Abstract
Hodgkin’s lymphomas belong to the most curable tumor diseases in adults. About 80% of patients in all anatomical stages and of all histological subtypes can be cured with modern treatment strategies. In spite of the great clinical progress, the pathogenesis of this peculiar lymphoproliferative entity has not been elucidated completely up until now.
In Section I Drs. Stein, Hummel, and Zollinger describe the different pro-proliferative and antiapoptotic pathways and molecules involved in the transformation of the germinal center B-lymphocyte to the malignant Hodgkin-Reed-Sternberg cell. They use a comprehensive gene expression profiling (Affymetrix gene chip U133A) on B- and T-Hodgkin cell lines and state that the cell of origin is not the dominant determinant of the Hodgkin cell phenotype, but the transforming event. H-RS cells lack specific functional markers (B-T-cell receptors) and physiologically should undergo apoptosis. Why they do not is unclear and a matter of intensive ongoing research.
In Section II Dr. Diehl summarizes the commonly used primary treatment strategies adapted to prognostic strata in early, intermediate and advanced anatomical stages using increasing intensities of chemotherapy (two, four, eight courses of chemotherapy such as ABVD) and additive radiation with decreased doses and field size. ABVD is without doubt the gold standard for early and intermediate stages, but its role as the standard regimen for advanced stages is challenged by recent data with time- and dose-intensified regimens such as the escalated BEACOPP, demonstrating superiority over COPP/ABVD (equivalent to ABVD) for FFTF and OS in all risk strata according to the International Prognostic Score.
In Section III, Dr. Connors states that fortunately there is a considerably decreased need for salvage strategies in Hodgkin’s lymphomas since primary treatment results in a more than 80% tumor control. Nevertheless, a significant number of patients experience either a tumor refractory to therapy or an early or late relapse. Therefore, one of the continuing challenges in the care for Hodgkin’s lymphomas today is to find effective modes for a second tumor control. High-dose chemotherapy followed by autologous stem cell support has proved to be the treatment of choice when disseminated tumors recur after primary chemo- and or radiotherapy. Nodal relapses respond well to local radiation when they recur outfield of primary radiation without B-symptoms and in stages I–II at relapse. Allogeneic stem cell support needs further intensive evaluation in controlled studies to become an established alternative.
I. Classical Hodgkin’s Lymphoma: Gene Expression Profiling and Biologic Risk
Harald Stein, MD,* Michael Hummel, PhD, and Raphael Zollinger, Cand. Biol.
Institut fur Pathologie, Univ. Veinikum Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany
Hodgkin’s lymphoma (HL), which accounts for approximately 30% of all malignant lymphomas, is composed of two different disease entities: the rare lymphocyte-predominant Hodgkin’s lymphoma (LPHL), making up approximately 5% of cases, and the more frequent classical HL, representing approximately 95% of all HLs. A common factor of both HL types is that neoplastic cells constitute only a small minority of the cells in the affected tissue, often corresponding to less than 2% of the total tumor load.1 This makes the tumor cells of HLs difficult to study. Because the tumor cells are so rare, large scale gene expression profiling has, so far, only been possible with cell lines derived from HLs. Currently, HL-derived cell lines are only available from classical HL, and therefore the report is restricted to classical HL. However, to link the gene expression data to the in vivo situation we also include data which were obtained by “conventional techniques” such as immunohistochemistry in conjunction with monoclonal antibodies, in situ hybridization, and polymerase chain reaction in this report.
Features of Classical Hodgkin Lymphoma
Classical HL (cHL) is a fatal disease with 90% of untreated patients dying within 2 to 3 years. With modern polychemotherapy, more than 80% of patients suffering from cHL are cured. Despite this treatment success rate, the pathogenesis of cHL is still largely unknown. What is known is that cHL nearly selectively arises and disseminates in lymph nodes, and that the cellular origin of the tumor cells of cHL, designated Hodgkin-Reed-Sternberg (HRS) cells, is not homogeneous. The vast majority (> 98%) are derived from germinal center or postgerminal center B cells and a very small minority (< 2%) from T cells.2–,5 It has also been shown that HRS cells do not resemble their normal cellular counterparts morphologically or immunophenotypically. The antigens specific or characteristic for B cells or T cells are more or less completely missing in the majority of cases, with the HRS cells acquiring a number of antigens (CD30, CD15, CD70, TARC, IRF4 [MUM1], etc.) which are not usually expressed by normal B cells or T cells. An intriguing characteristic of B-type HRS cells is their consistent inability to transcribe Ig, despite the presence of functional immunoglobulin (Ig) gene rearrangements in the majority of cases.2,4,6– 8 Since normal B cells die of apoptosis if they lose their capacity to express Ig, the HRS cells’ inability for Ig transcription points to a deregulation of the apoptotic pathway in these cells.
Questions to Be Answered
In view of the above findings, the question arose as to whether large scale expression profiling can throw any light on the above-mentioned characteristics of HRS cells. The concern that primary HRS cells present in tissue biopsies might differ too much from cultured HRS cells is arbitrary, since the cHL cell lines referred to in this report, in many aspects, display a very close similarity to in situ (primary) HRS cells. We are therefore confident that the cHL cell lines L1236, L428, KM-H2, L540, and HDLM2 used in this and other similar studies represent true cHL cell lines. Below, each of the above described characteristics of the HRS cells is addressed separately. The study was undertaken by using U133A Affymetrix GeneChips to analyze 15 B-non-Hodgkin’s lymphoma (NHL), 9 T-NHL, 5 plasmacytoma, and 5 cHL cell lines.
Do classical HL of B-cell and classical HL of T-cell type represent one disease or different diseases like B-NHL and T-NHL?
Two-dimensional clustering involving 46 highly B-cell and T-cell characteristic genes revealed that the 3 B-type cHL cell lines (L428, KM-H2 and L1236) had lost nearly all B-cell characteristic antigens (Figure 1; see Appendix, page TK). A similar finding was obtained for the 2 T-type cHL cell lines (L540 and HDML-2) in that they had lost their T-cell identity (Figure 1; see Appendix, page 614). Furthermore, with the same gene set, the different cHL cell lines proved to be indistinguishable from each other, confirming previous findings9,10 that B-type and T-type cHLs represent a single distinct entity irrespective of their B- or T-cell origin. It also implies that—as opposed to NHL—the cell of origin is not the dominant determinant for the phenotype of HRS cells, but the transforming event.
However, when we searched for genes that are differentially expressed in the B-type cHL and the T-type cHL cell lines, we found differences between the B-type and T-type cHL cell lines (Figure 2; see Appendix, page 614). The differences, however, were relatively minor because only 29 genes from more than 22,000 studied were found to be different. Hence it follows that B-type and T-type cHLs are very closely related, but probably do not represent identical disease entities. This conclusion raises the question of whether the B-type and T-type cHL cases also differ in clinical behavior and outcome. This question cannot regrettably be answered yet because clinical data are not available on the T-type cHL due to its rarity, and the only very recent identification of the latter type.
How closely are HRS cells related to plasma cells?
