Abstract
This chapter describes the clinical presentation and molecular basis of two inherited bone marrow failure syndromes, Fanconi anemia (FA), and Diamond-Blackfan anemia (DBA). It also provides an update on diagnostic and therapeutic approaches to bone marrow failure of all types (inherited and acquired) in pediatric patients.
In Section I, Dr. Alan D’Andrea reviews the wide range of clinical manifestations of Fanconi anemia. Significant advances have been made in understanding the molecular pathogenesis of FA. On the basis of these advances, new diagnostic assays and treatment options are now available.
In Section II, Dr. Niklas Dahl examines the clinical features and molecular pathogenesis of Diamond-Blackfan anemia. The possible links between the RPS19 gene (DBA gene) and the erythropoiesis defect are considered.
In Section III, Drs. Eva Guinan and Akiko Shimamura provide an algorithm for the diagnostic evaluation and treatment of children with inherited or acquired aplastic anemia. Through the presentation of a case study of a pediatric patient with bone marrow failure, he provides an overview of the newest tests and treatment options.
I. Molecular Pathogenesis of Fanconi Anemia: Implications for Diagnosis and Treatment
Alan D. D’Andrea, MD*
Dana-Farber Cancer Institute, Department of Pediatrics Oncology, 44 Binney Street, Mayer 640, Boston, MA 02115
Fanconi anemia (FA) is a rare autosomal recessive disease characterized by congenital abnormalities, progressive bone marrow failure, and cancer susceptibility.1,2 FA has an incidence of less than 1 per 100,000 live births. The recent cloning of seven FA genes has altered the diagnostic and therapeutic management of FA patients. FA children often develop pancytopenia during the first decade of life, and death often results from complications of bone marrow failure. The treatment of choice for patients with FA remains an allogeneic bone marrow transplant from a histocompatible sibling.
Clinical Features of FA
The clinical features of FA have been extensively reviewed.3–,5 FA patients often have skeletal abnormalities (e.g., thumb or limb abnormalities) and abnormal skin pigmentation (e.g., hypopigmentation or café au lait spots). Other organ systems involved include the cardiac, renal, and gastrointestinal (GI) systems. FA patients have decreased fertility.6 Accordingly, the FA genes, discussed below, regulate the development of multiple organ systems.
The hematologic consequences of FA often develop in the first few years of life. Progressive megaloblastic anemia ensues, often accompanied by elevated expression of the i antigen on the red cells. Anemia and thrombocytopenia often precede the neutropenia. FA patients also develop clonal chromosomal abnormalities in the bone marrow progenitor cells (i.e., chromosome 1 or 2 abnormalities or monosomy 7), which may evolve into myelodysplasia (MDS) or acute myeloblastic leukemia (AML). FA is often considered a genetic model system for the study of MDS or myeloid leukemia.
FA patients are predisposed to many types of malignancies. While AML is the most common cancer, older FA patients may develop squamous cell carcinomas of the head and neck or gynecologic system. Because of the underlying hypersensitivity of FA patients to DNA crosslinking agents and radiation, the treatment of these malignancies is especially difficult. The wide range of cancers observed in FA patients has previously been reviewed.7
Diagnosis of Fanconi Anemia
FA cells are hypersensitive to DNA crosslinking agents, such as diepoxybutane (DEB) and mitomycin C (MMC). Cellular exposure to these genotoxic agents results in an abnormal elevation of chromosome breaks and radial forms. The DEB chromosome breakage test, performed on peripheral blood lymphocytes, remains the clinically certified diagnostic tool for FA.8 The DEB test should be performed for any child with the characteristic clinical stigmata of FA (congenital abnormalities, pancytopenia). Since FA has a wide range of clinical phenotypes, however, the test should be considered in other isolated cases (e.g., sporadic childhood AML in a child with some congenital abnormalities, head and neck squamous cell carcinomas in young adults).
In addition, constitutive elevation of serum alpha-feto protein (AFP) has been reported in FA patients, although this finding is not specific to FA and can be seen in other pathological conditions, such as malignancies, liver disease, neural tube defects, and Down’s syndrome.
Additional diagnostic tools are now available for the clinician. First, the DEB test can be performed on primary fibroblast cultures.9 Fibroblast studies should be performed for FA patients who exhibit peripheral blood lymphocyte mosaicism. Mosaicism refers to the setting of two distinct populations of peripheral blood lymphocytes.10 One population is resistant to MMC and the other population remains hypersensitive to MMC. Mosaicism is common in FA and results from somatic reversion of a mutation in an FA gene.11 Second, several FA genes have been cloned. Sequencing of the genes is useful in some FA families and for prenatal diagnosis. However, most FA mutations are “private” mutations, and only a few mutations are found with any appreciable frequency in some ethnic populations.12,13 More recently, retroviral complementation with cloned FA cDNAs and immunoblots for FA proteins have added to the diagnostic accuracy and subtyping of FA patients and family members.14,15
Treatment of Fanconi Anemia
The treatment of FA is largely directed to the complications of bone marrow failure. Some FA patients respond initially to hematopoietic growth factors (EPO, G-CSF).16,17 Other FA patients respond to androgen therapy, although long-term androgen exposure may result in complications of hirsutism and liver disease. The best treatment for the pancytopenia of FA is matched allogeneic bone marrow transplantation. In the absence of a histocompatible matched sibling, unrelated donor transplants and cord blood transplants18,19 are routinely performed at some centers. There have been recent improvements in survival, resulting from the use of unrelated donor transplants for FA patients. In addition, newer approaches have been developed in the fields of preimplantation genetic diagnosis (PGD) and gene therapy20,21 for select patients with FA.
FA patients who survive bone marrow disease, perhaps by allogeneic bone marrow transplantation (BMT), remain prone to malignancies, usually in the second or third decade of life. Early diagnosis and excision of these cancers is critical. The use of chemotherapy and radiation in the treatment of FA patients with cancer is limited, due to the underlying cellular sensitivity of FA cells to these genotoxic agents.
