Abstract
Since the terrorist attack of September 11, 2001, preparation by the health care system for an act of terrorism has been mandated by leaders of governments. Scenarios for terrorist acts involving radioactive material have been identified, and approaches to management (based on past experience from atomic weapons detonations and radiation accidents) have been developed. Because of their experience in managing patients with profound cytopenia and/or marrow aplasia, hematologists will be asked to play a significant role in evaluating and treating victims of mass accidental or deliberate exposure to radiation. This review provides a framework for understanding how radiation levels are quantified, how radiation alters the function of hematopoietic (and nonhematopoietic) cells and tissues, and how victims receiving a significant radiation dose can be identified and managed.
In Section I, Dr. Nicholas Dainiak reviews four components of the Acute Radiation Syndrome: the hematopoietic, neurovascular, gastrointestinal and cutaneous subsyndromes. Clinical signs and symptoms are discussed for exposed individuals at the time of initial presentation (the prodromal phase) and during their course of disease (the manifest illness). In Section II, he presents clinical and laboratory methods to assess radiation doses, including time to onset and severity of vomiting, rate of decline in absolute blood lymphocyte count and the appearance of chromosome aberrations such as dicentrics and ring forms. Potential scenarios of a radiation terrorist event are reviewed, and methods for initial clinical assessment, triage, and early management of the acute radiation syndrome and its component subsyndromes are summarized.
In Section III, Dr. Jamie Waselenko reviews the hematopoietic syndrome, and presents guidelines for the use of cytokine therapy, antibiotics, and supportive care that have been developed by the Strategic National Pharmaceutical Stockpile Working Group. Results of preclinical and clinical growth factor therapy studies with G-CSF, GM-CSF, pegylated G-CSF, SCF, and IL-3 are summarized. When and how potassium iodide should be used after exposure to radioiodines is also reviewed.
In Section IV, Dr. James Armitage describes a narrow “window” of 7 to 10 Gy where therapy with stem cell transplantation may be appropriate. Victims who are candidates for allotransplantation should not have major trauma or significant injury to other (nonhematopoietic) tissues. Rarely, victims may have an identical sibling or autologous stored marrow or blood stem cells, in which case the threshold for transplantation is 4 Gy.
In Section V, Dr. Thomas MacVittie describes new directions for therapy, using cytokines such as IL-7, keratinocyte growth factor, and FLT-3. The potential for combinations of cytokines to enhance hematopoietic recovery is also reviewed.
I. Acute Radiation Syndrome
Nicholas Dainiak, MD*
The Bridgeport Hospital, 267 Grant Street, Bridgeport CT 06610
The threat of terrorist events involving radioactive material and the potential for radiation accidents mandate that the health care system develop and implement a preparedness plan of response. In addition to radiology professionals (including radiation safety officers, radiation oncologists, and nuclear medicine physicians), hematologists will be asked to play a significant (if not major) role in evaluating and treating victims of an accidental or deliberate exposure to radiation. Their experience in managing patients with cytopenias and/or marrow aplasia places hematologists in a unique position among physicians consulting victims who have received a significant radiation dose. Owing to the expected high prevalence of hematologic abnormalities, in many scenarios, hematologists will be asked to take primary responsibility for medical treatment of individuals with a moderate or high dose of radiation. Therefore, it is imperative that hematologists have an understanding of how radiation levels are quantified, how radiation alters the function of cells, tissues, and organ systems, and how victims receiving a significant radiation dose can be recognized and treated. They must also be aware of local, state, and national resources that may be accessed in the case of a radiological event. Accordingly, management of radiation victims will become a priority for practicing hematologists.
Radiation Measurements
Radiation dose is highly predictive of an effect on hematologic (and other) tissue of the body. Definitions for radiation units are provided in Table 1 . The absorbed dose is a measure of the energy departed per unit mass at a specific point.1– 3 A “rad” is the old unit of absorbed dose: it has been superseded by the gray (Gy). One Gy is equivalent to 100 rad. By contrast, the rem is the old unit for the equivalent or effective dose, and represents the product of absorbed dose (in rads) and a weighting factor for tissue. The effective dose accounts for differential sensitivity among tissue types. One hundred rem is equivalent to 1 Sievert (Sv). Since radiation therapy does not employ neutron exposure, 1 Sv is approximately equivalent to 1 Gy in patients undergoing treatment. Likewise, since most experimental studies employ low photon energy, 1 Sv is approximately equivalent to 1 Gy in radiobiology publications.
The average annual dose to persons residing in the United States is approximately 3.6 mSv (ie, the dose equivalence of 3.6 mGy).2 The majority of the average effective annual dose is due to indoor radon daughter products (55% of total dose). The next largest contribution to annual dose is due to man-made sources of radiation, with medical imaging studies contributing the largest amount of dose.2 A standard chest x-ray has a dose equivalence of 0.02 rem, while a Sesta Mibi stress test has a dose equivalence of 0.04 rem. By contrast, a barium enema provides a dose equivalence of approximately 2 rem.
The “dose rate” refers to radiation dose delivered per unit of time. It is often measured in Gy per hour. Geiger counters typically provide an estimate of dose rate (not dose) that may be used to estimate the level of hazard. Diminishing radiation dose rate results in a decreased radiation response. For example, it is believed that the carcinogenic effect of radiation delivered at a lower dose rate is less than that of the same dose delivered at a higher dose rate.
Sources of Data on Radiation Effects
Our understanding of the acute effects of total-body radiation is derived from analysis of the clinical course of individuals exposed to radiation after the detonation of atomic bombs over Japan and radiation accidents that have occurred throughout the world. In some cases, this includes a large affected population (for example, the Marshallese exposed in 1954 and individuals in the former Soviet Union and Europe exposed during the Chernobyl nuclear power plant disaster in 1986), while in other cases, relatively low numbers of individuals have been exposed. Recently, the registry of serious radiation accidents maintained by the Radiation Emergency Assistance Center/Training Site (REAC/TS) was updated at its 25th anniversary of continuous operation.4 Since December 1, 1990, over 50 accidents have occurred worldwide (including the United States), involving more than 650 individuals, more than 250 of whom had a significant exposure and more than 30 of whom who had a fatal outcome. In addition, updates of accidents are periodically made at Advanced Radiation Research Workshops that have been and are being held in Europe.5–,7 International meetings have also been held which review clinical information regarding significant accidents, such as the Chernobyl catastrophe.8,9
Phases of Radiation Injury
Early symptoms resulting from an acute total-body exposure constitute the prodromal radiation syndrome. Duration of symptoms and signs and mortality rate are dependent on the magnitude of radiation dose and the presence of additional injury (such as trauma or burns). Virtually all individuals receiving a dose of 10–20 Gy or higher develop prodromal signs and symptoms within 1–72 hours after exposure.10– 12 These include anorexia, nausea, vomiting, diarrhea, fever, fluid loss, and electrolyte imbalance. Although many of these are gastrointestinal symptoms, the control site for anorexia, nausea, and vomiting is located in the brain where abnormalities in the EEG are evident at much lower doses. These symptoms gradually merge into loss of consciousness, hypotension, and death (components of the cerebrovascular syndrome that is characterized by neurologic failure and cardiovascular collapse) before toxicity to other organ systems (such as the gastrointestinal and hematopoietic systems) can develop. Death occurs within a few days after exposure to 10–20 Gy. A rapid, severe prodromal response is the harbinger of a poor clinical outcome that is complicated by severe leukoneutropenia, thrombocytopenia, and anemia with reticulocytopenia, accompanied by hemorrhage, infection, and death. At lower doses (2–10 Gy), it is difficult to establish a prognosis based on the prodromal syndrome.
The prodromal phase is followed by a phase of manifest illness wherein syndromes specific to various organ systems emerge. Four major organ systems are known to be of critical significance in the development of acute radiation syndrome: the neurovascular system, the hematopoietic system, the cutaneous system, and the gastrointestinal system. Evaluation of system-specific signs and symptoms is required for triage of victims, selection of therapy, and determination of prognosis.
Component subsyndromes
The neurovascular syndrome at low doses of radiation results from localized, transient changes in the central nervous system. These changes include impaired capillary circulation, damage to the blood-brain barrier, interstitial edema, acute inflammation, petechial hemorrhages, meningitis, and hypertrophy of perivascular astrocytes.13 Paroxysmal spike and wave discharges may be evident on EEGs, and the presence of swelling and edema may be documented by head CT scans and MRIs.14 The presence of transient nausea, anorexia, vomiting, and fatigue augers a relatively good prognosis. Moderate damage to the central nervous system presents with persistent and more severe nausea and vomiting, and is accompanied by headache, neurological deficits, and abnormal cognition. The presence of severe nausea and vomiting, severe headaches, drowsiness, fever, and hypotension auger a poor prognosis.