The extension of our hierarchical cluster analysis involving 46 B-cell and T-cell typical genes to plasmacytoma cell lines disclosed that the cHL cell lines, as well as plasmacytoma cell lines, displayed a near complete loss of B-cell typical characteristics in the absence of T-cell typical genes and thus cluster very closely together (Figure 3; see Appendix, page 615). This could suggest a close relationship of the cultured HRS cells to plasma cells. This possibility prompted us to extend our analysis to the identification of differentially expressed genes in cultured HRS cell line cells and plasmacytoma cell lines. This search disclosed at least 216 significantly differentially expressed genes (Figure 4; see Appendix, page 615) indicating that the relationship between HRS cells and plasma cells is not as close as assumed from the consideration of only B-cell and T-cell typical genes (Figures 1 and 3).
Does the extinction of the B-cell gene expression program in HRS cell reflect a physiological differentiation program like the one seen in plasma cells?
Despite the differences between HRS and plasma cells described above, the question whether the extinction of the B-cell gene expression program in HRS cells is regulated by the same mechanism as in plasma cells appears to be justified. This is supported by the fact that HRS cells share a similarly high expression of several genes such as IRF4/MUM-1.11 IRF4/MUM-1 is a transcription factor that is consistently expressed in all normal and neoplastic plasma cells. Its extinction in mice blocks the differentiation of B-cells into plasma cells,12 indicating that IRF4/MUM-1 is significantly involved in the regulation of the differentiation of B cells toward plasma cells. Recent studies have shown that the physiological differentiation of plasma cells is associated with the expression of another important transcription factor, Blimp-1, and a complete downregulation of PAX5.13 Blimp-1 has been shown to promote plasma cellular differentiation by extinguishing the gene expression of B cells while allowing the expression of important plasma cell genes such as XBP-1.13 However, our study shows that Blimp-1 is low in all cHL cell lines, and PAX5 expression is retained in primary HRS cells and in some cHL cell lines.14 These and other findings imply that the mechanism by which the B cell gene expression program is switched off in HRS cells is different from that in plasma cells, and is probably related to the pathogenic event which transforms germinal center B cells into HRS cells.
Does the gene expression program of HRS cells explain the highly preferential dissemination of classical HLs in lymph nodes?
To clarify the preferential dissemination of cHL in lymph nodes, cultured and primary HRS cells were studied for the expression of chemokines and chemokine receptors. This undertaking revealed a high overexpression of CCR7 and CXCR4 in the cHL cell lines. The overexpression of CCR7 is of special interest since in CCR7-deficient mice, the T cells do not home to lymph nodes, but to the red pulp of the spleen instead.15 A similar homing pattern shows hairy cell leukemia, a human B cell leukemia that spares lymph nodes and disseminates to the red pulp of the spleen. Interestingly gene expression profiling revealed a loss of CCR7 in the hairy cell leukemia cells (B. Falini, Perugia, Italy: personal communication). These data convincingly demonstrate that CCR7 dominantly attracts B cells and T cells to lymph nodes. Thus the overexpression of CCR7 in HRS cells appears to be an important factor in the preferential dissemination of cHLs in lymph nodes, and the usual sparing of extranodal sites. The reason for the overexpression of CCR7 could also be clarified, as it is shown to be a consequence of the over-activation of NF-κB and AP-1.16,17
Does the gene expression program of HRS cells explain the resistance to apoptosis?
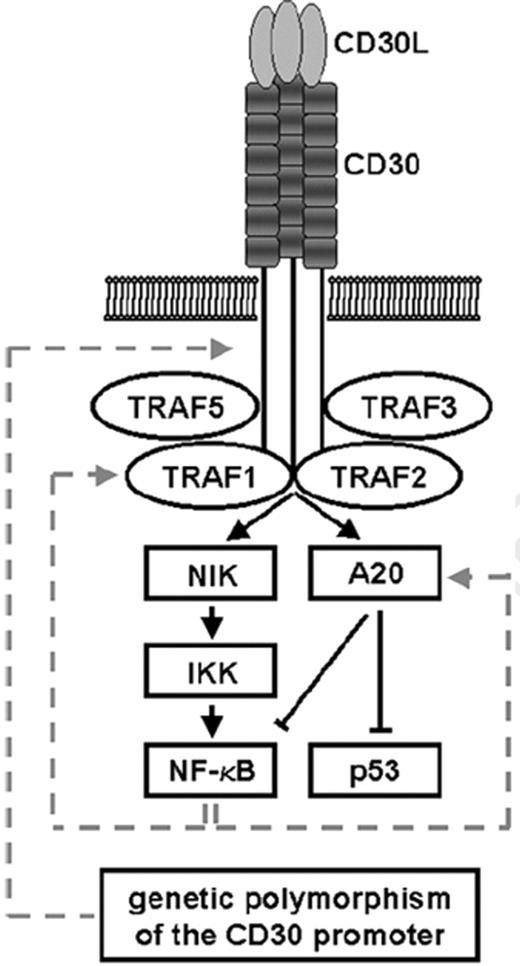
As mentioned above, B cells undergo apoptosis if they become unable to express Ig receptors on their surface. HRS cells lose the capacity to express Ig and therefore should die. The fact that this does not happen points to a deregulation of the apoptotic pathway in HRS cells. The almost selective overexpression of CD30 by HRS cells—a member of the tumor necrosis factor (TNF)-receptor family—steered attention to the TNF-signal transduction system at an early stage. At first sight, the overexpression of CD30 appears to be a paradox because activation of CD30 physiologically promotes apoptosis rather than resistance against it.18,19 However, when we and others investigated HRS cells for the expression and activity of the members of TNR-receptor signalling system, several molecules were identified in HRS cells which, directly or indirectly, inhibit apoptosis, i.e. NF-κB, TRAF1, A20, cIAP2, BCL-X, cFLIP, and others.20– 22 These findings may explain why overexpression of CD30 does not lead to apoptosis in HRS cells (Figures 5 and 6 ).
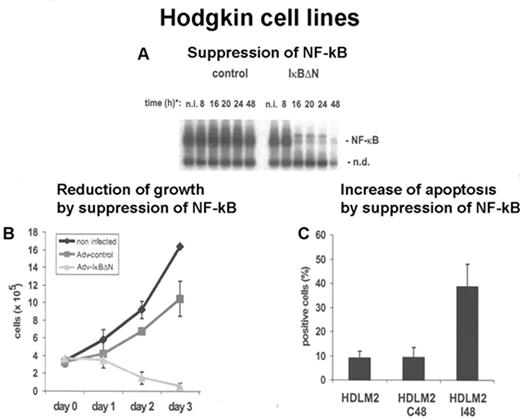
To clarify its role and to identify the target genes of NF-κB in HRS cells, cHL cell lines with suppressed and unsuppressed NF-κB activity were subjected to cHL gene expression profiling using the Affymetrix GeneChip U95A. The suppression of NF-κB activity was achieved by transfecting the dominant negative IκBΔN suppressor into cHL cell lines (Figure 7 ). The suppression of NF-κB proved to have a significant effect. It reduced the growth and increased the rate of apoptosis of cHL cell line cells (Figure 7 ). The result of the gene expression profiling shown in (Figure 8; see Appendix, page 616) revealed that a large number of genes were downregulated, and only a smaller number of genes were upregulated (data not shown). The data obtained indicate that the following regulators of apoptosis are expressed in dependence of NF-κB: IEX-1, BCL-XL, A1, CD95, cIAP2, and TRAF1.23
The identification of cIAP2 as one of the apoptosis inhibitors in HRS cells is of interest since HRS cells contain high amounts of the active form of caspase 3.24,25 cIAP2 is a direct inhibitor of caspase 3 (Figure 6 ). We were able to demonstrate that the cHL cases with active caspase 3 in the HRS cells also express large amounts, cIAP2, suggesting that HRS cells are directly protected from caspase 3–induced apoptosis by cIAP2 (Dürkop et al, unpublished data).