Genetics of Fanconi Anemia
The past decade has witnessed considerable progress in the molecular understanding of FA. This progress resulted, in large part, from the subtyping of FA patients and cell lines into discrete complementation groups.22– 24 On the basis of somatic cell fusion studies and correction of cellular sensitivity to MMC, FA was classified into at least 8 distinct complementation groups (A, B, C, D1, D2, E, F, G).
The identification of these 8 distinct subtypes set the stage for the cloning of the FA genes (Table 1 ). The first FA gene, FANCC, was expression cloned in 1992;25 subsequently, genes were cloned corresponding to complementation groups A,26 E,27 F,28 G,29 and D2.24 The structures of the 6 encoded FA proteins are novel, and recent studies indicated that these proteins cooperate in a common cellular pathway (see below). Most recently, a seventh FA gene has been identified as BRCA2, the well-known breast cancer susceptibility gene.30 Biallelic mutations in BRCA2 have been observed in FA-B and FA-D1 cells, suggesting that BRCA2 is the FA gene corresponding to both of these subtypes.
The identification of 7 FA genes has altered the diagnostic approach to the disease. Once FA patients are identified (or suspected) by a positive DEB test, mutational screening of the FA genes may be warranted. Subtyping of FA patients is first performed by retroviral complementation of primary or immortalized cells with the corresponding FA cDNAs. Retroviral complementation with, say, the FANCA cDNA indicates that the patient is assigned to subtype A. Subtyping is important for clinical decision making. For example, FA-A patients tend to have milder disease (i.e., later onset of bone marrow failure), while FA-C and FA-G patients have a more severe clinical course, perhaps warranting earlier bone marrow transplantation.31
Once subtyping has been completed, the determination of the precise FA gene mutation is possible. Identification of the FA gene mutation in an FA family is helpful for prenatal diagnosis, preimplantation genetic diagnosis, and FA carrier detection. FA subtyping and FA mutation verification are also necessary preconditions for the use of FA gene therapy.
The Six FA genes (A, C, D2, E, F, G) Interact in a Common Pathway
FA patients and FA cell lines derived from the 8 complementation groups of FA have striking phenotypic similarities. These FA cells are hypersensitive to MMC and develop characteristic chromosome breakage (radial forms) upon cellular exposure to MMC. Investigators therefore reasoned that the 6 orphan FA proteins (A, C, D2, E, F, G) may function in a common cellular pathway. Consistent with this hypothesis, 5 of the FA proteins (A, C, E, F, G) form a constitutive nuclear protein complex.32,33 Absence of any one of these 5 protein subunits results in loss of the nuclear FA complex and degradation of the other protein subunits (Figure 1, see Color Figures, 511).
The FANCD2 protein, while not a component of this complex, is modified posttranslationally by the complex.33 The FANCD2 protein is expressed as two isoforms in normal cells: FANCD2-L (monoubiquitinated) and FANCD2-S (unubiquitinated). The FANCD2-L isoform is not observed in cells derived from A, C, E, F, or G patients, suggesting that the FA complex is required for the monoubiquitination of FANCD2. Screening for FANCD2 monoubiquitination, by a simple western blot, therefore provides a powerful alternative to the DEB test. In normal cells, DNA damage results in a dose-dependent and time course–dependent monoubiquitination of FANCD2. The FANCD2 protein is targeted to nuclear subfoci, where it interacts with BRCA1 and BRCA2. These foci are believed to be required for normal homologous recombination DNA repair activity.1
Interaction of FA Proteins with BRCA1 and BRCA2
How the FA protein complex (A,C,E,F,G complex) regulates FANCD2 monoubiquitination remains unclear. Since none of these subunits has an obvious ubiquitin ligase catalytic domain, it has been hypothesized that an additional subunit(s) may perform this enzymatic function. The BRCA1 protein contains a ring finger ubiquitin ligase domain34 and therefore is a candidate enzyme in the FA pathway.
Several other lines of evidence also suggest that BRCA proteins (BRCA1 and BRCA2) may be genetically linked to the FA pathway. The BRCA1 and BRCA2 genes were originally cloned by position, at chromosomal sites corresponding to inherited breast and ovarian susceptibility foci. BRCA1 (+/-) and BRCA2 (+/-) carriers have an elevated risk of developing cancer during their lifetime. Tumors derived from these individuals have lost the normal copy of the BRCA1 or BRCA2 gene, respectively, causing a biallelic disruption.
Interestingly, BRCA1 (-/-) and BRCA2 (-/-) tumor cells have a striking resemblance to FA cells (Figure 2, see Color Figures, 511). In response to MMC, these tumor cells develop increased chromosome breaks and radial forms, suggesting a genetic interaction of FA genes and BRCA genes. Complementation of a BRCA2 (-/-) rodent cell line with the BRCA2 gene corrected the MMC-induced chromosome breaks.35 Finally, a homozygous disruption of the 3′ end of the murine BRCA2 gene, sparing the 5′ end of the gene, resulted in a mouse with a Fanconi-like phenotype (skeletal abnormalities, cancer predisposition, and MMC sensitivity).36
These genetic interactions, coupled with the presence of a ubiquitin ligase catalytic domain in the BRCA1 protein, suggested that BRCA1 may be an FA gene. We therefore initially sequenced the BRCA1 gene in unassigned FA patients or FA patients from subtype B and D1. No BRCA1 mutations were observed.
BRCA2 was also considered a candidate FA gene because of its close interaction with BRCA1.37 The BRCA1 and BRCA2 proteins colocalize in DNA damage-inducible and S phase–specific nuclear foci. Accordingly, the BRCA2 protein may modulate the ubiquitin ligase activity of BRCA1. Sequencing of the BRCA2 gene in 5 FA patients (non-A, C, D2, E, F, G patients) revealed biallelic BRCA2 mutations.30 Moreover, at least one of these cell lines (EUFA423) was corrected by microcell fusion with human chromosome 13 containing the wild-type BRCA2 gene. Taken together, these results indicate that BRCA2 is an FA gene.