The gastrointestinal syndrome occurs at doses of between 5 and 12 Gy.3 Mild gastrointestinal symptoms limited to 1 or 2 episodes of diarrhea with associated abdominal pain are accompanied by virtually certain recovery. Intermittent diarrhea and bleeding are associated with extensive sloughing of the epithelial cell layer, leading to denudation of the bowel. More severe damage to the gastrointestinal tract is associated with persistent diarrhea, gastrointestinal bleeding, and crampy abdominal pain, resulting in abnormalities of fluid and electrolyte balance, inflammation, and sepsis. Impaired barrier function of the gastrointestinal tract results in the passage of bacterial toxins through the intestinal wall into the bloodstream. Severe complications include ulceration and necrosis of the bowel wall, leading to stenosis, ileus, and perforation. In the latter case, recovery is most unlikely, as radiosensitive stem cells in the crypts of the gastrointestinal tract are permanently damaged. Consequently, there is no replacement of cells that are lost from the surface of the villi through the sloughing process of normal bowel use.15,16
The hematopoietic syndrome develops at doses of between 2–3 Gy and 8 Gy.1,3,11 Low-dose (< 2 Gy) radiation induces mild cytopenias without significant bone marrow damage.12 Peripheral blood lymphopenia may develop within the first 6–24 hours after a moderate- to high-dose exposure.16–,18 In addition to inducing apoptosis (whose effect is not seen before the first cell cycle), radiation alters recirculation properties of lymphocytes.19– 21 Based on the level of lymphocyte, polymorphonuclear leukocyte, and platelet counts, as well as the presence of infection and/or blood loss, the relative severity of hematotoxicity has been described (see Table 2 ). Approaches to therapy for different levels of severity of the hematopoietic syndrome are described in Section III.
The cutaneous syndrome may develop after early exposure (within 1–2 days) or may take years before becoming fully manifest. Nonuniform exposure results in local radiation injury. Early lesions include erythema and moist desquamation of the skin. Such lesions may be isolated or may appear simultaneously in several locations, depending on the amount of skin receiving direct exposure. Target cells of radiation reside at multiple levels (i.e., epidermis, dermis, hair follicle canals and subcutaneous tissues) within the skin; hence, the severity of the cutaneous reaction depends upon the “depth dose distribution” of the radiation source.13 Signs and symptoms include pruritus, blisters, and bullae (with or without hemorrhage), ulceration (limited to the epidermis or involving the dermis, subcutaneous tissue, muscle and/or bone), hair loss, and onycholysis.22,23 Epilation occurs 10–20 days after a single localized exposure to 3–4 Gy or greater. Gusev and coworkers estimate that the threshold for erythema is a localized exposure dose of 10–15 Gy, while moist desquamation and ulceration are seen with doses of 20–25 Gy.24 Blisters and bullae with or without necrosis appear 1–3 weeks after localized exposure to doses of > 30 Gy.24,25
Estimations of the 50% lethal dose (LD50) have been made in various scenarios. Depending on the incident, the LD50 ranges from 1.4 Gy among atomic bomb survivors in Japan to 4.5 Gy based upon bone marrow for uniform total-body exposure to external photons.25,26 Based upon a summary analysis of all available data, Lushbaugh has estimated the LD50 at 60 days (LD50/60) for humans to be approximately 3.5 Gy for young healthy adults.27 Vriesendorp and van Bekkum have estimated the LD50 to be approximately 4 Gy.28 Noteworthy is that among victims receiving appropriate supportive care and antibiotics, the LD50 increases to 6–7 Gy.15
II. Hematologic Response to Radiation Exposure
Nicholas Dainiak, MD
Scenarios have been developed for terrorist events resulting in small volume and mass casualties. The Acute Radiation Medical Management Working Group for the Strategic National Stockpile Program has recently developed a consensus opinion to provide guidance for estimation of radiation dose, clinical assessment of exposed individuals and medical management (Waselenko J, MacVittie TJ, Blakely WF, et al, submitted).
Potential Dispersal Scenarios
Uncontrolled radiation exposure may result from the release of radioactivity from small sources (i.e., nuclear medicine, brachytherapy, industrial gauges, small calibration sources, etc), transportation accidents, nuclear power plant accidents, nuclear weapons, and terrorist attacks using a radiological dispersion device (RDD).30 An RDD may involve a small or large isotopic source or spent nuclear fuel. According to the US Government Accounting Office, approximately 10 million “sealed sources” of radioactive material exist in 50 countries, including the US.31 These sources of radioactive material (i.e., Cs-137, Sr-90, Cobalt-60, Pu-238, Pu-239, etc) are encased in metal and are used in equipment for medicine, industry, agriculture, and research. Of the 612 sealed sources that were reported to be lost or stolen since 1995, 254 had not been recovered. Many additional sealed sources may be unaccounted for as well. Such material may be used by terrorists for making an RDD (or “dirty bomb”). When used with conventional explosives, radioactive substances may be dispersed over a relatively limited area (a few city blocks) with the intent to cause fear or panic.30,32 RDDs prepared with a radioactive powder, solution, or gas may be also dispersed (without initiating a nuclear reaction) over a larger area, thereby affecting a large population.
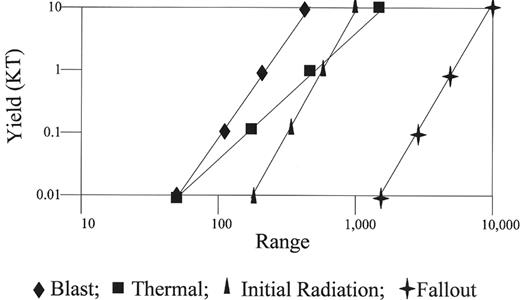
An improvised nuclear device (IND) employs radioactive material which, following detonation, results in a nuclear reaction. Three forms of energy are released from a nuclear explosion: heat (accounting for 35% of total energy), shock or bomb blast (50%), and radiation (15%). The distribution of energy released from the “Little Boy” atomic bomb explosion over Hiroshima is depicted in Figure 1 (see Appendix, page 604).33 The blast wave from a nuclear explosion results in fractures, lacerations, rupture of viscera, pulmonary edema, and hemorrhage and emboli, while the thermal energy causes flash burns, flame burns, flash blindness, and retinal burns. Based on the type of energy released, injuries and fatality rates vary by distance from the explosion (see Figure 2 ). Regardless of the source of radioactive material, the presence of multiple injuries (i.e., “combined injury”) is a mortality multiplier, owing to predisposition to infection from trauma and burns, and immunosupression by radiation at absorbed doses as low as 0.5 Gy.
Assessment of Radiation Dose
Radiation dose from external exposure can be assessed by physical, biological, and clinical dosimetric techniques. Physical dosimetry can provide an estimate of individual dose, using a whole-body radiation dosimeter.15 While sensitive and precise measurements of dose can be made, few whole-body dosimeters are available for rapid assessment of dose. Physical dosimeters can also measure dose in common materials (including air, soil, water, brick, etc). Reconstruction of dose can be made with considerable sensitivity, using environmental measurements combined with time-integrated activity.34,35 However, this is a time consuming process that is impractical in an emergency situation, particularly in the case of mass casualties.