All the NF-κB dependent regulators of apoptosis found by NF-κB suppression experiments have antiapoptotic functions except CD95. Overexpression of CD95 triggers apoptosis rather than protects against it. To clarify this paradox, the CD95 gene was analyzed for any loss of function mutations; however, no deleterious mutations were found.26 This prompted the question as to whether CD95 is activated in HRS cells, and this indeed proved to be the case, as revealed by the detection of the death-inducing signalling complex (DISC). This complex, however, proved not only to contain the FAS-associated death domain-containing proteins (FADD), caspase 8 and caspase 10, but also the cellular FADD-like IL1B-converting enzyme inhibitory proteins (cFLIP) (Mathas et al, unpublished data). Expression studies showed that cFLIP is overexpressed in cultured and primary HRS cells.27 Thus cFLIP proves to be a candidate for the blockage of the death-inducing effect of CD95. The fact that cFLIP exerts a very strong protective effect against apoptosis in HRS cells could be demonstrated by suppression experiments. Suppression of cFLIP dramatically induced apoptosis in the HRS cell lines (Mathas et al, unpublished data). Thus cFLIP may indeed be the molecule that neutralizes the effect of activated CD95 in HRS cells.
Can the gene expression program of HRS cells explain the high sensitivity to chemotherapy?
So far we do not have any data, meaning that at the moment we can only speculate. The above mentioned studies have shown however, that there is a fine balance between proapoptotic and antiapoptotic mechanisms in HRS cells. It might well be that the drugs used in the polychemotherapy cocktails for the treatment of cHLs disturb this balance in favor of the apoptotic mechanisms. This possibility needs to be studied by further experiments.
II. Early, Intermediate, and Advanced Hodgkin’s Disease: Modern Treatment Strategies
Volker Diehl, MD,* for the German Hodgkin Lymphoma Study Group
Chair, Medizinische Klinik I, University of Cologne, Joseph Stelzmann Str. 9, 50924 Cologne, Germany
Questions to be answered:
A: Early Hodgkin’s disease (HD): Stages I + II A, B without risk factors (RF: B-symptoms, high ESR, bulk > 10 cm, LMM [≥ 1/3 of the greatest thorax cross-section]), E-stage
Is radiation therapy (RT) alone obsolete?
If combination chemotherapy (CT)/RT:
What CT, how many cycles?
RT: field and dose?
Is CT alone sufficient?
B: Early Unfavorable (Intermediate) HD: Stages I + II A, B with RF
Do we need an intermediate group?
Is combination CT-RT the gold standard?
Which CT and how many cycles?
RT: dose and field?
C: Advanced HD: Stages IIB (+ LMM or bulk > 10 cm), and stages III + IV
Do we have better RF than those of the International Prognostic Score (IPS)?
Is ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) the gold standard?
Do we need RT after effective CT?
Choice of Treatment
Prognostic factors and treatment groups in early favorable and unfavorable stage HD
In spite of an enormous effort to define clinically relevant and generally acceptable prognostic factors, there are still two major determinants for dividing HD patients for a risk- or prognosis-adapted therapy: anatomical stage, and systemic symptoms like fever, night sweat, and weight loss. A third factor has recently emerged and meets general transatlantic acceptance: massive local tumor burden, i.e., bulky disease > 10 cm in diameter. In the US, most centers still treat Hodgkin patients according to the traditional separation in “early stages” (I–IIA) and “advanced stages” (III–IVA, B and usually stage IB and IIB, bulky, i.e., > 10 cm in diameter).
In most centers or trial groups, patients with stage I–IIA, “early stages,” are treated with combined modality strategies. An exclusion is the nodular lymphocyte predominant Hodgkin’s disease (NLPHD) subtype in favorable stage IA without risk factors which can be treated by lymph node excision followed by a “wait and see” strategy or IF radiotherapy with 20–30 Gy. Patients with NLPHD stage IIIB or IV or with B symptoms or bulky (> 10 cm) disease, “advanced stages,” have been associated with the poorest prognosis and assigned an extensive chemotherapy protocol of 6 to 8 months’ duration, sometimes followed by additive radiotherapy. Their prognosis is comparable to that of patients in similar stages in the classical Hodgkin’s disease subtypes.
Further prognostic factors were often used to assign stage CS I–II patients to a more unfavorable group.
In Europe, the EORTC and the GHSG have defined CS I–II (supradiaphragmatic only) patients as unfavorable (intermediate) and assigned them to combined modality treatment if they had any of the factors depicted in Table 1 , summarizing the prognostic groups according to how the EORTC and the GHSG tailor their treatment strategies.
The Canada Clinical Trials Group and the ECOG subdivide early stage HD into a low- and a high-risk category:
Low risk: NLPHD and nodular sclerosing histology, age < 40yrs, ESR < 50, involvement of 3 or fewer disease-site regions.
High risk: all others in stages I–II, excluding bulky disease > 10cm.
The NLPHD in stage I–IIA, also described in Table 1 , are treated by the EORTC and the GHSG with IF-RT 30 Gy, stages III+IV are treated like the classical HD subtypes. NLPHD is generally being recognized as a distinct clinicopathological entity. Mostly NLPHD cases occur in early stages I A, mainly in the peripheral lymph nodes, and tend to have frequent multiple relapses, even up to 15 years, which are less aggressive and result in good survival rates.
In 2003, most centers and groups in the US as well as in Europe tend to favor combined modality treatment in early favorable stages with a moderate chemotherapy (typically 2–4 cycles of ABVD) and a reduced radiation, involved field, and dosage (typically 30–35 Gy IF).
The EORTC includes in its advanced stage cohorts stages III and IV only, without regard to other factors, as did the US National Cancer Institute and several US cooperative groups. In the GHSG, all stage III and IV patients plus stage IIB with LMM or E-lesions (extralymphatic extension of the disease that is not any more curable by radiation alone) are included in the “advanced” group. Certain other trial groups include further stage I–II B patients with bulk > 10 cm in the “advanced” prognostic group.
Figures 9 and 10 (see Appendix, page 616) demonstrate the freedom from progression (FFP) and overall survival (OS) data for the early, intermediate, and advanced stages of HD according to the experience of the GHSG in 2001. The risk-adapted treatment strategies result in freedom from treatment failure (FFTF) rates in all strata of more than 80% after 6 years’ follow-up and OS rates of more than 90%.
Prognostic factors for advanced stage HD
The International Prognostic Factor Project produced an International Prognostic Score (IPS),1 which is not necessarily completely comprehensive, but at the moment it is an internationally widely accepted and used score that can be taken as reliable.
In conclusion, the 3-level scheme of division into early favorable, early unfavorable (intermediate), and advanced stage cases remains a suitable instrument to tailor risk-adapted therapy according to current knowledge. Since clinical and biological factors up to now do not discriminate the 15%–20% of advanced stage patients who will progress during therapy or experience an early relapse (< 12 months), molecular markers—hopefully generated by the gene-expression profiling techniques—are urgently needed to save the majority of patients from overtreatment or allow even more intensive treatment for the 15%–20% of patients with resistant disease under best modern treatment modalities.