Interestingly, all of the BRCA2 (-/-) FA patients had at least one BRCA2 allele with a mutation in the 3′ region of the gene. In each case, this mutant allele encoded a carboxy terminal truncated BRCA2 protein, presumably with partial activity. Homozygous disruption of the 5′ end of the human BRCA2 gene probably results in embryonic lethality, similar to the outcome in the mouse model.38
The BRCA2 (-/-) FA patients have the characteristic clinical appearance of FA (i.e., thumb abnormalities, bone marrow failure, cancer predisposition), strongly suggesting that BRCA2 functions in the FA pathway (Figure 1, 511). Although the precise functions of BRCA1 and BRCA2 in the pathway remain unknown, several models are possible. Because BRCA2 and RAD51 have a strong interaction, it has been assumed that the FA pathway regulates a downstream DNA repair function.
Summary
In conclusion, FA is the most common inherited bone marrow failure syndrome. FA displays a wide range of clinical features. The diagnostic test for FA remains the DEB chromosomal breakage test; however, the identification of at least 8 FA complementation groups and the cloning of 7 FA genes have provided new opportunities for diagnostics and subtyping. Subtyping has become an important feature of FA diagnostics, and it sets the stage for clinical gene therapy protocols. The treatment of choice remains allogeneic bone marrow transplantation, although recent progress with unrelated donor transplantation has been made. Finally, the interaction of the FA genes with the BRCA1 and BRCA2 genes has provided unexpected insights into the molecular mechanisms of DNA repair and the pathogenesis of cancers in the general (non-FA) population. Additional information regarding the diagnosis and treatment of patients with Fanconi anemia can be obtained online.39,40
II. Molecular Basis of Diamond-Blackfan Anemia
Niklas Dahl, MD, PhD*
Uppsala University Children’s Hospital, Department of Genetics and Pathology, The Rudbeck Laboratory, S-751 85 Uppsala, Sweden
Diamond-Blackfan anemia (DBA) is characterized by a chronic constitutional aregenerative anemia with absent or decreased erythroid precursors in the bone marrow but otherwise normal cellularity.1,2 The disease may serve as a model for a differentiation arrest of the erythroid lineage. The specific reduction of erythroid precursor cells in the bone marrow suggests that the deficient protein(s) has a key regulatory role in erythropoiesis.
Most patients present with anemia in the neonatal period or in infancy. Approximately 30% of affected children present with a variety of associated physical anomalies. Thumb and upper limb malformations as well as craniofacial abnormalities are common. Other defects frequently observed include atrial or ventricular septal defects, urogenital anomalies, and prenatal or postnatal growth retardation.1–,5 A moderately increased risk of developing hematological malignancies also exists.5,6 Most cases with DBA are sporadic, with an equal sex ratio, but 10-25% of patients have a positive family history for the disorder.4,7,8 The pathophysiology and the basic molecular defect(s) of DBA remain unclear. Different in vitro assays as well as molecular studies have failed to demonstrate a direct role of hematopoietic growth factors or their receptors in the pathogenesis of DBA.9–,12 A normal erythropoiesis can be restored in DBA by bone marrow allografting,13 which indicates that the anemia can be ascribed to an intrinsic defect of hematopoietic progenitor cells.
Hematological Findings
The minimal diagnostic criteria for DBA generally include normochromic anemia in infancy (< 2 years), low reticulocyte counts, absent or decreased bone marrow red cell precursors (< 5% of nucleated cells), and a normal chromosome fragility test (mitomycin C or diepoxybutane). Additional features include presence of malformations, macrocytosis, elevated fetal hemoglobin, and elevated erythrocyte adenosine deaminase (eADA) level.14,15 Some patients, usually mildly affected familial cases, are identified after the age of 2 years and when a more severely affected family member is first diagnosed. The anemia in DBA is usually severe at the time of diagnosis, and macrocytosis is seen in a majority of patients.1,2 The bone marrow aspirate is usually normocellular, but erythroblasts are markedly decreased or absent. The other cell lines are normal, but mild to moderate neutropenia, thrombocytopenia, or both may occur later in the course of the disease. Progression of the single-lineage erythroid deficiency of DBA into pancytopenia and aplastic anemia is rare, but it does occur. In a series of 379 published cases of DBA, 8 were reported with malignant transformation, corresponding to a 2% incidence.16
Transient erythroblastopenia of childhood (TEC) is usually the only diagnosis that is confused with DBA.17 The diagnosis of TEC requires that a viral etiology, such as parvovirus B19, has been excluded. Both TEC and DBA show similar morphological features in the bone marrow, but TEC is self-limited, with a recovery usually within 5-10 weeks without recurrence.
Initial treatment in DBA is transfusions, but long-term administration of red cells may cause serious and sometimes lethal side effects due to iron overload. Corticosteroids are also administrated at the onset of disease and at least 50% of DBA patients respond.1,2,8,16 There is no known predictor of steroid responsiveness, and later relapses occur. During treatment, some patients may recover sensitivity to corticosterids or even proceed to a spontaneous remission.8,16 Allogeneic BMT is a treatment option for DBA in steroid-resistant patients, with risks that are similar to those observed for other congenital diseases.13,18 Cytokine therapy using interleukin-3 (IL-3) or EPO has been attempted for DBA.6 Only IL-3 has shown some effect in a subgroup of patients, and it has been suggested that IL-3 may play a role early in the treatment.19
Genetics
Different patterns of inheritance are observed in familial forms of DBA. Both autosomal dominant transmission and autosomal recessive inheritance are described.20–,22 In addition, there are several observations of autosomal dominant inheritance in which there appears to be a partial penetrance.4,8,22 The unpredictability of the transmission is also illustrated by families in which both mild and severe forms of anemia coexist.