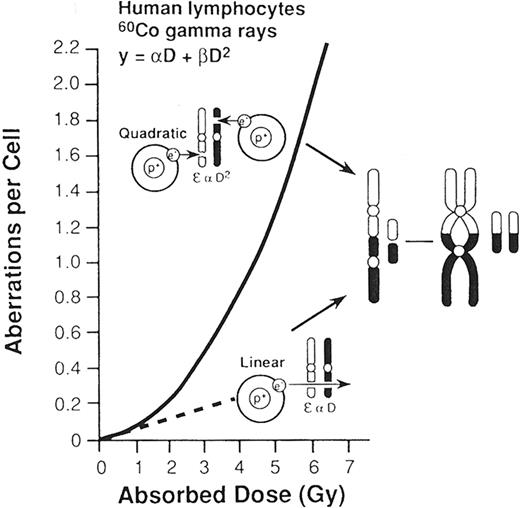
Individual “biomarkers” for a radiation exposure have been sought from the beginning of the nuclear age. Bender and Gooch made a landmark observation by showing that the frequency of chromosome aberrations in lymphocytes correlates well with radiation dose.36 The formation of dicentrics involves an interchange between 2 separate chromosomes, while ring formation involves a break in the arm of a single chromatid, followed by rejoining to form a ring and a fragment.37 The frequency of these asymmetric aberrations in circulating lymphocytes correlates with radiation dose, and best fits a linear-quadratic relationship (Figure 3 ). At low doses, chromosome breaks result from passage of a single charged particle, the consequence of which is a linear function of dose. At high doses, chromosome breaks are caused by the passage of multiple charged particles, resulting in an interaction that is a quadratic function of dose.1 Chromosomal aberrations have become the “gold standard” for biodosimetry. Their detection is facilitated by the application of hybridization probes for centromeres and automated metaphase detectors.38,39 For triage of victims of terrorist events, as few as 20 metaphases may be scored to provide a preliminary estimate of dose.40
Other forms of biological dosimetry include lymphocyte depletion kinetics, interphase aberrations (detected by premature chromosome condensation induced by agents such as okadaic acid and p34cdc2/cyclin B kinase41), and electron spin resonance of dental enamel. Of these methods, monitoring for a decrease in absolute lymphocyte count has been found to be a reliable and practical method to assess dose soon (within hours or days, depending on dose) after a radiation exposure.16,42 Goans et al developed a simple algorithm for estimating whole-body dose (between > 50 cGy and < 10 Gy) from acute exposure to gamma radiation, using the rate of decline in circulating lymphocytes.42 A comparison of selected methods for estimating radiation dose is presented in Table 3 .
Recently, the importance of documenting clinical signs and symptoms has been emphasized in assigning risk from radiation exposure.13 These include the time of onset and intensity of nausea and vomiting, the appearance and type of skin changes, the development of anorexia and fatigue and the severity of depression in circulating blood counts, including the polymorphonuclear cell count, lymphocyte count and platelet count.43–,45 Of these clinical features, the time to emesis and lymphocyte depletion kinetics are amenable to quantitative analysis with respect to dose.46 Since the incidence of nausea, vomiting, skin lesions, and fatigue is not 100%, estimating dose by clinical signs and symptoms loses sensitivity at low doses, and may lead to underestimation of dose. Whenever possible, incorporation of data from 3 key elements (i.e., time to onset of vomiting, lymphocyte depletion kinetics, and chromosome aberrations) is required for assignment of prognosis and selection of therapy.
In contrast to measurement of dose from external radiation, estimation of radiation dose from internal exposure due to the deposit of radioactive materials (such as alpha emitters, including plutonium, americium, and californium, and beta-gamma emitters, including cesium, cobalt, and iodine) into the lungs, gastrointestinal tract and other tissues, requires detection with special instrumentation (such as ion chambers and spectroscopes). In this case, measurements are made on body fluids (blood, urine, saliva, etc), nasal swipes, fecal samples, and/or expired air.47
Cellular Effects
Ionizing radiation may interact directly with intracellular targets or may interact with other molecules (such as H2O) to produce free radicals that, in turn, reach a target (such as DNA and the plasma membrane). Depending on the amount of ionization deposited along a unit length of track of radiation, the chances of achieving a “hit” will vary. Ionization is sparse for low linear energy transfer (LET) sources of radiation such as x-rays and gamma-rays. High LET radiation characterized by dense ionization is observed with alpha particles and neutrons. As with all tissues composed of short-lived cells, hematopoietic tissue is directly and indirectly affected by radiation. Depending upon the dose and dose rate, effects are primarily exerted through cell renewal, apoptosis, and redistribution of lymphohematopoietic cells.48
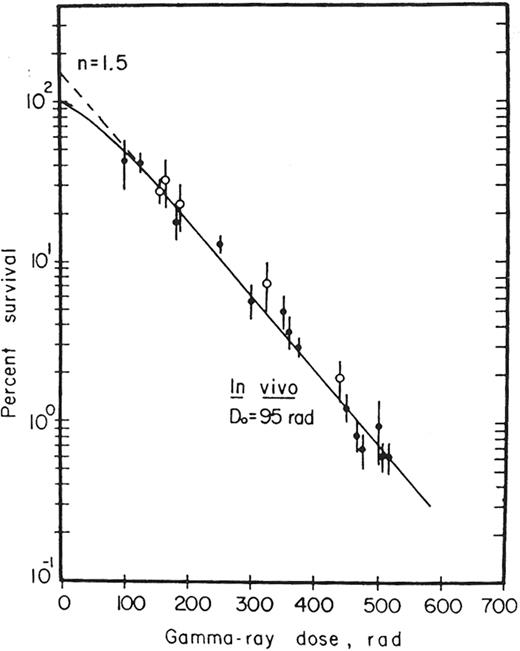
Survival curves for normal clonogenic cells have been derived by McCulloch and Till, using the CFU-S assay.49Figure 4 shows that a single-exponential radiation survival curve is evident for murine hematopoietic stem cells. These stem cells were determined to be the most sensitive of all mammalian cells undergoing mytotic death (D0 = 0.95 Gy). Nevertheless, some CFU-S appear to be extremely radioresistant, surviving doses as high as 6 Gy.50,51 Inoue and colleagues51 showed that a larger than expected surviving fraction of day 9 CFU-S is detectable after exposure to 4–6 Gy (see Figure 5 ). It is possible that such radioresistant stem cells may play a role in the hematopoietic response that is observed in individuals receiving cytokine therapy after exposure to external radiation (see next section). In addition, hematopoietic reconstitution may occur from unirradiated (or relatively “under-irradiated”) areas of bone marrow that have been “shielded” from the source of radiation by physical materials, heavy clothing, or other body tissues.
Dose-dependent effects on various organs have been identified. Table 4 shows that the threshold absorbed dose for a “deterministic effect” on bone marrow (i.e., an effect wherein severity is determined by dose) is lower than that for all organs, except for the testes. In contrast to such deterministic effects, the carcinogenic effects of radiation occur after a prolonged and variable delay (or latency) after exposure. The latter “stochastic effects” represent outcomes for which the probability of occurrence (rather than severity) is determined by dose; these effects do not have an apparent threshold dose. The mechanisms for deterministic and stochastic effects remain unknown. Recent studies showing the impact of radiation on gene function may shed light in this area.52–,54 In addition, studies employing cDNA microarrays may help to identify profiles that may serve as biomarkers for an exposure to radiation.53– 55
Clinical Assessment and Early Triage
In the case of a terrorist incident or accident involving nuclear material, first responders will include members of a HAZMAT team, local fire and police departments, and individuals knowledgeable in radiological monitoring from a state agency such as the State Department of Environmental Protection. Their responsibilities are to manage the consequences of the incident by (1) limiting further damage, (2) protecting the public, (3) performing decontamination, and (4) disposing of radioactive material.30,32 For larger incidents, a national system is available through the Department of Energy (i.e., the Radiological Assistance Program) in order to provide additional expertise. During this phase of consequence management, the lead federal agency is the Federal Emergency Management Agency.56 The lead agency for handling the crisis (i.e. insuring that no further threat is present and managing the site using law enforcement measures) is the Federal Bureau of Investigation.57 Hospitals must develop radiation response plans that coordinate their efforts with those of local response teams.
First responders and all other health care providers must use universal precautions (disposable gowns, gloves, masks, etc) for personal protection. Early management in the field and the emergency room should follow basic triage principles. First, life-threatening injuries should be treated (even before assessing exposure). It should be assumed that victims are contaminated (i.e., by absorption of radiation through the skin after being physically covered by radioactive material or by ingestion or inhalation of radioactive material). Victims should be stabilized and transported to a medical facility. Second, injured victims should be treated by standard triage guidelines. Preliminary decontamination (removal of clothing, washing of the victim, etc) should be performed before or during transport to a medical facility. Third, persons who are only externally contaminated (without other injury) should be relocated to an upwind site (other than the hospital) in order to decongest the hospital care system. Thereafter, assessment and prevention of contamination, treatment of minor injuries, and evaluation and treatment of internal contamination should be addressed.30
After arrival in the emergency department, victims should be classified based on whether or not they have been contaminated, received a significant external exposure without contamination, and/or received physical injury. The vast majority of individuals arriving in the emergency department after an event involving an RDD are likely to have insignificant or no exposure. Clinicians should be able to identify those victims whose exposure is significant. When necessary (and if not already performed), decontamination should be performed after clinical assessment has been made. Depending on the situation, a trauma surgeon, burn specialist, dermatologist, and/or neurologist may be consulted, in addition to the hematologist. Information regarding level of risk should be provided to medical caregivers by the hospital radiation safety officer, radiologist, nuclear medicine physician, and/or radiation oncologist.