Early Stage Favorable Disease
Treatment strategies of early stage HD have changed during the past years. Until recently, extended field (EF) irradiation has been considered the standard treatment. However, due to the recognition of the high relapse rate and the fatal long-term effects, EF radiotherapy (radiation to initially involved and adjacent lymphnode areas) is now being abandoned by most study groups. Instead, for favorable early stage disease, short duration chemotherapy for control of occult lesions is combined with involved field (IF) irradiation (restricted only to initially involved lymph node areas). Most groups and centers give 4 courses of ABVD followed by IF-RT (30–35 Gy).2
Many of the ongoing and recently completed studies were developed in an attempt to reduce the long-term complications of treatment without increasing mortality from HD. These include studies that evaluate reduction of radiation dose or field size, evaluate combined modality treatment in an attempt to identify the optimal chemotherapy regimen, the optimal number of cycles of chemotherapy, and to determine the optimal radiation volume and dose when combined with chemotherapy. Table 2 summarizes the most prominent ongoing or recently terminated international trials.4-16,10– 11
Answers to the following questions (early favorable stage I–IIA,B, no RF)
Is RT alone obsolete?
If combination CT-RT:
What CT, how many cycles?
RT: field and dose?
Is CT alone sufficient?
Answer to 1: RT alone is no longer the treatment of choice in most centers in Europe and North America.
Answer to 2: Combination CT-RT is the most common treatment strategy in Europe and US. 2a) 2–4 cycles of ABVD are considered the international gold standard for early stage HD. 2b) 302–35 Gy IF is the modern standard.
Answer to 3: This problem is currently being investigated in clinical trials; the answer is pending. At the ASH meeting 2003, results of the HD-6 trial of the National Cancer Institute of Canada Clinical Trials Group (ECOG trial JHD06) will be reported.
Early Stage Unfavorable (Intermediate) HD
It is generally accepted that early stage unfavorable (intermediate) HD patients (CS/PS I and II with certain risk factors) (see Table 1 ), qualify for combined chemotherapy and radiotherapy. However, the prognostic impact of a single risk factor, the optimal chemotherapy regimen, the number of chemotherapy cycles, the field sizes, and the dosage of radiation within these fields are subjects of ongoing studies and continuing debates.
Trials to identify the best chemotherapy regimen
Based mainly on results of trials in advanced HD, ABVD has become the standard regimen for CS I–II patients. Three current trials analyze combined modality protocols comparing ABVD with more intense, novel regimens. Both the EORTC H9U and the GHSG HD11 studies are comparing 4 cycles of ABVD with 4 cycles of BEACOPP-baseline (bleomycin, etoposide, doxorubicin (Adriamycin), cyclophosphamide, vincristine, procarbazine and prednisone) and radiotherapy is limited to IF at a dose of 20 Gy or 30 Gy, respectively (see below). The ECOG 2496 trial compares 6 cycles of ABVD to 12 weeks of Stanford V, followed by radiotherapy. All these studies, except the GHSG HD11 trial, are ongoing. Interim analysis data of this trial will be reported at the 2003 meeting of the American Society of Hematology.
Radiation field and dose
In preceding studies, the GHSG had randomized responding patients in early unfavorable (intermediate) stages to either 40 Gy EF or 20 Gy EF + 20 Gy IF (HD1) with no outcome difference. In the follow-up trial (HD5), patients received 30 Gy EF + 10 Gy on bulky sites. These trials demonstrated that radiation dose in the EF can safely be reduced to at least 30 Gy (with 10 Gy on bulky tumors) when given after 2 cycles of alternating COPP/ABVD.17
The question as to whether radiation fields can be reduced to the involved sites after adequate chemotherapy was sufficiently answered by the Milan trial9 (Table 2 ) that did not differentiate between favorable and unfavorable early HD patients, and the HD 8 trial of the GHSG (Table 3 ).14 This trial compared radiotherapy of 30 Gy EF + 10 Gy to bulk (> 5 cm) and 30 Gy IF + 10 Gy to bulk after 2 alternating cycles of COPP/ABVD. The 1204 patients were randomized between 1993 and 1998. For the arm comparison, 1064 patients were informative. The median observation time was 54 months. The overall survival for all eligible patients was 91% and freedom from treatment failure (FFTF) was 83%. Comparisons of both arms showed similar rates for FFTF (85.8% and 84.2%) and OS at 5 years (90.8% and 92.4%). There were also no significant differences between the 2 arms in terms of complete remission (98.5% and 97.2%), progressive disease (0.8% and 1.9%), relapse (6.4% and 7.7%), death (8.1% and 6.4%), and secondary neoplasias (4.5% and 2.8%). In contrast, acute side effects including leukopenia, thrombocytopenia, nausea, and gastrointestinal and pharyngeal toxicity were more frequent in the EF arm. The Milan trial and the GHSG HD-8 trial comparing 30 Gy radiotherapy in EF or IF technique defines a new standard of treatment for patients in early unfavorable stage HD, i.e., 4 cycles of effective chemotherapy followed by IF radiotherapy.9,14
The shortcoming of this strategy, however, is that about 5% of those patients with intermediate stage will suffer from progressive disease while on ABVD-like chemotherapy and another 15% will relapse within the following 5 years. Despite this fact, based mainly on trials in advanced HD and its reduced acute and long-term toxicities in comparison to protocols including alkylating agents, ABVD has become the standard regimen used in patients with unfavorable (intermediate) CS I–II disease. The recently closed HD11 trial of the GHSG compared the efficacy of two different chemotherapy regimens: 4 ABVD versus 4 BEACOPP baseline, to test whether the dose-equivalent but time intensified BEACOPP baseline regimen would decrease the still unsatisfactory 102–15% relapse and progression rate after 4 ABVD in this unfavorable prognostic setting. The fourth interim analysis of this study was done in August 2003 with 1047 patients. After a median observation time of more than 28 months the FFTF rate for the total group was 90% and the OS rate was 97%. There was no difference between the 4 ABVD arm and the 4 standard BEACOPP arm, nor for the comparison between 20 Gy IFRT and 40 Gy IFRT. The GHSG in January 2003 has started the HD14 trial which compares 2 courses of intensified BEACOPP, followed by 2 courses of ABVD in comparison with 4 ABVD courses, in both arms supported by 30 Gy IF (Table 3 ). Two large randomized trials are currently evaluating whether 4 cycles of combination therapy are equally effective compared to 6 cycles of combination therapy. The EORTC H8U18 study randomized patients to combined modality with either IF or STLI radiation and 4 or 6 cycles of MOPP/ABV: neither relapse-free survival nor overall survival differed significantly among the 3 groups (Table 3 ). The EORTC H9U trial randomized patients to 4 or 6 cycles of ABVD (or 4 cycles of BEACOPP). At the first interim analysis in October 2002, an FFP rate of 90% (without arm comparison) was found.
The ongoing National Cancer Institute of Canada (NCI-C) HD6 trial evaluates HD patients in stages I+II with favorable and unfavorable disease, but excludes patients with LMM or bulky disease (> 10 cm). Patients are randomized to receive combined modality therapy with 2 cycles of ABVD followed by irradiation (an extended mantle plus splenic irradiation or mantle plus paraaortic and splenic irradiation) or 4 to 6 cycles of ABVD alone (depending upon the rapidity of response). This trial will be reported at the American Society of Hematology meeting in December 2003 and hopefully will answer the question for which subgroup of patients in this setting chemotherapy without radiation suffices.