Cytogenetic aberrations are rare in DBA. Only a few examples have been reported, including structural abnormalities of chromosome 1.23 An important finding was the identification of a balanced reciprocal translocation t(X;19) in a sporadic case of DBA.24 Identifying translocation breakpoints from patients with a specific rare phenotype has proven to be a powerful method for the positional identification of disease genes. Accordingly, it was hypothesized that the chromosome 19 breakpoint disrupted the function of a structural gene. Subsequent linkage mapping studies in multiplex families (i.e., families with more than one affected individual) confirmed that the chromosome 19 region did cosegregate with the disease.22 This was further supported by the identification of microdeletions in this region on chromosome 19 in a few patients affected by DBA.25 The combined results localized a gene responsible for DBA to 19q13. However, some multiplex families did not show linkage to chromosome 19, which indicates genetic heterogeneity in DBA but without correlation between the 19q13 locus and the clinical expression of the disease.25
The subsequent cloning of the 19q13 breakpoint region associated with DBA in an isolated case revealed a gene that encodes the ribosomal protein (RP) S19 associated with the ribosomal 40S subunit.26 The involvement of the RPS19 gene in DBA has been verified by the analysis of DNA from other affected individuals. Different studies identified gene mutations in a proportion of patients (up to 25%) analyzed.27–,29 The probands carry mutations that predict an altered structure or function of RPS19. The RPS19 mutations identified in multiplex families cosegregate with the DBA phenotype in consecutive generations, with an autosomal dominant mode of transmission. A variable clinical expression for RPS19 mutations is observed in several families, and the penetrance appears incomplete. Recent findings suggest that familial forms of TEC may be allelic with DBA on chromosome 19q.30 This observation is consistent with the mild clinical presentation in some obligate carriers for DBA.
Further linkage studies in DBA families without RPS19 mutations and without linkage to chromosome 19 have shown the presence of a second DBA locus on human chromosome 8p22-p23.31 In this region, no candidate gene for the disease is yet identified. There is also strong evidence for a third gene locus, as linkage analyses in some multiplex families have excluded both chromosome 19 and chromosome 8.31
Ribosomal Protein S19 in Development and Erythropoiesis
Human ribosomal protein (RP) S19 consists of 145 amino acids with highly significant homologies to diverse organisms.26 The RPS19 is 1 of 79 RPs. RPs constitute a major component of cellular proteins, but their functions, apart from being part of the ribosome, are barely known.32 As the ribosome is known to be mandatory for cellular growth, one should expect a haploinsufficiency for an RP to result in protein synthesis rate limitation in most tissues with high proliferative activity.
The finding of an RP as a cause of DBA is unexpected in view of the fact that clinical symptoms in the majority of patients are confined to the erythropoiesis, and in some cases to organs during embryogenesis. Although a human RP gene mutation is expected to have a generalized effect, there is now accumulating evidence that some RPs have a second and extraribosomal function.33 In Drosophila melanogaster, evidence for extraribosomal functions of RPs has emerged from the identification of mutations in the genes encoding RPS2, RPS6, and RPL19, respectively. The RPS2 (string-of-pearls) mutations result in an arrest of oogenesis at stage 5 of development,34 RPS6 mutations result in hypertrophied hemopoietic organs,35 and RPL19 mutants display abnormal wing blade development.36 Analogous to the Drosophila RPS2, RPS6, and RPL19 mutants, the clinical features of DBA could suggest extraribosomal and tissue-specific functions for RPS19. The 3 Drosophila mutants for RPS2, RPS6, and RPL19 also share some features with a set of about 50 other Drosophila mutations designated Minute.37 Flies with the Minute phenotype have delayed larval development, diminished viability, reduced body size, decreased fertility, and thin bristles. Several Minute genes have been identified; all encode RPs. In view of the Drosophila Minute phenotype, it is tempting to speculate that the unspecific features frequently associated with DBA (e.g., short stature) reflect a general dysfunction of the translational apparatus or a second function for this RP. There is now evidence for the existence of extraribosomal RPS19 in mammalian cells. In vitro studies suggest that RPS19 may form a homodimer that can act as a chemotactic factor.38,39 In addition, free intracellular RPS19 can interact with fibroblast growth factor 2 (FGF-2) in NIH3T3 cells.40 FGF-2 is involved in the differentiation process of different cell types. An interaction between FGF-2 and RPS19 may suggest a link for RPS19 in embryogenesis.40
Using DNA sequence analysis of RPS19 in patients with DBA, several studies have identified mutations in 25% or less of these patients, with similar numbers for sporadic and familiar cases. The low proportion of RPS19 mutations may have several explanations. One possibility is that mutations in regulatory elements or in other noncoding regions of the RPS19 gene are involved. Furthermore, small deletions involving one or both primers used to generate sequencing templates from genomic DNA may have escaped identification. A more likely possibility is that the DBA phenotype is caused by genes unlinked to chromosome 19q13.
The phenotype associated with RPS19-deficient DBA may result from haploinsuffiency of a ribosomal function and/or altered extraribosomal functions. Whether a partial DBA phenotype, or the entire complex of clinical features, is caused by either one or both of these mechanisms remains to be clarified. The hematological observations in DBA patients suggest that RPS19 may be involved in the developmental program of early definitive erythroid progenitor cells. This is strongly supported by the results from gene transfer of the RPS19 gene into CD34+ cells from RPS19-deficient DBA patients. Expression of the normal RPS19 transgene increases the number of BFU-E and CFU-E several times over.41 Interestingly, a recent study shows that RPS19 mRNA and protein expression decrease during terminal erythroid differentiation.42
Erythroid commitment involves the actions of several regulatory factors, including TAL1, LMO2, GATA-2, and GATA-3.43 A principal extracellular regulator of erythropoiesis is erythropoietin, which activates specific intracellular kinases through the dimerized erythropoietin receptor. These kinases include Janus family tyrosine protein kinase 2 (JAK2), phosphoinositol-3 (PI3) kinase, and mitogen-activated protein kinase. Substrates for these kinases are tyrosines in the erythropoietin receptors themselves as well as signal transducer and transcription activator proteins. At a later stage in erythroid-specific differentiation, additional transcription factors are required, including GATA-1 and FOG-1.44 One hypothesis is that extraribosomal RPS19 may participate in a critical multiprotein complex, possibly involving one or several known factors in the commitment and early stage of erythropoiesis (Figure 3 ). However, such a role for RPS19 remains to be clarified in the poorly understood mechanisms behind erythroid differentiation.