Historical information that should be documented in the medical record include the location of the incident, duration of exposure, interval between exposure and clinical evaluation, activity at the time of exposure, and occupation of the victim. Other information (such as a general description of the accident, source of radiation, and numbers of individuals involved in the exposure) should be documented, if possible.
In general, the clinical signs and symptoms of a radiation exposure are nonspecific (see discussion above). The physical examination should focus on vital signs (fever, hypotension, orthostasis), skin examination (erythema, blistering, edema, desquamation), neurological examination (ataxia, motor/sensory deficits, papilledema), gastrointestinal examination (abdominal tenderness) and hematologic examination (ecchymoses and/or petechiae of mucous membranes and skin). A schema has been developed to assess severity of changes in the clinical examination (see Table 5 ).
Initial laboratory testing should include a CBC, routine chemistry tests, and an extra sample of blood in a heparinized tube to be held for cytogenetics, if necessary in the future. For victims in whom internal contamination is suspected, peripheral blood (for the same tests as ordered for an external exposure), urine, nasal smears, spontaneous vomitus, and stools should be obtained for radiological monitoring.
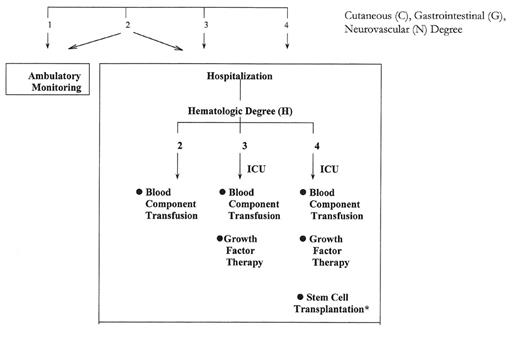
Management decisions must be made for high-risk individuals at a time when results of chromosome analysis for individual biodosimetry are not yet available. As a guide to the clinician, one approach to the decision-making process that was developed by the Medical Treatment Protocols (METREPOL) team, is to assign a score based upon clinical and routine laboratory findings.13 Integration of clinical information regarding the severity of signs and symptoms described in Table 5 with changes in peripheral blood counts described in Table 2 permits one to assess hazard in quantitative terms. A “response category” is assigned to each victim, determined by the highest degree of severity in any of the signs or symptoms.13 Using this information and results of hematologic monitoring, victims can be triaged to the (1) ambulatory setting, (2) routine care medical/surgical floor, (3) intensive care unit or (4) transplantation unit, as outlined in Figure 6 .48
Dose estimates provide an added level of certainty with regard to prognosis, and impact on selection of therapy. In order to facilitate the integration of clinical, laboratory, and dosimetric data, a radiation casualty management software program (i.e., the Biological Assessment Tool) has been developed by the Armed Forces Radiobiology Research Institute and is available at the Web site www.afrri.usuhs.mil.58 Background information and advice on clinical management of radiation incidents can be obtained at this Web site and at the Web site for REAC/TS (www.orau.gov/reacts).
The psychosocial impact of an act of terrorism cannot be overemphasized. Many victims will have some psychological symptoms, ranging from insomnia and hypervigilence to social withdrawal.59 Posttraumatic stress disorder may occur among victims, families, and friends. High-risk victims include children, pregnant women, mothers of young children and victims with a prior medical history of psychiatric disorders. The principle of therapy is to establish trust through open communication. Approaches to management of behavioral and mental health problems have been discussed.60
III. Management of the Hematopoietic Syndrome
Jamie K. Waselenko, MD*
Walter Reed Army Medical Center, WD78: Hematology/Oncology Clinic, 6900 Georgia Avenue, NW, Washington DC 20307
The lymphohematopoietic elements are among the most highly replicated tissues in mammals and as such are among the most radiosensitive. Irradiation of bone marrow stem and progenitor cells results in exponential death.1,2 While the hematopoietic syndrome (HS) may be seen with significant partial-body or whole-body radiation exposures > 1 Gy, it is not usually clinically significant below this level.2 Mitotically active hematopoietic progenitors are unable to divide after a whole-body exposure > 2–3 Gy, which results in a hematologic crisis in the ensuing weeks.1 This results in lymphopenia, bone marrow atrophy, pancytopenia, and its attendant sequelae; infection, bleeding, and poor wound healing, which contribute to its lethality.
While most bone marrow progenitors are susceptible to dose-dependent decrements in viability, subpopulations of selectively radioresistant stem cells and/or accessory cells exist.3,4 These cells may play an important role in recovery of hematopoiesis after exposure to doses as high as 6 Gy, albeit with a reduced capacity for self-renewal.
Inhomogeneity of dose is also a critical tenet which may contribute to future reconstitution of hematopoiesis, if potentially life saving pockets of viable bone marrow are spared, and the dose to other organs is not too great (i.e., < 10 Gy). This arises due to the uncontrolled nature of an exposure to radiation that would occur due to an IND detonation. The patient’s physical environment and proximity to the source may afford partial shielding, resulting in dose variability.
A predictable decline in lymphocytes is known to occur after radiation exposure. Indeed, a 50% decrease in absolute lymphocytes within the first 24 hours, followed by a second drop within 48 hours, characterizes a lethal injury for ionizing radiation. This predictability has led to the development of a model using lymphocyte depletion kinetics as an element of biodosimetry.6,7 Patients suffering from burns8 and trauma,9 common in nuclear detonations, may develop lymphopenia as a result of these injuries. Additional perturbations include a great decline in T helper cells relative to other subsets,10 which contributes to the profound immunosuppression that these patients may experience.
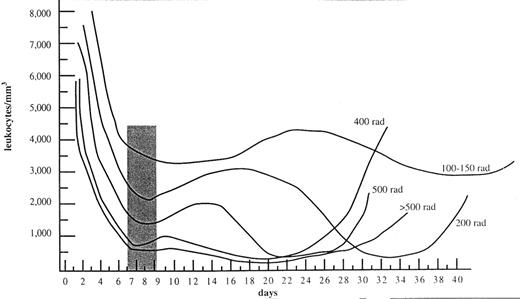
The onset of cytopenias is variable and dose dependent.5Figure 7 shows that granulocytes may transiently increase prior to falling in patients with < 5 Gy exposures.5 This transient increase prior to decline is termed an “abortive rise,” a finding that may be clinically helpful as it may indicate a more survivable exposure. Time to onset and duration of the nadir are variable. Indeed, the nadir may not occur for 3–4 weeks,1,5 particularly at low doses. The duration of neutropenia may be prolonged, requiring prolonged administration of hematopoietic growth factors, blood product support, and antibiotics.
Burns and combined injuries are expected to occur in 60%–70% of patients after an IND detonation.2,11 These injuries significantly complicate the management of patients with the HS and significantly lower the LD50/60. The converse is also true. Patients with burns and/or wounds suffer from poor wound healing, bleeding, and infection because of lymphohematopoietic suppression.12
The management of patients with combined injuries is intense, and requires the help of physicians who are experienced in the management of radiation toxicity and prolonged aplasia. With significant casualties, supportive care measures will need to be prioritized based on resources and the probability of a survivable exposure (see Table 6 ).
Cytokine Therapy
Currently, the only hematopoietic colony-stimulating factors (CSFs) which have FDA marketing approval for the management of treatment-associated neutropenia are the recombinant forms of granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), and its pegylated form (PEG-G-CSF or pegfilgrastim). None of these cytokines currently have marketing approval for the management of radiation-induced marrow aplasia. The rationale for the use of CSFs in this setting is derived from 3 sources: their enhancement of neutrophil recovery in oncology patients, their perceived benefit in a small number of radiation-accident victims, and, most importantly, the survival benefit observed in several carefully conducted, prospective trials in canines and nonhuman primates exposed to radiation.
The value of CSFs in the treatment of radiation-induced marrow myelosuppression lies in their ability to increase the survival, proliferation, amplification, and differentiation of granulocyte progenitors to produce neutrophils. Both cytokines can activate or prime neutrophils to enhanced function such as microbicidal activity,14,15 an important element in the host’s nonspecific response to opportunistic infection.