Answers to the questions (Early unfavorable [intermediate] HD Stages I+II A,B + RF)
Do we need an intermediate group?
Is combination CT-RT the gold standard?
Which CT and how many cycles?
RT: dose and field?
Answer to 1: The EORTC, the GELA, and the GHSG continue to treat the early unfavorable (intermediate) group differently from the early favorable and advanced group. There is evidence that allocating these patients into the early stage favorable group would undertreat a certain subgroup and overtreat another if one moves them up to the advanced group. Ongoing subgroup analyses try to discriminate these special subgroups for even better custom-tailored therapy.
Answer to 2: Yes, CT+RT is the gold standard internationally at the moment.
Answer to 3: ABVD is considered the gold standard at the moment, but this assumption is challenged by the fact that 5% of patients progress under therapy and 5–10% relapse rather early, many of those appearing resistant to salvage therapy.
Answer to 4: Recent studies have shown that IF-RT after 4 courses of effective CT (typically ABVD) suffices and is less toxic and equally effective as EF-RT. Current and recently closed trials have investigated the question whether 20 Gy IF-RT is sufficient after f.e. 4 × ABVD or whether in certain risk groups one needs 30 Gy or more. CT alone has not been sufficiently tested to testify its potential to cure patients in this setting.
Advanced Stage Hodgkin’s Disease
ABVD: the second pioneer combination
In 1975, Bonadonna and colleagues introduced the ABVD regimen19 in an attempt to develop a regimen for patients whose disease had recurred after MOPP. The Milan group started to compare MOPP and ABVD, using 3 cycles of each drug combination, followed by extended field irradiation and 3 additional cycles of the same chemotherapy. This comparison demonstrated a significant superiority for ABVD with FFP rates of 63% for MOPP versus 81% for ABVD. Since both regimens were highly active and had no overlapping toxicities, it was therefore consequent to test MOPP and ABVD in various combinations to further increase cure rates.
Hybrid regimens
Investigators in Vancouver20 and Milan21 independently designed 2 hybrids of MOPP and ABVD in order to test the Goldie-Coldman hypothesis prospectively. The NCI-C compared the MOPP-ABV hybrid with alternating MOPP/ABVD in patients with stage IIIB or IV HD. At 5 years there was no significant difference in the overall survival rates between both arms; however, the hybrid regimen was associated with higher hematologic and nonhematologic toxicities.
Subsequently, large multicenter trials were started in the US and Europe to compare MOPP/ABV hybrid versus alternating MOPP/ABVD and sequential MOPP→ABVD. These multicenter trials demonstrated that MOPP/ABV hybrid was equally effective as alternating MOPP/ABVD, but more effective than sequential MOPP→ABVD.22
As a conclusion to the sequence of these comparative trials, first the CALGB, as reported by Canellos and then later the North American Intergroup, as reported by Duggan et al, have addressed the important question of whether the inclusion of MOPP in the conventional setting and scheduling add therapeutic benefit to ABVD or merely enhances toxicity.23 The authors concluded that ABVD alone is equally effective as MOPP/ABV hybrid but less toxic, and all combinations are more effective than MOPP alone. In addition, ABVD alone has the advantage of less acute toxicity, especially no sterility and few or no secondary acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS). But one has to keep in mind the cardiotoxicity due to doxorubicin and pulmonary side effects due to bleomycin if one applies 6–8 courses of ABVD, even more if one adds consolidative radiotherapy. However, at present, it is internationally accepted that ABVD should be the standard regimen against which all experimental drug combinations are tested.
New chemotherapy regimens
Stanford V, a 7-drug regimen, was developed as a short-duration, reduced-toxicity program including doxorubicin, vinblastine, mechlorethamine, bleomycin, vincristine, etoposide, and prednisone. The program was applied weekly over 12 weeks. Consolidative radiotherapy to sites of initial bulky disease was employed. In this Phase II trial, 142 patients were recruited. The estimated 5-year freedom from progression was 89% and the overall survival was 96% at a median observation time of 5.4 years in this single center study.24 An intergroup trial testing Stanford V versus ABVD has been initiated for patients with advanced HD.
Similarly, the Manchester group developed an abbreviated, 11-week chemotherapy program, VAPEC-B (vincristine, doxorubicin, prednisone, etoposide, cyclophosphamide, bleomycin). In a randomized trial VAPEC-B and the hybrid ChlVPP/EVA (Table 4 ) were compared with radiotherapy applied to previous bulk disease or residual disease.27 This study was stopped after 26 months due to a 3-fold increase in the rate of progression after VAPEC-B. After a median follow up time of 4.9 years FFP, EFS and OS were all significantly better with ChlVPP/EVA than with VAPEC-B (FFP: 82% versus 62%; EFS: 78% versus 58%; OS: 89% versus 79%, respectively).
Regimens increasing dose-intensity and dose-density
In 1992, the GHSG designed the BEACOPP regimen that used similar drugs as in the COPP/ABVD regimen, excluding velban and dacarbazin and adding etoposide, trying to increase efficacy by two modifications: dose-density and dose-intensity by squeezing the drug application to 14, respectively 9 days and recycle already on day 21 or respectively day 15.
After dosefinding and feasibility studies, the GHSG designed a 3-arm study, the HD9 trial, comparing COPP/ABVD, standard BEACOPP and escalated BEACOPP in patients with advanced HD. Radiotherapy was prescribed for bulky disease at diagnosis (30 Gy) or for residual disease (40 Gy) after 8 cycles of chemotherapy and about two thirds of patients received consolidative radiotherapy. In the final analysis in June 2001, 1201 patients were evaluated. There was a significant superiority over the COPP/ABVD arm for freedom from treatment failure with 87% for escalated BEACOPP versus BEACOPP baseline with 76% and COPP/ABVD with 69% at 5 years median observation time, a highly significant result. A major difference was observed in the rate of primary progressive disease during initial therapy which was significantly lower with escalated BEACOPP (2%) versus BEACOPP baseline (8%) and COPP/ABVD (12%) (P < .001).
The OS rates for COPP/ABVD were 83%, for BEACOPP baseline 88% and for escalated BEACOPP 91%, the survival differences were highly significant in the global test (P < .002), the survival difference between COPP/ABVD and escalated BEACOPP again reached high significance (P < .002).
As expected, escalated BEACOPP was associated with greater hematological toxicity including a higher number of red blood cell and platelet transfusions. Second malignancies, including acute myeloid leukemia possibly related to etoposide were reported, with BEACOPP escalated 9 AML/MDS, BEACOPP baseline 4 AML/MDS, and COPP/ABVD 1 AML/MDS. However, the total rate of secondary neoplasias was highest in the COPP/ABVD arm with 4.2% compared to 3.4% in the BEACOPP escalated arm. The death rates at 5 years, including all acute and late causes of deaths, were for the COPP/ABVD arm (49/260) 18.8%, for BECOPP baseline (61/469) 13%, and for escalated BEACOPP (40/460) 8.6%. That means 10 more patients out of 100 died in the COPP/ABVD arm.28
The 14-day variant of the BEACOPP-21 regimen: the BEACOPP-14
Increase in dose intensity can by obtained by 2 means: (1) increasing the dosage in the same time frame (dose-intensity), or (2) shortening the intervals between the treatment courses and shortening the timescale in which drugs are applied (dose-density).
