatment will translate into a greater response rate. Several examples demonstrate this concept. Cytogenetic relapse following allogeneic transplant has effectively been treated with a low dose of interferon (1-3 × 106 U/m2/d).45 In this study 14 patients were treated, and remarkably, 12/14 achieved a CCR. Of 9 patients in CCR tested for bcr-abl by RT-PCR, 4 also had evidence of a molecular remission. Eight patients (57%) remained in a durable CCR lasting up to 5 years. The treatment of MRD with T-cell immunotherapy has been demonstrated by van Rhee et al, who treated 14 relapsed CML patients (hematologic, cytogenetic, or molecular) with DLI.46 Of the 7 patients with hematologic relapse, 3 had a CCR to DLI; however, 2 of these patients developed aplasia. Three patients with hematologic relapse and 2 patients with only bcr-abl molecular positivity were treated; all had a complete response, and there were no aplastic events. There was no clear difference in the incidence of severe GVHD among the 3 groups. Thus, patients treated earlier in the course of relapse had improved response and fewer complications. Further studies of DLI administration posttransplant have also suggested the potential increased efficacy of treating a lower disease burden afforded by MRD testing.47,48
In the near future, novel therapeutic approaches may be used to treat MRD in the post-transplant setting. Given the promise of early trials in treating cytogenetic or molecular relapse, the potential use of less toxic agents (imatinib) would seem quite promising. However, it cannot be emphasized enough that any treatment of MRD post-transplant is investigational and should take place only in the context of a research trial.
Molecularly Defined Risk Groups
Treatment response in CML is variable, to both non-transplant and transplant modalities. For example, in accelerated phase CML, only a small percentage of patients will obtain a complete response to imatinib or interferon, and approximately one half will relapse after transplantation. Why is there such heterogeneity in response and relapse? Why do some patients in accelerated phase behave like chronic phase patients (survive without relapse), while others behave like blast crisis patients (relapse shortly after transplant)? Presumably part of this heterogeneity is based on the individual genetics of the particular leukemia. The genetic basis of these differences in gene expression may reside in the controls of the cell cycle or apoptotic pathways, or alternatively, genes involved in drug metabolism and drug resistance. Regardless of the specific mechanism, we hypothesize that different patterns of gene expression detected prior to transplant will be associated with different outcomes. If this proves true, we would be able to predict high- and low-risk patients prior to transplant and tailor therapy appropriately.
Much is said about the promise of tailoring individual therapy to the genetic specifics of each patient’s leukemia, but in most diseases, the effective therapeutic modalities are so limited as to make such a promise a tad optimistic. Not so in CML, as patients may enjoy a considerable long-term benefit, if not cure, with several approaches (interferon, imatinib, transplantation). How does one pick the best treatment? Prognostic scores, such as those proposed by Hasford et al49 and Sokal et al,50 are useful but do not disclose information about the pathogenesis of disease progression or disease response.
Several studies have used DNA microarrays to study the differences in gene expression seen from different types of leukemia or different subsets of leukemia.51,52 Comparisons may be made between disease types, such as acute lymphoblastic versus acute myeloid leukemia, or among different phenotypes within a specific disease, such as treatment responders versus non-responders. Such an approach should be quite informative in CML. For example, a comparison of gene expression differences between chronic phase and accelerated phase or blast crisis may detect genes involved in the progression of the disease; these genes could then be studied in biological context, as well as serve as targets for more sensitive assays of disease state. In comparing a pool of blast crisis samples to a pool of chronic phase samples, we have found approximately 500 genes significantly different between the two disease states.53 Blast crisis and chronic phase patients demonstrate clear differences in gene expression, and occasional cases occur in which the clinical and pathological diagnosis is quite discordant from the gene expression pattern (Figure 8, see Color Figures, page 514). In addition, gene expression studies may give insights into the biology of response (e.g., by defining a subset of genes associated with imatinib response). Patients could be screened for the presence of these genes, prior to therapy, to determine the best treatment strategy. Studies in Ph+ ALL have suggested a defined subset of genes associated with the unresponsive imatinib phenotype.54
Of course, gene expression studies are only part of the biological picture, but the rapidly evolving technology to document chromosomal and DNA changes (by comparative genomic hybridization or single nucleotide polymorphism studies) and the rapidly evolving field of proteomics are bound to make a mighty contribution to both our biological understanding and our treatment algorithms.
Conclusion
Patients and physicians face a difficult choice for treatment of early CML. Imatinib is a very exciting drug, but a number of unknown elements should at least give the clinician pause. First, it will take several years to establish whether imatinib and its predictable spinoffs (new tyrosine kinase inhibitors, and combinations of imatinib with old drugs) are curative. Second, it is not known if imatinib treatment failures will return to a chronic phase or will evolve with a more aggressive disease difficult to treat with transplantation. With frequent monitoring (e.g., quantitative RT-PCR) one would expect to detect patients bound to relapse early. It is also unclear what, if any, long-term side effects from imatinib might become unmasked with transplantation. As part of this reflection, it is important to report that success in transplantation has also improved over the past years; it remains an important treatment option that should not be neglected.