Currently, no prospective, randomized trials have examined the benefit of CSFs in humans exposed to nontherapeutic forms of radiation. G-CSF and GM-CSF have been shown to hasten neutrophil recovery by approximately 3–6 days in humans after intensely myelotoxic therapies,16 including bone marrow and stem cell transplantation.17,18 In fact, neutrophil recovery times are similar for both early and delayed G-CSF posttransplant,19– 21 suggesting no added benefit to earlier use.
Both G-CSF and GM-CSF have been used in radiation accident victims, and neutrophil recovery appeared to have been hastened in 25 of 28 cases in the registry maintained by REAC/TS. In most cases, these individuals received both G-CSF and GM-CSF concurrently for significant periods of time. However, there was considerable variation in the time of CSF administration (often delayed for weeks after the exposure) and in how CSFs were used. Some patients also received interleukin-3. Based on these results, no definitive conclusions can be made.
A number of studies examining the role of G-CSF, GM-CSF, pegylated G-CSF, and a chimeric molecule in an irradiated rhesus macaque’s model22–,25 demonstrated significant neutrophil enhancement when these agents were employed on day 1 postexposure and continued for 14–21 consecutive days. Studies performed in irradiated rhesus macaques also suggested a benefit to early initiation of G-CSF or GM-CSF, similar to those noted in the canine studies. One study suggested no diminished efficacy in a delay,26 the other suggested a lessened therapeutic effect relative to the respective early treatment cohorts. Neutrophil recovery parameters were also diminished in the delayed treatment cohort.
While enhanced neutrophil recovery in irradiated animals provided the proof of principle for use in humans, an important survival advantage was seen in nonhuman primates and canines27,28 given CSF that occurred in addition to that observed with supportive care alone, especially if given early (< 24 hours after exposure).29 The time interval required before the survival advantage is lost is unknown, but it suggests that CSF should be initiated as early as possible in those exposed to a survivable whole-body dose of radiation who are at risk of the hematopoietic syndrome (> 3 but < 10 Gy) and in any patient who becomes neutropenic, defined as an absolute neutrophil count (ANC) < 500, and who is not already on a CSF.
Schuening et al27 and Nash et al28 used a comparable irradiated canine model to investigate the treatment efficacy of G-CSF, GM-CSF, and stem cell factor (SCF) on lethality and hematopoietic recovery after otherwise lethal TBI doses of 4, 5, or 6 Gy.27–,29 In the 4 Gy TBI cohorts, 1 of 28 (3.6%) control animals survived with supportive care alone. Of the 7 dogs receiving G-CSF (0–21 days post exposure), 5 (71%) survived with complete hematopoietic recovery,27 a significantly better survival than that noted with administration of GM-CSF, in which only 1 of 10 survived.28 SCF was also administered in this study and resulted in 5 of 10 (50%) surviving animals.27 Increasing the dose to 5 Gy (100% lethal) and G-CSF treatment resulted in the survival of 3 of 10 (30%) animals whereas treatment with SCF alone or in combination with G-CSF resulted in hematopoietic recovery and survival in 2 of 7 (28.6%) and 2 of 5 (40%) respectively. Treatment with either G-CSF or SCF (n = 5 each) could not stimulate hematopoietic recovery in dogs exposed to 6 Gy irradiation.
Treatment with the cytokines G-CSF, GM-CSF, or SCF plus supportive care, over the radiation dose range noted above allowed determination of the hematopoietic CSFs impact on the LD50/30 value. CSF treatment administered within 24 hours post exposure increased the LD50/30 to 5.1 Gy representing dose modification factors of 2 and 1.5 (unpublished data) over the respective 2.6 Gy LD50/30 of unsupported canines and 3.38 Gy LD50/30 for the supportive care cohorts.
These data collectively demonstrate that CSFs may not only ameliorate radiation-induced neutropenia but may also shorten the duration of neutropenia, resulting in a survival advantage, especially if employed early. These data justify the treatment recommendations presented in Table 7 . Finally, victims at extremes of age (children < 12 years of age and the elderly) may be more susceptible to irradiation and have a lower LD50/60.1 These groups may benefit from CSF administration at a lower dose threshold, 2 Gy.
Supportive Care
Successful administration of supportive care in the nontransplant scenario is dependent upon 3 conditions: (1) radiation-induced damage to stem and progenitor cells being reversible, (2) a surviving fraction of stem cells being capable of spontaneous regeneration, and (3) the other (nonhematopietic) injuries being survivable. Hematopoietic regeneration must result in the production of functionally normal neutrophils and platelets within the critical, clinically manageable period or the patient will not survive.
Supportive care is essential, and includes the administration of cytokines, blood products, antibiotics, antiemetics, antidiarrheals, fluid, and electrolytes and topical burn creams. In addition, management of combined injuries is of paramount importance. The focus of the following recommendations is the management of the HS.
Experimental work performed more than 2 decades ago demonstrated the efficacy of supportive care. This included the use of systemic antibiotics against Gram-negative bacteria and fresh, irradiated, platelet transfusions.30–,32 Several studies indicated that antibiotics given alone or in combination were somewhat effective in reducing mortality of irradiated dogs in the LD50/30 range.30–,33 Controlling infection during the critical neutropenic and thrombocytopenic phases is the limiting factor in successful treatment.31 MacVittie et al34 extended the evaluation of supportive care studies in irradiated dogs over a complete hematopoietic syndrome dose range, thereby determining the shift in LD50/30 due to supportive care. The LD50/30 value increased significantly from 2.59 Gy to approximately 3.37 Gy, measured as midline tissue dose with supportive care alone. Similar studies, showing an increase in the LD50/30 threshold, have also been reported for non-human primates22,23,26,35 as compared to historic studies of the LD50/30 without supportive care.36– 38
Blood products support is required for patients with severe bone marrow damage resulting from radiation-induced aplasia. Fortunately, this complication does not typically occur before 2–4 weeks, during which time blood donors may be rapidly identified. All cellular products should be leukoreduced and irradiated (25 Gy) to prevent transfusion-associated graft-versus-host disease (TA-GVHD), a life threatening form of acute graft-versus-host disease (aGVHD). The intense immune suppression that some of these patients may suffer will prevent clearance of these alloreactive passenger lymphocytes, which are acquired during a transfusion. This may lead to proliferation in the recipient with ensuing lethal aGVHD. This latter process may be difficult to distinguish from other organ toxicities seen in radiation victims (i.e., fever, pancytopenia, skin rash diarrhea, and hyperbilirubinemia and other liver function test abnormalities.
Leukoreduction is known to lessen febrile nonhemolytic reactions and the immunosuppressive effects of blood transfusion.39,40 Moreover, leukoreduction affords some protection against platelet alloimmunization and protection against acquiring cytomegalovirus infections.41 Life-saving products should not be withheld but should be leukoreduced and irradiated whenever possible.
Susceptibility to local and systemic infection after radiation arises as a result of (1) breeches in the integument and mucosal barriers and (2) immune suppression resulting from declining lymphohematopoietic elements. In nonneutropenic patients, antibiotics should be directed toward the foci of infection and the most likely pathogens. For those who experience significant neutropenia (ANC < 500/μL) broad spectrum prophylactic antimicrobials should be employed as the neutropenic duration is likely to be prolonged. Prophylaxis should include a fluroquinolone (FQs) with streptococcal coverage (with penicillin or amoxicillin if not inherently covered by the FQ), as an antiviral agent if the patient is herpes simplex virus (HSV) positive, and an antifungal agent.
Studies in irradiated mice have demonstrated that the gut flora also undergoes a dose-related reduction in numbers within the first 4 days post-radiation.51,52 This is followed by a relative increase of Enterobacteriaceae compared to anaerobic bacteria by the 12th day. Fatal bacteremia may then result from the bacterial translocation of these organisms. The use of quinolones was effective in controlling systemic endogenous Gram-negative infections after radiation.53,54 Supplementation with penicillin prevented treatment failures due to Streptococci and increased survival in animals. A similar benefit has also been observed in cancer patients suffering treatment related neutropenia.55 Quinolones given for 21 days were also effective at preventing endogenous Klebsiella and Pseudomonas infections.51,53,54 While controversy exists over FQ prophylaxis, FQs have been explored extensively for prophylaxis in high-risk neutropenic patients and have demonstrated a reduction in the incidence of bacteremic episodes,56– 60 further serving as the basis for our recommendation.