The experiences with the high efficacy but also increased toxicity of the escalated BEACOPP principal (given in 21-day intervals) led the GHSG to consider a BEACOPP variant, in which the drug dosage and time architecture according to the effective dose model of Hasenclever et al33 would accomplish the same efficacy, but have a reduced toxicity, especially concerning the rate of AML/MDS. The result was the construction of a time intensified BEACOPP-baseline regimen given in 14-day intervals, applied with the help of granulocyte colony stimulating factor (G-CSF) support for advanced HD (BEACOPP-14) (Table 4 ).
In a multicenter pilot study with 32 centers, the GHSG tested the feasibility, toxicity, and efficacy in 99 patients in stage IIB with LMM/extranodal disease (23%), stage III/IV (77%), from July 1997 to March 2000. The final analysis with 94 evaluable patients was performed in August 2002.29
Treatment: 91% of the 94 patients received 8 cycles, 77% were given within 16 days, and 94% were given within 22 days. Seventy percent of the patients received consolidatory radiotherapy. Seven patients with initial bulky disease were not irradiated. Results: 88 patients (94%) achieved a CR, only 4 patients had progressive disease. With a median follow-up of 34 months, 5 patients relapsed, only 1 high-grade NHL developed, 3 patients died, one due to toxicity, two had progressive disease. The estimated FFTF was 90% and the OS 97% at 34 months median observation time. Toxicity: acute hematotoxicity was moderate, ranging between that of the escalated and the baseline BEACOPP-21 regime, with 75% of patients experiencing WHO grade 3 or 4 leukopenia, 23% thrombocytopenia and 65% anemia, in a few cases necessitating the use of erythropoietin or blood transfusions. In summary, treatment results with the BEACOPP-14 baseline regimen are promising and might help to treat advanced Hodgkin patients more effectively and safely.
Role of radiotherapy in advanced stage of HD
A number of Phase III trials investigated the role of consolidative radiotherapy after primary chemotherapy with divergent results. The GHSG analyzed the role of low-dose (20 Gy) involved field radiotherapy versus 2 cycles of further chemotherapy consolidation in 288 patients in CR after initial chemotherapy with COPP/ABVD. There was no significant difference in FFP or overall survival rates between the two treatment arms.
A similar approach with a potentially more active chemotherapy, BEACOPP, was performed by the GHSG (HD12 study) In this trial, patients were randomized to 8 cycles of intensified BEACOPP or 4 cycles intensified BEACOPP + 4 cycles of standard BEACOPP, followed by either radiotherapy to initial bulky and residual disease or no further treatment. The third interim analysis, in March 2003, with 908 patients after a median observation time of more than 24 months, showed an FFTF of 90% and an OS of 94% with a similar toxicity as described in the HD9 trial,15 but a reduced number of only 5 AML/MDS (0.6%) (oral presentation ASCO 2003). The comparison between the RT arm and the no RT arm showed no difference, while 13% in the no RT arm were assigned by a review panel to receive 30 Gy IF-RT due to either minor response or residual disease > 2.5 cm.
In the EORTC #20884 trial, patients with advanced stage HD achieving CR after initial 6–8 cycles of MOPP/ABV were randomly assigned to receive either involved field radiotherapy (24 Gy to all initially involved nodal areas, 16–24 Gy to all initially involved extranodal sites) or no further treatment. Those with PR after chemotherapy were treated with 30 Gy to nodal areas and 18–24 Gy to extranodal areas. Of all 739 patients included, 172 received involved field radiotherapy, 161 received no further treatment, and 250 patients with PR were treated with radiotherapy. The 5-year event-free survival and 5-year overall survival rates were 84% and 91% for patients with no further treatment and 79% and 85% in the group assigned to involved field irradiation, respectively. Among the patients with PR after chemotherapy, the 5-year event free survival rate was 97% and the 5-year overall survival was 87%.30
In the recently started HD15 trial the GHSG in advanced stages of HD compares 8 cycles of BEACOPP escalated to 6 courses of BEACOPP escalated and 8 courses of BEACOPP-14. In this trial RT is given only to PET positive residual tumors.
Answers to the questions for: Advanced HD stages IIB (+ LMM or bulk > 10 cm), and stages III, IV:
Do we have better RF as the IPS?
Is ABVD the gold standard?
Do we need RT after effective CT?
Answer to 1: IPS is still the internationally most accepted and used risk factor score for advanced HL. Many groups are working intensively to find new biologic or gene expression profiling markers for the identification of risk groups in advanced HL. The results are curiously awaited.
Answer to 2: As seen in Table 6 (data from the HD9 study of the GHSG), the early progression rate, the 5-year freedom from treatment failure and the overall survival rates were significantly inferior for the 0–2 and the 3–7 RF strata if one compares patients treated with COPP/ABVD and with escalated BEACOPP as also is demonstrated in Figure 11 .
For these reasons the GHSG has decided to treat even patients with a low-risk factor score with escalated BEACOPP. The pivotal international study headed by the EORTC comparing 8 ABVD versus 4 escalated BEACOPP + 4 baseline BEACOPP in advanced HD patients will add valid information about the feasibility, toxicity, and the equality or superiority of the regimen in question. Although there are no randomized studies comparing ABVD versus COPP/ABVD, one can assume that both regimens have similar efficacy in advanced HD. Therefore, it seems justified to take the results with COPP/ABVD in analogy to ABVD.
Answer to 3: The recently published paper by the EORTC30 demonstrated that after reaching a CR after 8 cycles of effective chemotherapy, patients with advanced HL patients do not benefit from additive RT. That said, 80% of the secondary AML/MDS in this study were seen in the RT arm. PR patients, however, had a benefit from complementary radiation and fared as well as the primary CR patients. Furthermore, the GHSG HD12 study has demonstrated that after 8 CT cycles there was no difference between the RT+ or RT− arms in an intention to treat analysis. There might be a risk group, however, which needs RT for elimination of the last tumor cell. This question is addressed in the HD 15 trial of the GHSG, where only patients with PET positive residual tumors get 30 Gy IF-RT.
Conclusion
Consolidative radiation should only be given to HD patients that only reached a partial response after 6–8 courses of anthracyclin containing chemotherapy (such as ABVD) or had a minor response (< 70%) with residual nodal lesions. Using the new dose and time intensified regimen (f.e. intensified BEACOPP) for advanced HD it seems that only a minority (< 20%) of patients need consolidative radiation to residual lesions of > 2.5 cm. PET imaging might help to discriminate between scary tissue or vital tumor tissue in residual lesions.
III. Treatment of Refractory or Relapsed Hodgkin’s Lymphoma
Joseph M. Connors, MD*
BC Cancer Agency, 600 West 10th Avenue, Vancouver BC V5Z 4E6, Canada
Treatment outcome for patients with Hodgkin’s lymphoma has steadily improved over the last half-century. Only two or three decades ago, it was common to encounter initially refractory disease or see patients relapse from apparent complete remissions. In such a circumstance, secondary treatment was a major part of the management of many patients. However, progress in primary treatment has brought dramatic change. As can be seen in Figure 12 , which depicts our experience in British Columbia over the past 4 decades, failure of initial treatment has become very uncommon. Whereas a patient treated in the 1960s had an 80% chance of subsequent progression of disease, one treated in the 1990s has less than a 20% chance of developing the same problem. The need to have a strategy for the treatment of Hodgkin’s lymphoma not cured by primary treatment remains important. Fortunately, many fewer patients must deal with this complication. In this section we will focus on the treatment of Hodgkin’s lymphoma not cured by initial treatment.