Conclusions
The molecular background of DBA is likely to be heterogeneous. The bone marrow characteristics in DBA range from total erythroid aplasia to a maturation arrest of proerythroblasts. The different mutant genes are expected to be of importance for erythroid commitment and early differentiation. Lessons from other diseases, including FA, tell us that mutant proteins in a common metabolic pathway may result in similar phenotypes.45
A better understanding of mechanisms for the commitment to the erythroid lineage may reveal novel candidate proteins behind DBA. Functional genomics has also revealed genetic systems in other mammals that, in parallel, may be used for the identification of mutants in erythroid differentiation. Clarification of the molecular mechanisms behind such mutants will require biochemical analyses of protein-protein interactions and the identification of molecules involved in early erythroid differentiation, including those in the intracellular response to erythropoietin. Novel candidate genes and gene regions behind DBA may also be identified by further genetic studies of affected individuals and families.
The link between erythropoiesis and RPS19, the only factor identified in the pathogenesis of DBA, remains to be clarified. This will require a combination of experimental approaches, including studies of animal models deficient for RPS19 and other specific proteins in erythroid differentiation. Animal models are also important for future therapeutic trials of DBA, including studies of gene transfer.41
III. Clinical Approach to Bone Marrow Failure in Children
Eva C. Guinan, MD,*
Dana-Farber Cancer Institute, Department of Pediatrics Oncology, Children’s Hospital Boston, Division of Hematology/Oncology, 44 Binney St., Dana 367, Boston, MA 02115
Bone marrow failure syndromes consist of a number of diverse diseases, initially presenting with either single lineage or multilineage cytopenia. A routine approach can be taken to the evaluation of these patients, and a diagnostic algorithm for pancytopenia is available in many standard texts.1 In applying this algorithm to children and young adults, the diagnostic evaluation must include the evaluation of congenital bone marrow failure syndromes and should consider myelodysplasia. These diagnoses have implications for family donor testing as well as for treatment options.
The following case description and ensuing questions and answers are not meant to be inclusive. Rather, because the absolute number of children with marrow failure is small and thus the number presenting to any pediatric hematology practice limited, we have chosen to highlight important issues and those areas where new information and approaches will rapidly be incorporated into practice.
Case 1
A 13-year-old girl presents to the emergency room after playing full-court basketball with what is described as funny red dots on her feet and soles. She has not had any other recent medical problem. A physical exam reveals petechiae on the dorsum and plantar surfaces of her feet and pallor. Laboratory studies obtained urgently include a hemoglobin of 4 g/dL, an MCV of 103, a WBC of 4,000/μL with absolute neutrophil count of 1100/μL and a platelet count of 7,000/μL. Coagulation studies are all within normal limits, as are tests of liver and renal function. A bone marrow aspirate and biopsy are obtained.
What findings on physical exam might help to establish a diagnosis?
Because the diagnosis of a congenital marrow failure syndrome has implications for genetic counseling, donor ascertainment, treatment triage and patient/family education, attention to the details of examination is important. In addition to abnormalities of the radial anlage and renal anomalies, short stature, skeletal, otic and cardiac abnormalities, and hypogonadism are often found in virtually all marrow failure syndromes. Also, there are some physical findings that may suggest a particular bone marrow failure syndrome. Findings of epiphora, esophageal stricture, premature graying of the hair and phimosis, as well as nail dystrophy and leukoplakia, suggest a diagnosis of dyskeratosis congenital (DC). In this regard, it is critical to remember that females such as this patient are at risk for DC, albeit at low risk for clinical presentation of the X-linked form caused by dyskerin mutations. Pigment abnormalities, including diffuse hyperpigmentation and café au lait spots, should prompt consideration of FA. Craniofacial abnormalities, including jaw/dental findings, various eye anomalies and pterygium colli, are often seen in DBA4,5 (although these may be seen in FA2 and DC as well). Central nervous system anomalies, including cerebellar and cerebral atrophy, occur with some frequency in amegakaryocytic thrombocytopenia.6
It is also important to recognize that substantial numbers of patients will have no characteristic physical finding. An analysis of the International Fanconi Anemia Registry found that nearly 40% of patients had no reported findings.2,3 As DNA testing for diagnosis becomes widespread, this proportion is likely to rise. Interestingly, the correct diagnosis of patients with obvious congenital physical anomalies is often delayed until the appearance of hematologic abnormalities. Physical examination, when abnormal, should therefore prompt a diagnostic evaluation for a congenital marrow failure syndrome and, conversely, a normal physical examination should not be used to exclude these diagnoses.
What history might help to distinguish the diagnostic possibilities?
Family history is often confined to questioning about similar hematologic disorders or consanguinity in the family. Obviously, a like syndrome in a sibling is highly suggestive of an inherited disorder. While a history of multiple family members with any of the above physical findings is provocative, concordance of presence, severity and/or type of defect within families appears uncommon.2 A broader approach is indicated. For example, it is becoming recognized that patients with congenital marrow failure disorders and, therefore, undiagnosed cases within the extended kindred have an excess incidence of cancer. Not only is there a predilection for myelodysplasia and acute myeloid leukemia,5,7,8 but a history of family members with any early-onset malignancy—particularly squamous cell carcinoma (both of the head and neck and the genitourinary tract), liver cancer, or (likely) gastrointestinal or breast cancers—should be actively pursued.7,9 A careful history taken from the patient’s mother may reveal frequent miscarriages5 and/or babies small for their gestational age.
Do hematologic parameters distinguish between acquired or congenital marrow failure?