HSV serological status should be determined if resources allow, and empiric therapy should be employed, using acyclovir or one of its congeners in those who are serologically positive for types I or II HSV. During intense periods of immunosuppression, these patients are at high risk for HSV reactivation, which may be confused with radiation stomatitis and may complicate its management. While patients undergoing local head and neck therapy did not show a significant risk of HSV reactivation,42 patients receiving immunosuppressive therapies such as bone marrow transplant have a high incidence of reactivation,43 which can mimic or add to the severity of mucosal injury. If serologies are not known, then it would be reasonable to offer HSV prophylaxis based on any previous history of oral or genital herpes infections. Additionally, any patient not receiving prophylaxis and suffering from severe mucositis, especially if unusually severe or prolonged, should be assessed for possible HSV reactivation.
Fluconazole has been shown to lessen invasive fungal infections and mortality in patients undergoing allogeneic bone marrow transplant44,45 at a dose of 400 mg po daily. Data in patients receiving conventional forms of severely myelotoxic chemotherapy also have demonstrated benefit,46 although conflicting results exist.47,48 Fluconazole prophylaxis is ineffective against aspergillus, molds, Candida krusei, and resistant Candida species.
These antimicrobials should continue until the patient fails them, i.e., experiences a neutropenic fever or experiences neutrophil recovery (ANC > 500/μL). Any foci of infection that developed during the neutropenic period will require a full course of therapy. In patients who experience first fever, traditionally the FQ is stopped and therapy directed at Gram-negative bacteria (in particular, Pseudomonas aeruginosa) as infections of this type may be rapidly lethal. Therapy of patients with neutropenia and infection should be guided by the recommendations of the Infectious Diseases Society of America (IDSA)49 and should take into consideration other foci of infection such as mucosal or integument injury.
Potassium Iodide
Owing to their short half-lives, radionuclides are unlikely to be components of an RDD or “dirty bomb.” Therefore, iodine prophylaxis is not indicated. Nevertheless, for an incident involving a nuclear power plant or an IND, it is probable that radioiodines will be released. Early prophylaxis is indicated in the latter situation.
The thyroid is a radiosensitive organ at risk. Exposure to radioiodine can result in thyroid cancer, a delayed consequence, which may be more aggressive than de novo forms.50 Exposure could begin immediately if the released plume is near ground level. The main route of radioiodine exposure is inhalation in those in the near field and via ingestion of contaminated food and drink (particularly milk) for those farther away (far field). Exposure via the latter route could last longer, cover a larger area, and affect a larger population.
Thyroid blocking with potassium iodide (KI) affords protection when radioisotopes of iodine are components of the exposure. Dosing guidance is summarized in Table 8 and is also available online at: www.bt.cdc.gov/radiation/ki.asp. Oral administration of KI should occur as soon as possible after exposure (within 6 hours). Caution should be taken with those individuals who are allergic to iodine because severe reactions have been reported. KI should be dosed daily, until the exposure risk no longer exists. Thyroid protection for pregnant women exposed to radioiodine is crucial. In the first trimester with a near field exposure, stable iodine will protect the mother. Pregnant women in the far field may be able to avoid contaminated foods and milk. The fetal thyroid normally does not begin to function until around the 12th week of gestation, although it is iodine-avid once it has developed. Thus, pregnant women in the second and third trimesters should receive KI in both near and far field exposures to protect the maternal and fetal thyroids.
IV. Role of Transplantation in Victims with Bone Marrow Failure
James O. Armitage, MD*
University of Nebraska Medical Center, Dean’s Office, School of Medicine, 976545 Nebraska Medical Center, Omaha NE 68198
Historical Perspective
The intravenous infusion of hematopoietic stem cells (HSCs) in an attempt to rescue patients with bone marrow failure was reported in 1939 in a patient who received 18 mls of intravenous marrow from his brother to treat aplastic anemia.1 In the 1950s, the scientific basis for this treatment was developed by demonstrating that rodents could be protected against lethal hematopoietic injury by intravenous infusion of bone marrow cells.2 The discovery of transplantation antigens (i.e., the HLA system in humans) laid the ground work for the difficult and time-consuming clinical trials that have made hematopoietic stem cell transplantation a widely utilized treatment approach. The ability to freeze and thaw hematopoietic stem cells has led to the use of autologous hematopoietic stem cell transplantation (SCT) to support intensive therapy of a number of malignancies. Allogeneic hematopoietic SCT, syngeneic hematopoietic SCT (i.e., with an identical twin donor), and autologous hematopoietic SCT have all been used to reestablish hematopoiesis in patients who received otherwise lethal doses of therapeutic total body irradiation.
Barriers to Transplantation
Given this background, it would seem obvious that hematopoietic stem cell transplantation might be utilized to treat patients with severe bone marrow injury from accidental or intentional (e.g., a terrorist attack) radiation overdose. However, the use of hematopoietic SCT in these patients is complicated by a variety of factors. Radiation exposure is often not homogeneous. For example, patients might have bone marrow ablative doses of radiation to parts of their body, but other marrow-containing structures might be minimally or not irradiated. This can come about because the patient was partially shielded by an automobile, desk, wall, etc. Concomitant injuries such as burns or trauma can greatly complicate the care of patients who also have bone marrow failure induced by radiation. In a compilation of 58 patients who had potentially lethal radiation exposure, the major causes of deaths were burns (55%), hemorrhage (41%), infection (15%), and acute respiratory distress syndrome (15%). In many patients, more than one major factor contributing to death was identified.3 Obviously, in many of these patients hematopoietic SCT would not have repaired the injury that led to death.
Finally, a major terrorist event with the explosion of a nuclear device leading to mass casualties would also destroy the infrastructure necessary for the care of these patients. The likelihood of identifying patients who might benefit from transplantation, conducting typing, and providing the sophisticated care for a successful transplant seems slim.
Another major problem of the use of hematopoietic SCT to treat victims of accidental or intentional radiation exposure is to accurately diagnose those patients who might benefit. Doses below 3 Gy of uniform total body exposure would usually not be fatal with excellent nursing care. The upper dose limit that can be survived without hematopoietic SCT might be in the range of 7–8 Gy with prompt use of hematopoietic growth factors and aggressive supportive care. Doses in excess of 10 Gy are likely to be fatal because of injury to organs other than bone marrow. Thus, there is not a large “window” of opportunity for hematopoietic stem cell transplantation to be utilized. All of this is complicated by the difficulty in accurately determining the patient’s dose of radiation—a factor that is dealt with elsewhere in this manuscript.
Case Selection
A variety of scenarios would be possible in which a patient might benefit from hematopoietic stem cell transplantation after accidental or intentional radiation exposure. The most favorable situation would be a patient who had autologous HSCs stored for some other reason. While a few patients might have HSCs stored for military purposes, this would be unusual. However, in an attack in a large city, it is possible that some patients in whom autologous hematopoietic SCT as a treatment for cancer had been considered would have cells stored. It is also possible that patients with identical twins might be the victim of such an attack. In either circumstance, if logistics made it possible, it would seem wise to be liberal in the use of hematopoietic SCT to treat these patients and to err on the side of overutilization, since complications of either autologous or syngeneic hematopoietic SCT in this setting should be minimal.
It is unlikely that patients would be the victims of accidental or intentional radiation overexposure and know that they have an HLA matched sibling. It is even more unlikely that a patient would have had a search previously done for a matched unrelated donor. The ability to carry out either of these activities after a radiation event depends upon the size of the explosion and the number of patients injured. However, it is possible that HLA typing of siblings or an accelerated search for an unrelated donor might be accomplished. Particularly in children, the use of haplotype-matched donors might be considered. Unfortunately, available data to treat radiation injury with hematopoietic stem cell transplantation are not encouraging. In 29 patients who underwent hematopoietic SCT after accidental radiation overdose, the median survival was only 33 days.3 All 29 patients had some evidence of engraftment (i.e., 10%–100% of bone marrow cells being recipient type) 14 days after the transplant, but in none of the surviving patients was there permanent engraftment. Three patients survived for more than 1 year (2 with infusion of bone marrow stem cells and 1 with fetal liver cells) but in none was there permanent engraftment. It is impossible to determine whether or not these patients’ survival was related to the transplant. Unfortunately, in 7 of the 29 patients (24%) graft-versus-host disease (GVHD) was felt to be a major contributing cause of death.