Which Patients with Hodgkin’s Lymphoma Require Secondary Treatment?
During and after initial treatment of Hodgkin’s lymphoma, it is helpful to keep in mind an estimate of the likelihood that secondary treatment will ever be needed. Patients differ in their probability of having the disease re-emerge. One simple way of estimating this risk is to focus on initial stage. Table 7 shows the risk of manifesting primarily refractory disease or relapse of disease after initial complete remission for all 711 patients with Hodgkin’s lymphoma seen in British Columbia during the 1990s. Patients are divided by stage and whether the Hodgkin’s lymphoma proved refractory to primary treatment or relapsed after it was complete. Limited stage includes only those with stage IA or IIA disease without any tumor mass exceeding 10 cm in greatest diameter. Advanced stage includes those with B symptoms, bulky disease (greatest diameter 10 cm or larger), or stage III or IV disease. Refractory means that the lymphoma progressed during primary treatment or was proven by biopsy to have persisted despite that treatment. Relapsed means the disease progressed after completion of primary treatment that resulted in a complete remission. During the 1990s, patients with limited stage lymphoma were offered brief chemotherapy (2 cycles of ABVD or equivalent) followed by radiation. Patients with advanced stage disease were offered extended chemotherapy (6 to 8 cycles of ABVD or equivalent) with radiation to initially bulky sites of nodal disease (> 10 cm). These data show very clearly that very few patients with limited stage Hodgkin’s lymphoma demonstrate refractory or relapsed disease. Thus, a need to find effective secondary treatment for Hodgkin’s lymphoma is confined almost entirely to patients presenting with advanced stage lymphoma.
Even among patients with advanced stage Hodgkin’s lymphoma, failure to cure the disease is not equally distributed across all patient subgroups. The landmark study published by the International Prognostic Factors Project on Advanced Hodgkin’s Disease1 showed that for the nearly 30% of patients with advanced stage Hodgkin’s lymphoma who present with 0 or 1 of the factors listed in Table 8 , the risk of refractory or relapsed disease is less than 20% but for the 19% of patients with 4 or more of these factors this risk exceeds 50%. By keeping these stage and prognostic model factors in mind, the clinician can maintain a readiness to identify the minority of patients with Hodgkin’s lymphoma who will demonstrate refractory or relapsed lymphoma and be prepared to intervene as early as possible.
Choice of treatment for Hodgkin’s lymphoma refractory to or relapsing after primary chemotherapy
High dose chemotherapy and irradiation plus autologous hematopoietic stem cell transplantation (HDC/HSCT) has, over the past two decades, become established as the most effective treatment for patients whose Hodgkin’s lymphoma has proven incurable with standard chemotherapy and radiation. Phase II trials, collected series from bone marrow transplantation registries2–,24 and two Phase III randomized trials25,26 have demonstrated that the effectiveness of HDC/HSCT is sufficiently clear that HDC/HSCT has become widely accepted as the best treatment approach for most patients who are not cured by primary treatment programs based on multi-agent chemotherapy.
Identification of candidates for HDC/HSCT
The high levels of toxicity and cost associated with HDC/HSCT demand that it be reserved for patients where it clearly increases the chance of cure compared to alternative treatments. This describes two groups of patients: first, those whose disease progresses during primary chemotherapy or fails to enter a complete remission as proven by biopsy demonstrating persistent disease; second, patients who relapse after completing a full course of multi-agent chemotherapy with or without radiation. The first group, usually referred to as having refractory or chemotherapy resistant disease, has very little chance of cure with any program of standard dose chemotherapy with or without irradiation.27– 29 This group, lacking reliably curative alternatives, is best treated with HDC/HSCT because it offers a definite chance of cure.
The use of HDC/HSCT for patients in first relapse after primary chemotherapy is somewhat more controversial, especially if the relapse occurs long after completion of the primary treatment or in an isolated nodal area easily amenable to irradiation. However, when relapse occurs after primary chemotherapy consisting of a regimen as effective as ABVD, the chance of inducing long-term disease-free survival with standard dose chemotherapy is small, probably less than 20 percent.27,28,30 Two special subgroups may not share this poor prognosis: those who relapse solely in originally involved but unirradiated lymph node groups;31–,40 and those who relapse more than 1 year after completion of the primary chemotherapy.7,27,28,41 In the first of these 2 subgroups, wide field irradiation with or without additional chemotherapy may cure 40% to 50% of very carefully selected patients.31–,40 However, very few patients fit the ideal pattern of having nonbulky disease confined to lymph nodes at diagnosis and relapse, absence of B-symptoms at diagnosis and relapse and, preferably, a long interval from primary treatment to time of relapse. Although those relapsing more than a year after completion of primary chemotherapy may do well with a switch to potentially noncross-resistant chemotherapy with or without irradiation, this approach will only cure 20% to 40% of these specially selected patients.7,27,28,41 In contrast, however, this same subgroup is the one with the very best outcome with HDC/HSCT. Of particular relevance is the experience of the German Hodgkin’s Study Group.25 This group found that HDC/HSCT not only produced a superior progression-free survival for all patients in their study, but this was equally true for both those who relapsed early and those who relapsed late. Table 9 gives an overview of the characteristics of patients who should receive HDC/HSCT for relapse of Hodgkin’s lymphoma arranged by whether the approach is currently accepted or controversial. Relatively few patients fall in the controversial group and even for them, the case for use of HDC/HSCT is strong. Thus, in my opinion, the standard treatment for relapse of Hodgkin’s lymphoma after primary chemotherapy should be HDC/HSCT.
Technique of HDC/HSCT
Although most of the initial experience employing HDC/HSCT for Hodgkin’s lymphoma was acquired using autologous bone marrow cells, most groups now use autologous peripheral blood stem cells for the reasons shown in Table 10 .22,42–,44 In addition, most groups currently employ at least some standard dose chemotherapy prior to the high-dose chemotherapy for two reasons. First, it brings the Hodgkin’s lymphoma under control while the logistics of stem cell collection and the hospitalization for HDC/HSCT are arranged. Second, it provides priming for the peripheral blood stem cell collection enhancing the effectiveness of hematopoietic stem cells. However, it is important to remember that the purpose of this pre-HDC/HSCT chemotherapy is not to test for chemosensitivity. Hodgkin’s lymphoma, almost uniquely among human neoplasms, can be cured with the use of HDC/HSCT even when the disease does not respond to standard dose chemotherapy.45
Although a variety of HDC regimens have been described, no one regimen has been shown to be clearly superior. Currently, popular regimens include CBV (cyclophosphamide, carmustine [BCNU] and etoposide [ETOP]),22,24,46–,49 BEAM (carmustine [BCNU], etoposide, cytarabine and melphalan)17,19,25,50 or high-dose melphalan with or without total body irradiation.15,18 Because none of these regimens has been shown to be superior, it is more important for investigators at an individual center to master the management of the acute and chronic toxicities of their chosen regimen than to switch from one to another seeking some modest but unproved advantage. Most patients with Hodgkin’s lymphoma have previously been exposed to thoracic irradiation, bleomycin, nitrosoureas, or other agents with potential pulmonary toxicity. For this reason, it is best to avoid total body irradiation because it may be associated with a high risk of life-threatening interstitial pneumonitis.16,19,76 Selected results achieved using HDC/HSCT for refractory or relapsed Hodgkin’s lymphoma are summarized in Tables 11 and 12 .