Although trilineage cytopenia and aplastic anemia (AA) are generally seen as being equivalent, the absence of absolute neutropenia in this patient does not exclude the diagnosis. The diagnosis of acquired AA is most appropriately made by using accepted hematologic criteria. Among the most widely used are the Camitta criteria: (1) marrow cellularity < 25% overall or < 50% with < 30% hematopoiesis; and (2) two of the following: neutrophil count less than 500/μL, anemia with corrected reticulocyte count < 1%, or platelet count < 20,000/μL.10 Severity is further defined by the degree of neutropenia, with severe AA generally defined by neutrophil counts < 500/μL and very severe AA by neutrophil counts < 200/μL.11 What is often referred to as moderate AA is a loosely defined entity of cytopenia that is milder than the above and accompanied by some degree of marrow hypoplasia. These criteria are exceedingly important because they have been used to define the populations studied in establishing the efficacy and toxicity profile of each potential therapy and have become the major predictors of outcome.
While macrocytosis is often seen and associated with congenital marrow failure syndromes, notably FA and DBA, it need not be present. A large retrospective analysis of patients with DBA found that while untransfused patients 5 months of age or older were frequently more macrocytic at diagnosis than their age-matched counterparts in the general population, this was not true of younger children. In patients under age 5 months, the MCV was almost always in the normal range for age.5 Conversely, the reticulocytopenia of AA does not always result in normocytic indices; a fair proportion of patients will be macrocytic.12 It is felt that this may result from high erythropoietin levels driving the residual compromised red cell production.
Are there marrow findings that can distinguish between different etiologies for marrow failure?
The histology of marrow failure syndromes may be nonspecific. Acquired AA can appear modestly dysplastic. The small, hypolobulated megakaryocytes characteristic of amegakaryocytic thrombocytopenia are seen in some myelodysplasias. The practicing hematologist should be particularly aware of the difficulties in distinguishing so-called hypoplastic myelodysplasia from AA. While the observation of ringed sideroblasts, Auer rods, or excess blasts defines the presence of hematologic malignancy, the observation of hypoplasia and mild to moderate dyserythropoiesis and/or dysmyelopoiesis presents a more challenging situation. Such findings may suggest the diagnosis of myelodysplasia but are quite common in marrow failure. Also, patients with marrow histology entirely consistent with AA have been found to have clonal chromosomal abnormalities consistent with myelodysplasia.13 The implication of clonal chromosomal findings or myelodysplastic histology is unclear at present, particularly because there are patients with both acquired and congenital marrow failure in whom these findings have been transient or stable for protracted periods.1,18 Recent studies suggest that immunosuppressive agents, such as those used in treatment of AA, may also be effective in myelodysplasia (particularly refractory anemia), and thus reinforce the difficulties that may be encountered in distinguishing a diagnosis in this setting.14–,16 Specific cytogenetic abnormalities, such as involvement of chromosome 1 in FA7 or presence of isochrome 7 in Shwachman-Diamond syndrome (SDS),17,18 may portend a more prolonged course prior to potential leukemic conversion. This literature is certain to evolve over the next years and should be followed closely to aid in clinical management.
What novel tests can help to establish a specific diagnosis?
There are no molecular assays that establish the diagnosis of either acquired AA or SDS. AA remains a diagnosis of exclusion in which the patient’s clinical picture, hematologic parameters, and bone marrow histology fulfill the commonly accepted diagnostic criteria. SDS may be characterized by abnormal marrow stromal function in addition to the hematopoietic defect in in vitro assays, but these are unwieldy for diagnostic purposes and the divergence of different congenital syndromes in these assays is not established.8
While there is significant confusion regarding the importance of detecting evidence of paroxysmal nocturnal hemoglobinuria (PNH) during the diagnostic evaluation,19–,21 the assays have improved significantly. Flow cytometric analysis detects evidence of PNH, defined by absence of glycophosphatidylinositol (GPI) anchored cell surface proteins as measured by CD55/59, in up to 50% of patients diagnosed with AA.22 If recently transfused, care must be taken to make sure the flow cytometry is gated to assess GPI-linked antigens on leukocytes. The GPI anchor is also the receptor for the channel-forming protein aerolysin; in one study, aerolysin-based assays demonstrated detectable PNH cells in more than 60% of aplastic patients.23
Identification of molecular pathogenesis of some marrow failure syndromes coupled with technologic innovation is leading to the development of diagnostic tools that are likely to move into clinical practice in the near future. In addition to traditional testing for chromosomal breakage in the presence of either mitomycin C or DEB, the diagnosis of FA can be both established and refined using additional techniques. As discussed in Section I, the recent molecular characterization of the multiple proteins in the Fanconi pathway permitted the development of an immunoblot assay that can detect whether that pathway is intact. If the pathway is not intact, retroviral gene transfer of specific genes can potentially be used to correct function and precisely define the defective gene and thus the Fanconi subtype. In addition to defining the molecular subtype, the immunoblot approach lends itself to possible screening of larger numbers of patients than the labor-intensive chromosomal breakage assay. Parenthetically, this approach can also be used to screen for acquired abnormalities in the Fanconi pathway that may contribute to oncogenesis in the general population.
Abnormalities of the c-mpl gene have been found in some patients with congenital amegakaryocytic thrombocytopenia24 and, recently, abnormalities of two genes have been linked with the diagnosis of dyskeratosis congenita.25–,27 As a result, sequence analysis of c-mpl, dyskerin and telomerase RNA (hTR) genes can now be performed to aid in diagnosis, although it remains unknown what proportion of patients, respectively, bear one of these mutations. Similarly, abnormalities of the RPS19 gene,28– 30 as discussed in Section II, are found in approximately 25% of patients with DBA and can, when present, be used to establish this diagnosis.
It will be particularly important to follow literature linking specific genotype to clinical phenotype and outcome. These relationships are still unclear, and patients (and siblings) who have the identical mutations appear to have different clinical expression of hematologic and other somatic findings and divergent natural histories.28,29,31
Case 1, continued
While in the emergency room, the patient has an episode of epistaxis. Direct pressure is applied and the rate of bleeding slows but does not completely resolve.