Summary
At the present time, it is difficult to be optimistic about the contribution of hematopoietic SCT to treat patients of accidental or intentional radiation overdose. The chance of identifying patients with lethal marrow injury but no lethal injury to other organs and lacking severe burns or trauma, and with the medical infrastructure intact, seems poor. However, in the few patients who might have autologous or syngeneic HSCs available, this treatment may be lifesaving in patients where a significant (e.g., perhaps 4 Gy or more) exposure is felt to have occurred and might be utilized in patients in whom much higher doses than would be usually survivable are estimated but where there is some uncertainty. The use of matched sibling or unrelated allogeneic hematopoietic SCT in this setting will only occasionally be an appropriate treatment.
V. New Directions in Growth Factor Therapy
Thomas J. MacVittie, PhD,* and Ann M. Farese, MS
Greenbaum Cancer Center, University of Maryland, 655 W Baltimore Street, BRB 7-039, Baltimore MD 21201
Therapy for Lymphopenia and Immunosuppression
Both early-term and long-term myeloid reconstitution are dependent upon the administration of G-CSF, GM-CSF, or pegylated G-CSF as soon as possible postradiation exposure. Nevertheless, the effect of supportive care and CSF therapy on long-term immune reconstitution is unknown in the context of severely irradiated animals. There is, however, a substantial clinical database showing that the multicycle, myelosuppressive chemotherapy or myeloablative conditioning prior to stem cell transplant may have deleterious effects on immune recovery.1–,3 To date, there is no effective treatment for the prolonged T cell deficiencies associated with cytotoxic therapy. The significant delay in regeneration of CD4+ T cells, marked imbalance in the CD4/CD8 ratio, and a limited T-cell receptor repertoire leave the patient at risk for infectious complications. New therapeutic strategies are required in order to promote the naïve, thymic-dependent T-cell regeneration that is essential for long-term immune reconstitution. Several cytokines including IL-2, -4, -7, -17, c-kit ligand (KL), flt-3 (FL), thymic stromal lymphopoietin (TSLP), and keratinocyte growth factor (KGF) are associated with T-cell differentiation, proliferation, and enhanced thymopoiesis and functional recovery of peripheral T cells.4– 9 Of these, IL-7, TSLP, FL, and KGF are potential therapeutic agents for enhancing recovery of thymopoiesis and immune reconstitution.
Therapy for Immune Reconstitution
Immune suppression as profound cytotoxicity to the T-cell compartment, will be a common problem consequent to high dose, total-body irradiation, similar to that noted with multiple cycle chemotherapy and myeloablative conditioning for stem cell transplant. Potential new therapies include IL-7, KGF, and FL.
IL-7
IL-7 is produced by a subset of thymic epithelial stromal cells (TEC) and bone marrow cells and is a stimulus for proliferation, survival, and differentiation of immature thymocytes.7,10–,14 IL-7 administration increased the rate of T-cell immune reconstitution in murine models of autologous bone marrow transplant as well as chemotherapy or radiation-induced myelo and immune suppression.15–,19 Studies performed more than a decade ago demonstrated the therapeutic potential of IL-7 administered to mice with chemotherapy or radiation-induced lymphopenia.16,19,20 Sublethally irradiated mice treated with IL-7 showed accelerated lymphocyte recovery as well as increased white cell recovery in peripheral blood and spleen. IL-7 treatment of mice irradiated at higher doses resulted in preferential expansion of CD8+ T cells and more rapid normalization of the CD4/CD8 ratio. Morrisey et al noted an accelerated regeneration of CD4 and CD8 T cells with IL-7 administration in both spleen and lymph nodes of cyclophosphamide-induced lymphopenic mice.16
Bolotin et al showed that relative to controls, bone marrow–transplanted mice treated with IL-7 had more rapid normalization of thymic cellularity, normal proportions of thymic cellular subsets, peripheral CD4+ cells, and improved antigen-specific T and B cell function.17 Abdul-Hai et al noted that IL-7 treatment increased thymic cellularity 12-fold after syngeneic BMT.15 More recently Mackall and colleagues18 extended these studies to show that IL-7 administration exerts its beneficial effect on immune reconstitution via increased thymopoiesis and a direct increase in the magnitude of antigen-driven peripheral T-cell expansion. Thus, treatment with IL-7 enhances thymopoiesis following chemotherapy or myeloablative conditioning for BMT as well as antigen-driven, peripheral T-cell expansion in T-cell depleted or thymic-deficient hosts.15–,18,21
A cautionary note should be applied considering the use of IL-7 therapy in the context of GVHD following allogeneic SCT. Pharmacologic doses of IL-7 may have potent effects on mature T cells.18,21 IL-7 treatment may induce peripheral T-cell expansion and effectively enhance the response of mature T cells to alloantigen. These consequences may well predict enhancement of GVHD and graft rejection. Mackall’s group18 utilized a murine model of parent stem cells into F1 progeny to study the effect of IL-7 on immune reconstitution and GVHD following allogeneic bone marrow transplantation (alloBMT). In this model, administration of IL-7 lowered the threshold dose of T cells required to induce clinical signs of GVHD as well as lethal GVHD. IL-7 administration was associated with a greater degree of inflammation and tissue damage at all T-cell doses employed. It was observed however, that in a setting of alloBMT in T cell–depleted hosts, IL-7 therapy enhanced thymic function although not to the degree noted with treatment of syngeneic BMT recipients. This study stands in contrast to that reported by Alpdogan et al22 in which posttransplant administration of IL-7 to recipients of alloBMT enhanced lymphoid reconstitution without aggravating GVHD. It was noted that the alloreactive donor-derived T cells from the alloBMT recipients expressed little IL-7R. Resolution of these disparate results will require further examination of the respective variations in IL-7 dose and time and duration of IL-7 administration.
In summary, the preclinical database, primarily in rodent models of myelosuppression or BMT with myeloablative conditioning, suggests a potentially effective role for IL-7 therapy for reconstituting the immune system of severely irradiated personnel. Two recent studies in nonhuman primate models underscore this potential. Fry and colleagues demonstrated significant increases in peripheral blood CD4 and CD8 T cells after IL-7 treatment of moderately CD4-depleted SIV-infected rhesus macaques and suggested that a major component of its effect is peripheral homeostatic expansion of mature cells.23 Storek et al showed that IL-7 administration after autologous BMT in baboons stimulated a significant expansion of peripheral blood CD4 T cells.24
KGF
The efficient, de novo, thymic-dependent production of naïve T cells takes place within a stromal microenvironment composed of a complex network of epithelial cells and humoral factors. High-dose irradiation may severely disrupt the structured milieu that is necessary for reconstitution of the immune system and development of a functional T-cell repertoire. Recently, several groups have approached the problem of diminished immune reconstitution through the use of KGF, to stimulate recovery of the radiation or chemotherapy-damaged thymic epithelium.25– 27
KGF, a member of the acidic fibroblast growth factor (FGF-7) family, is produced by thymic epithelial cells in both the cortical and medullary regions. KGFR (FGFR2IIIb) is expressed on TECs and in turn, TECs respond in vitro to KGF and support thymocyte survival.13,25,28,29 Mice that are lacking either FGF10 or the FGFR2 splice variant have significant defects in thymopoiesis, hypoplastic thymic tissue and impaired thymic epithelial cell development.28,29 The rationale for use of KGF is further enhanced by the fact that IL-7 is produced by a subset of TECs and IL-7 is required for normal thymopoiesis.13,14,27 The literature documents the treatment efficacy of KGF in various murine models of bone marrow transplant in which KGF is administered prior to BMT. However, there are no published reports of KGF administered therapeutically in models of radiation- or chemotherapy-induced myelo- and immune-suppression.