While many dwell on the question of “which is better” (usually pitting imatinib versus transplantation), we should rather rejoice that there are several effective treatment options in CML. One possible approach for decision making (Figure 9, see Color Figures, 515) revolves around the presence or absence of a stem cell donor. In the near future, treatment of CML may be guided by molecular studies (Figure 10, see Color Figures, page 515). After initial therapy would come molecular monitoring of the disease, with early detection of disease recurrence triggering the appropriate next level of therapeutic intervention. CML has always been the paradigm for “bench to bedside” translational research. Now, once again, the collision of new therapeutic options with advances in molecular biology offers a “next generation” of effective therapy for the vast majority of CML patients, and may show the way for the future treatment of other malignancies.
Schematic of point mutations in the Abl kinase domain.
The Abl kinase domain, from amino acid 240 to 500, is shown with the ATP binding domain (P), the catalytic domain (C) and the activation loop (A). The numbers below the kinase domain are amino acids that are mutated in patients who relapsed on therapy with imatinib. The vertical lines above the kinase domain indicate the number of times each amino acid has been found to be mutated as compiled from Shah et al,43 Hochhaus et al,42 Branford et al,44 von Bubnoff et al,45 and Hofmann et al.47 Adapted from Shah et al.43
Schematic of point mutations in the Abl kinase domain.
The Abl kinase domain, from amino acid 240 to 500, is shown with the ATP binding domain (P), the catalytic domain (C) and the activation loop (A). The numbers below the kinase domain are amino acids that are mutated in patients who relapsed on therapy with imatinib. The vertical lines above the kinase domain indicate the number of times each amino acid has been found to be mutated as compiled from Shah et al,43 Hochhaus et al,42 Branford et al,44 von Bubnoff et al,45 and Hofmann et al.47 Adapted from Shah et al.43
Survival of CML patients in chronic, accelerated and blast phase.
All patients were transplanted from matched related donors.
Survival of CML patients in chronic, accelerated and blast phase.
All patients were transplanted from matched related donors.
The effect of age on survival.
Figure 3A shows the influence of age on matched transplants for CML chronic phase after receiving a preparative regimen of total body irradiation/ (TBI/CY).
Figure 3B shows the survival after targeted busulfan/CY (BU/CY). There is no significant difference in the survival of these age cohorts up to age 65.
The effect of age on survival.
Figure 3A shows the influence of age on matched transplants for CML chronic phase after receiving a preparative regimen of total body irradiation/ (TBI/CY).
Figure 3B shows the survival after targeted busulfan/CY (BU/CY). There is no significant difference in the survival of these age cohorts up to age 65.
The IBMTR experience of chronic myelogenous leukemia (CML) chronic phase patients.
The curves demonstrate both the effect of a related versus unrelated donor, and the benefit of early transplantation on survival (Figure 4 reprinted with permission from the IBMTR).
The IBMTR experience of chronic myelogenous leukemia (CML) chronic phase patients.
The curves demonstrate both the effect of a related versus unrelated donor, and the benefit of early transplantation on survival (Figure 4 reprinted with permission from the IBMTR).
The effect of age on survival in unrelated donor (URD) transplants for chronic myelogenous leukemia (CML).
The results show a significant age effect after the age of 40. All patients were transplanted from a single center (Fred Hutchinson Cancer Research Center) and received a standard total body irradiation/cytoxan (TBI/CY) preparative regimen.
The effect of age on survival in unrelated donor (URD) transplants for chronic myelogenous leukemia (CML).
The results show a significant age effect after the age of 40. All patients were transplanted from a single center (Fred Hutchinson Cancer Research Center) and received a standard total body irradiation/cytoxan (TBI/CY) preparative regimen.
Comparison of matched related and unrelated transplants.
Chronic myelogenous leukemia (CML) chronic phase patients treated at National Marrow Donor Program (NMDP) Centers from 1988-1999. Figure 6A shows the disease-free survival for matched related transplants, divided by time from diagnosis to transplant.
Figure 6B shows the same effect for URD transplants. Figure 6A reprinted with permission from Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99(6):1971-1977.
Comparison of matched related and unrelated transplants.
Chronic myelogenous leukemia (CML) chronic phase patients treated at National Marrow Donor Program (NMDP) Centers from 1988-1999. Figure 6A shows the disease-free survival for matched related transplants, divided by time from diagnosis to transplant.
Figure 6B shows the same effect for URD transplants. Figure 6A reprinted with permission from Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99(6):1971-1977.
A comparison of related and unrelated transplants for chronic myelogenous leukemia (CML) chronic phase.
Figure 7A shows the experience from the Fred Hutchinson Cancer Research Center, and Figure 7B the experience from the University of Minnesota. There were no significant differences between the unrelated and related survivals.
Figure 7B reprinted with permission from Davies SM, DeFor TE, McGlave PB, et al. Equivalent outcomes in patients with chronic myelogenous leukemia after early transplantation of phenotypically matched bone marrow from related or unrelated donors. Am J Med. 2001;110(5):339-346..
A comparison of related and unrelated transplants for chronic myelogenous leukemia (CML) chronic phase.
Figure 7A shows the experience from the Fred Hutchinson Cancer Research Center, and Figure 7B the experience from the University of Minnesota. There were no significant differences between the unrelated and related survivals.
Figure 7B reprinted with permission from Davies SM, DeFor TE, McGlave PB, et al. Equivalent outcomes in patients with chronic myelogenous leukemia after early transplantation of phenotypically matched bone marrow from related or unrelated donors. Am J Med. 2001;110(5):339-346..