What approach should be taken to transfusion in the emergency room? After discharge?
Regardless of the choice of therapy, some elements of supportive care apply to all patients with marrow failure. In general, blood products should be used sparingly in order to minimize allosensitization, iron overload, and infection transmission. Family members should not be donors of blood cell products, as sensitization to minor antigens on passenger leukocytes, for example, might preclude successful stem cell transplantation (SCT). Normal procedures to minimize allosensitization from all products should be followed. The use of apheresis platelets may minimize donor exposure. A randomized, blinded study in patients receiving induction chemotherapy for acute myeloid leukemia found similar allosensitization rates for patients receiving either leukoreduced apheresis platelets or pooled platelet concentrates.32 However, the effects of concomitant chemotherapy make generalization of these results to the marrow failure population questionable. Refractoriness to platelets is a significant and potentially devastating complication with few management options. Nonetheless, potential sensitization must be balanced with consideration of bleeding risk. The central nervous system is, of course, the most dramatic and potentially devastating site of bleeding, and the hypertension caused by both calcineurin inhibitors and corticosteroids may exacerbate this risk. Patients on steroids are also at risk for gastritis and associated local hemorrhage. No consensus exists as to the best threshold for transfusion to avoid these complications. Withholding platelet transfusions until the platelet count falls to 5,000-10,000/μL in stable outpatients with AA has been shown to be both feasible and safe.33 This decision should be individualized for each patient, with consideration for chronicity, blood pressure, concomitant medications, and activity level. Prior performance is also an important indicator: many patients do not bleed at counts chronically but stably in the 5,000-15,000/μL range, while others bleed reproducibly at twice that level. Attention to good oral hygiene to minimize oral bleeding, use of stool softeners to minimize gastrointestinal bleeding, management of treatment-related hypertension, prophylaxis of menstrual bleeding by an appropriate therapy, and counseling with regard to activity and medication side effects are all important contributors to minimizing hemorrhagic complications.
Case 1, continued
The diagnostic evaluation does not reveal a specific diagnosis, and the patient and family are told that the patient has acquired AA. The therapeutic possibilities introduced to the family focus on watchful waiting and transfusions, transplantation, and immunosuppression.
What differences in acute and chronic toxicity should be pointed out to assist the family with a choice of therapy?
While other therapies exist and will be developed, the standard choice of treatment for newly diagnosed patients with severe AA is SCT or multiagent immunosuppression. Numerous comparisons have been undertaken.34,35 Given the current excellent 5-year survival rates with either approach, much of the decision is a matter of patient preference coupled with biological variables (timely availability of a suitable donor) and disease variables (severity, requirement for transfusions, treatment tolerability). A complete review of this topic is beyond the scope of this paper. In brief, results of SCT have improved over the past decades.36,37 Among 1699 patients reported to the International Bone Marrow Transplant Registry as having undergone matched sibling transplantation for AA between 1991 and 1997, the 5-year probabilities of survival ranged from 75% ± 3% for patients under 21, to 68% ± 4% for those aged 21-39 years, and 35% ± 18% for those over 40 years old. Factors associated with improved survival include younger patient age, decreased disease duration prior to SCT, a minimal history of prior transfusion and good clinical status, suggesting that early in the course of disease a management plan for excellent supportive care and rapid diagnostic evaluation and donor ascertainment should be put in place. Use of alternative donors, whether mismatched family members or unrelated marrow or cord blood donors, has resulted in inferior results to those obtained with matched siblings.34–,36,38 Presumably, the increased interval between diagnosis and SCT due to use of other therapies and time involved in finding unrelated donors contributes to declines in clinical condition, increased transfusions, iron-loading, and other factors that negatively impact outcome. As there has been substantial improvement in the outcome of alternative donor SCT for AA, this therapeutic option should perhaps be considered earlier in management than is the current standard of care.
It is most important to recognize that overall disease-free survival is only one parameter by which outcome is assessed. SCT for AA remains plagued by a rate of graft failure which has fallen over the years but persists at 5%, despite the advent of leukofiltration and intensification of the conditioning regimen for the highest risk children.37,39,40 In addition to graft rejection and other acute toxicities of SCT, long-term survivors of allogeneic SCT for AA may suffer additional complications including treatment-related malignancies, endorgan toxicities related to the conditioning regimen and various mild or severe sequelae of chronic GVHD.36,41 The fertility of this group of patients is poorly characterized, although fertility can be preserved after many commonly used conditioning regimens for AA. Quality of life is reported to be normal for the majority of long-term survivors.36,41,42
The mainstay of therapy for patients without a suitable donor or those who choose not to pursue SCT is immunosuppression. Both large multicenter and small single-center studies have demonstrated the efficacy of various antilymphocyte globulin (ALG) or ATG preparations.1 Rates of response (variably defined as any improvement or bi- or tri-lineage improvement to some level or transfusion independence) range from 24-85%. Other immunosuppressive agents have also demonstrated some efficacy in AA, yet none with the robustness of the various antithymocyte reagents. The addition of cyclosporin to ATG or ALG, with or without corticosteroids, has further improved the response rate.43 Even so, substantial numbers of patients require ongoing therapy, and declines in counts when cyclosporin is discontinued are common. A review of the potential additive effects of colony-stimulating factors, corticosteroids, and androgens lies outside the scope of this presentation.