In theory, the use of allogeneic stem cells, with their potential to add an immunologic attack on the malignant cells and provide a stem cell source free of contaminating tumor cells, should be even more effective that autologous stem cell transplantation following HDC for Hodgkin’s lymphoma. However, this improved potency is more than offset by increased toxicity leaving no net gain for the patient.51– 55 Any gain in disease control is overshadowed by increased toxicity, often lethal, from graft versus host disease and interstitial pneumonitis. Presently, with the availability of peripheral blood stem cells that appear to be free of clonogenic tumor cells and their proven efficacy and lower toxicity, autologous stem cells are the source of choice for hematologic engraftment when HDC/HSCT is used for Hodgkin’s lymphoma.
What can be achieved when HDC/HSCT is used to treat refractory or relapsed Hodgkin’s lymphoma? As shown in Tables 11 and 12 , short-term results indicate that at least some patients can do well. However, few data are available on long-term outcomes. To gain some perspective on the durability of responses to HDC/HSCT, I have examined the long-term follow-up of the 209 patients we have treated in British Columbia where we have had a dedicated program offering this technique to all eligible patients since 1985. Figure 13 shows the long-term survival for patients with refractory or relapsed Hodgkin’s lymphoma. The better outcome for patients receiving HDC/HSCT for relapsed disease than for refractory disease is obvious. Almost twice as many patients appear to be cured using this technique for relapsed than for refractory disease. However, the plateaus on both survival curves indicate that patients in each group can be cured. What about timing? Figure 14 shows our experience with HDC/HSCT focusing solely on its use for relapsed disease broken down by first, second, or third relapse. As would be expected, earlier use of HDC/HSCT produces a much better result than when it is delayed until patients have had multiple separate types of chemotherapy. This provides another reason to consider its use in first relapse.
Future Research
With more than 15% of patients still dying of progressive lymphoma despite optimal use of primary chemotherapy and secondary HDC/HSCT there is a clear need to find effective new therapeutic agents. Gemcitabine is the most promising traditional type chemotherapeutic agent currently under investigation for Hodgkin’s lymphoma.56–,61 In small series of heavily treated patients, an overall response rate of approximately 50% has been found with 10%–20% complete responses. Even more encouraging, 2 groups have found an overall response rate higher than 75% when gemcitabine was combined with cisplatin and a corticosteroid.56,61 This promising new agent will need further testing and integration into combinations with standard or other novel agents to exert its ultimate impact in the management of Hodgkin’s lymphoma.
One of the most promising new types of treatment for lymphoma, in general, is targeted immunotherapy. The anti-CD20 monoclonal antibody rituximab has proven useful for several different types of B cell lymphomas. The nearly universal expression of CD20 on the neoplastic cells of LPHL suggests rituximab may be useful. Preliminary data from several small series show response rates exceeding 50%;62– 66 however, the durability of these responses seems limited. Treatment with rituximab is attractive for this disease because of the lack of cumulative or late toxicity with this agent but will need to be integrated with conventional treatments to have a substantial impact.
Efficacy of one type of targeted immunotherapy hints that others may also be useful. Monoclonal antibodies aimed at other B cell or lymphocytic antigens,67 radio-immunoconjugates,67 and immunotoxin molecules67–,71 including bispecific antibodies and, eventually, tumor specific immunization strategies72 all hold promise. New immunotherapeutic approaches such as these hold substantial promise for Hodgkin’s lymphoma. However, because this disease is already so often cured, finding subjects for the testing of new agents is increasingly difficult. It would be unfortunate if this disease, which has served as a template for successful clinical research for more than three decades, were now to become neglected. Clinicians and clinical investigators should continue to work together to keep that from happening.
Schematic presentation of the CD30 receptor and intracellular binding partners as well as molecules presumably modified in their expression by activation (i.e., CD30 ligand) of the CD30 receptor.
Reprinted with permission from
Schematic presentation of the CD30 receptor and intracellular binding partners as well as molecules presumably modified in their expression by activation (i.e., CD30 ligand) of the CD30 receptor.
Reprinted with permission from
Schematic view of CD30 signal transduction.
The relationship of the listed components is characterized as activation by molecular interaction (normal arrow), inhibition by molecular interaction (line with terminal diagonal line), or induction of gene transcription (dotted arrow).
Schematic view of CD30 signal transduction.
The relationship of the listed components is characterized as activation by molecular interaction (normal arrow), inhibition by molecular interaction (line with terminal diagonal line), or induction of gene transcription (dotted arrow).
Adenovirus-mediated IκBΔN expression abrogates NF-κB activity and induces massive spontaneous apoptosis in HDLM2 cells.
(A) Whole cell extracts of HDLM2 cells infected with Ad5 control or Ad5-IκBΔN were analyzed by EMSA using an H2K binding site probe; (B) growth rates of uninfected or infected HDLM2 cells, as indicated, were determined in 5 independent experiments; and (C) apoptotic cells were determined by annexin V staining in uninfected or infected cells.
Abbreviations: N, novel NF-κB target genes
Reprinted with permission from
Adenovirus-mediated IκBΔN expression abrogates NF-κB activity and induces massive spontaneous apoptosis in HDLM2 cells.
(A) Whole cell extracts of HDLM2 cells infected with Ad5 control or Ad5-IκBΔN were analyzed by EMSA using an H2K binding site probe; (B) growth rates of uninfected or infected HDLM2 cells, as indicated, were determined in 5 independent experiments; and (C) apoptotic cells were determined by annexin V staining in uninfected or infected cells.
Abbreviations: N, novel NF-κB target genes
Reprinted with permission from
Kaplan Meyer curves for freedom from treatment failure for patients with Hodgkin’s lymphoma in advanced stage according to the International Prognostic Score (IPS) strata 0–2 and 3–7.
Kaplan Meyer curves for freedom from treatment failure for patients with Hodgkin’s lymphoma in advanced stage according to the International Prognostic Score (IPS) strata 0–2 and 3–7.
Hodgkin’s lymphoma in British Columbia showing the progression free survival for all patients diagnosed during the indicated decade.
Abbreviations: cum, cumulative
Hodgkin’s lymphoma in British Columbia showing the progression free survival for all patients diagnosed during the indicated decade.
Abbreviations: cum, cumulative
Overall survival after treatment with high-dose chemotherapy and hematopoietic stem cell transplantation for refractory or relapsed Hodgkin’s lymphoma. Long-term results seen in British Columbia.
Overall survival after treatment with high-dose chemotherapy and hematopoietic stem cell transplantation for refractory or relapsed Hodgkin’s lymphoma. Long-term results seen in British Columbia.
Overall survival after treatment with high-dose chemotherapy and hematopoietic stem cell transplantation for relapsed Hodgkin’s lymphoma. Long-term results seen in British Columbia by number of relapses prior to transplant.
Overall survival after treatment with high-dose chemotherapy and hematopoietic stem cell transplantation for relapsed Hodgkin’s lymphoma. Long-term results seen in British Columbia by number of relapses prior to transplant.