The critical issue is that the majority of patients who respond to multiagent immunosuppression have persistently abnormal hematopoiesis despite quantitative improvement in cell production, and the relapse rate is substantial.1,44,45 Between 30-60% of patients may suffer substantial decline in counts after an initial response.43,46 Patients who have a decline in counts or frank recurrence may respond to an increased dose or reinstitution of cyclosporin. Repeat courses of immunosuppresion (ATG/ALG) may also be attempted.47 Responses have been observed in up to half of patients so treated without undue side effects. Patients treated with multiagent immunosuppression are subject to a variety of side effects. Acute side effects are those that are attributable to each agent alone. Further important concerns arise in examining the literature on long-term outcome. The frequency and severity of late-steroid induced side effects on bone, including avascular necrosis and osteoporosis, are likely underestimated.41,48 As noted above, there is an increased risk of developing clonal hematopoietic disease. In one large analysis of 860 patients treated with immunosuppression, there were 11 cases of myelodysplasia (and not all patients have histologic monitoring of the bone marrow) and 15 cases of acute leukemia as well as 7 solid tumors and a non-Hodgkin’s lymphoma.49 The cumulative incidence of developing a malignancy was nearly 20% by 10 years. The risk of developing a clonal hematopoietic disorder was even higher (33% at 10 years) in a closely followed cohort of 122 NIH patients.1
Case 1, continued
Alternatively, the diagnostic evaluation demonstrates an abnormal DEB test and immunoblotting defines an abnormality of FANC-A. The therapeutic possibilities introduced to the family include observation and supportive care, androgens, CSFs, transplantation and potential gene therapy.
What medical issues in addition to hematologic concerns should be voiced to the family as they begin to evaluate therapeutic opportunities?
In this disorder, age, transfusion history, prior treatment history, disease status, physical functioning and, as it becomes established, more novel information such as genotype, all need to be considered in evaluating the potential choices of therapy and their relative risks and benefits. To date, the only curative therapy for the pancytopenia of patients with FA remains SCT. Those with an unaffected matched sibling donor may have prolonged survival rates upwards of 80%.50–,52 Outcomes of alternative donor SCT have been considerably less encouraging.50,53,54 Other therapies, including androgens, colony stimulating factors, and transfusions, have variable efficacy and are palliative with respect to hematologic status.
Improved supportive care and therapeutic options for patients with FA and other congenital marrow failure syndromes have resulted in the identification of other medical issues that must be addressed as part of the successful care of these patients, whether or not they go on to SCT. The most dramatic of these is the increased risk of malignancy. In addition to myelodysplasia and acute myeloid leukemia, patients with FA are at risk for developing squamous cell carcinomas of the head and neck, skin, GI tract, and genital tract.7 The average age of presentation is much younger than in the general population. New technologies should be considered as adjuncts to care; for example, computer-assisted evaluation of oral brushings for cellular atypia55 may be of particular value to FA and DC patients but has not yet been prospectively evaluated in this population. Other solid tumors of the breast, GI tract, and central nervous system are also overrepresented.7 Prospective studies of mammography are not yet available. Endocrine abnormalities, including growth hormone insufficiency, hypothyroidism, glucose intolerance, hyperinsulinism, and diabetes, were found in one prospective analysis of FA patients.56 Thus, not only must patients be screened for these medical complications but the effects of treatments on patients with these underlying predispositions (e.g., steroids on glucose tolerance, iron-overload from transfusion) must be considered.
Our patient, at 13 years old, should have a thorough physical exam, including pelvic, to search for physical abnormalities and/or evidence of malignancy. Any issues should be addressed and the relevance of any findings for current and future therapy should be thoroughly reviewed with the patient and family. For example, although FA patients may have premature menopause, normal cycling may also occur. A management algorithm for menstrual prophylaxis should be developed appropriate to degree of thrombocytopenia. Counseling regarding sexual activity should be initiated. Some sexual activities may have increased risk in neutropenic and thrombocytopenic patients. Barrier methods of contraception may be particularly pertinent in this patient already at risk for cervical and vulvar dysplasia and malignancy. FA patients have diminished, but not absent, fertility and with appropriate support can have successful pregnancies.57 As this patient matures, these issues become extremely important and enter into consideration of therapy. A well-developed plan for prospective, regular monitoring and patient education that recognizes the transition from pediatric patient to independent adult is critical to the longitudinal care of such a patient.
Summary
Today’s marrow failure patients can expect, on average, to have markedly improved response to treatment and survival in comparison to past decades. Accurate diagnosis through recognition of associated physical anomalies, family and past medical history, and incorporation of appropriate diagnostic tests must be emphasized, particularly in children and young adults. This will allow for proper individualized management. There are many therapeutic options with variable efficacy and costs of treatment in terms of end-organ toxicity, secondary malignancy, and altered quality of life. Increased understanding of the natural history of these disorders has also disclosed many chronic issues that were previously underappreciated, and more of these are likely to surface in coming years.
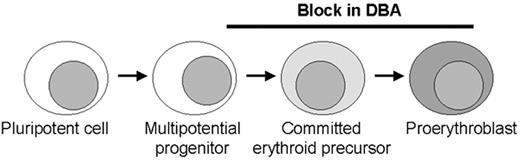
Model of erythroid production with possible sites of blockage (black bar) in Diamond-Blackfan anemia (DBA) along the pathway from early commitment and proliferation.
Most patients have no or very few recognizable erythroid elements whereas some patients retain a relatively normal number of erythroid precursors with an arrest at the proerythroblast stage. These stages in erythroid development require different molecules, including transcription factors and proteins in the EPO signalling pathway that may be involved in the pathogenesis of DBA. The role for ribosomal protein S19 at the stages of blockage is unclear.
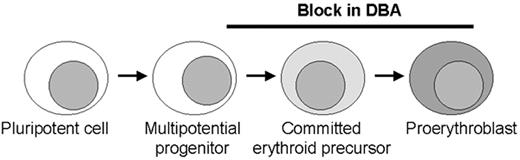
Model of erythroid production with possible sites of blockage (black bar) in Diamond-Blackfan anemia (DBA) along the pathway from early commitment and proliferation.
Most patients have no or very few recognizable erythroid elements whereas some patients retain a relatively normal number of erythroid precursors with an arrest at the proerythroblast stage. These stages in erythroid development require different molecules, including transcription factors and proteins in the EPO signalling pathway that may be involved in the pathogenesis of DBA. The role for ribosomal protein S19 at the stages of blockage is unclear.