KGF has been shown to protect epithelial cells from chemotherapy or radiation-induced injury.30,31 Subsequent studies demonstrated that pretreatment of mice with KGF ameliorated GvHD and manifestations of idiopathic pneumonia following intensive chemotherapy-induced conditioning and alloBMT.32–,34 Pretreatment with KGF reduced GVHD-induced weight loss as well as lesions in the skin and lungs of the long-term survivors resulting in significantly reduced morbidity and mortality.32 Blazar’s group extended these early studies and further demonstrated that KGF reduced GVHD, by mechanisms independent of repair of the conditioning-induced injury.35 Murine GVHD could be ameliorated in the absence of conditioning in SCID recipients of allo T cells. KGF could be administered prior to (3-day course) or after (7-day course) T cell transfer with no difference in survival although the posttreatment schedule promoted higher body weights. Blazar and Weinberg extended these studies by determining the ability of KGF pretreatment to enhance immune reconstitution via its restorative/protective effect on TECs and consequent production of intrathymic IL-7.27 In this report, the KGF-treated BMT recipients showed enhanced thymopoiesis and increased numbers of functional peripheral T cells. Furthermore, KGF pretreatment increased thymopoietic capacity of mice after congenic or alloBMT and after any of 5 different conditioning regimens that differed in radiation dose or cytotoxic therapy. Compared to PBS controls, KGF-treated groups had significantly increased thymic cellularity following radiation doses of 650, 1000, or 1400 cGy. KGF treatment increased thymocytes measured at 28 days after BMT by 3-fold over controls.27
Of significant note is the fact that a brief course of KGF pretreatment sustained the rise in cellularity of the thymus for at least 3 months after BMT. These differences in cellularity were due to an increase in donor-derived thymocytes, not from survival of radiation-resistant host thymocytes. Increased production of TEC-derived intrathymic IL-7 was suggested as the mechanism of KGF-induced post-BMT immune reconstitution. The KGF-treated mice had an increased frequency of intrathymic cells expressing IL-7 transcripts. Min et al27 substantiated this effect by analyzing the response to KGF treatment in IL-7−/− mice. They noted that the thymuses of either PBS- or KGF-pretreated IL-7−/− mice were hypocellular and consisted of only 20% donor-derived thymocytes. This suggests that IL-7 production by TECs is required for the observed effects of KGF on post-BMT thymopoiesis and immune reconstitution. Although the utility of pretreatment with KGF would be reserved for emergency first responders in the radiation accident scenario, its noted efficacy in BMT models and knowledge of its mechanism of action suggest its significant potential as a therapeutic.
FL
The pleiotropic FL acts at very early stages of hematopoietic and lymphoid development and appears essential in the development of a murine stem cell subset toward lymphoid-restricted progenitors in vivo and in vitro.4,36–,38 Furthermore, FL, through its action on dendritic cell expansion, may augment antigen-driven peripheral T cell homeostasis.39,41 Fry et al, utilizing FL in rodent models of BMT in both thymectomized and euthymic recipients, suggested that FL is capable of enhancing both thymic-independent homeostasis and thymopoietic pathways for T cell restoration.39 Additional studies using a class of engineered proteins called progenipoietins emphasized the potential of combination therapy.42 Progenipoietin, a chimeric composed of both flt-3 and G-CSF receptor agonists, administered after high-dose myelosuppressive irradiation significantly enhanced neutrophil recovery over that noted with FL or G-CSF monotherapy{930,1284|. The combined administration of FL and G-CSF was as effective in enhancing neutrophil recovery as progenipoietin. These data suggest that the combination of FL and one of the granulocyte CSFs—G-CSF, GM-CSF, or peg-G-CSF—would be effective in stimulating both neutrophil and immune recovery after severe, high-dose irradiation.
Cytokine combination therapy for enhanced myeloid recovery and survival after high-dose irradiation
A recent study by Herodin and colleagues focused on the use of a cocktail of early-acting cytokines to rescue mice irradiated with high-dose (90% lethal) total-body K-radiation.45 The cytokine combinations consisted of those with demonstrated in vitro, anti-apoptotic activity such as FL (F), TPO (T), IL-3 (3), SCF (S), and SDF-1. Lethally irradiated mice were administered a 4 (SFT3) component or 5 component (SFT3 + SDF-1) cocktail 2 hours and 24 hours after exposure. Either cocktail significantly increased survival from 8.3% in controls to greater than 80% in the treated cohorts. The authors noted, however, that long-term hematopoietic recovery was impaired while only 50% of the short-term survivors were alive 300 days after exposure and treatment. These data suggest that early administration of anti-apoptotic cytokines provide an anti-apoptotic survival effect on irradiated hematopoietic stem and progenitor cells assuring short-term reconstitution but limited long-term reconstitution. The authors suggested that supplemental treatment with selected cytokines or prolonged administration of the cytokine cocktail may be required for long-term hematopoietic reconstitution. However, the utility of this treatment protocol in the radiation accident scenario is marginalized due to the apparent requirement for 2, very early (2-hour and 24-hour) injections of cytokines postirradiation.
The early administration of survival promoting CSFs is worth consideration but also raises questions regarding the induced survival of genomically-damaged stem cells by diminishing the induction of the apoptotic pathway. The pivotal role of P53 in maintaining genomic integrity is reflected in the fact it is called the “guardian of the genome.”46 In response to radiation-induced DNA damage and other types of stress, P53 is stabilized, ensuring that cells carrying genomic damage are effectively eliminated. Activation of P53 gives rise to cell cycle arrest and apoptosis. The P53 gene is highly expressed in radiation-sensitive tissues such as the hematopoietic system. Wlodarski et al47 demonstrated the role of P53 in hematopoietic recovery after chemotherapy using P53 knockout and wild-type mice. Following 5-FU injection, a greater number of HSCs with repopulating ability and clonogenic activity were recovered in P55−/− mice than the P53+/+ counterparts. These results suggest that P53 suppression facilitates hematopoietic recovery by delaying exhaustion of the HSC pool, decreasing HSC sensitivity to apoptosis and enhancing the proliferative response to in situ CSFs. The P53−/− mice are also more resistant to higher doses of K-radiation than are the P53 wild type.48,49
Herodin and colleagues have compared the 4 (SFT3) and 5 (SFT3 + SDF-1) GF component cocktails to single injection of the component cytokines and show increased efficacy of the cocktail relative to the single CSF, suggesting additive or synergistic effects.45 For instance, the combination was indeed greater in effect, than TPO as a single injection. Previous investigations showed a single TPO administration within 2 hours or immediately after lethal exposure to mice significantly improved survival.50,51 Additional evidence for the role of CSFs in modulation of P53 activity is provided by Ritchie et al.52,53 This group showed that TPO upregulates the promoter conformation of P53 in Mo7e cells that have a diminished ability to mediate cell cycle arrest and apoptosis. This effect coincided with the downregulation of Bax and Mdm2 protein levels. The invocation of the P53-Mdm2 autoregulatory loop acts to keep P53 in tight check and possibly terminate the apoptotic signal.
Thus, in the case of antiapoptotic cytokines, the radiation-damaged HSCs, stimulated by appropriate cytokines, avoid apoptotic signals remain viable, repair genomic damage and remain available for renewal and differentiation.
Summary
Currently, there is only one treatment protocol for radiation-induced neutropenia in the accident scenario. There are two components. The first is aggressive supportive care. Preclinical studies in canines and nonhuman primates have documented the effect of supportive care consisting of antibiotics, platelets or whole blood transfusions and fluids on survival after lethal and supralethal doses of radiation.54– 59 The second is the administration of granulopoietic cytokines, G-CSF, GM-CSF, or pegylated filgrastim as soon as possible after the exposure. There is a substantial preclinical database showing the effect of these CSFs in stimulating granulopoiesis and survival after lethal doses of radiation. If IL-11 or TPO are considered, the same administration schedule applies. The available preclinical data with regard to IL-7, KGF, and FL suggests their utility in enhancing recovery of the immune system in severely irradiated personnel. It is reasonable to consider combination cytokine regimens given the lineage dominance of the CSFs available (Table 9 ). A combination of either granulopoietic CSF with IL-7, KGF, or FL may prove valuable in enhancing both long-term hematopoietic and immune reconstitution.
Distance at which 50% fatality occurs versus size of nuclear weapon.
Fatality from nuclear weapons occurs by different mechanisms, depending on the distance from the hypocenter. Fatality rates for a single type of injury are graphed. Fatality is increased by the interaction of multiple types of injuries.
Reprinted with permission of the National Council on Radiation Protection and Measurements, NCRP Report No. 138.30
Distance at which 50% fatality occurs versus size of nuclear weapon.
Fatality from nuclear weapons occurs by different mechanisms, depending on the distance from the hypocenter. Fatality rates for a single type of injury are graphed. Fatality is increased by the interaction of multiple types of injuries.
Reprinted with permission of the National Council on Radiation Protection and Measurements, NCRP Report No. 138.30
Frequency of chromosome aberrations (dicentrics and rings) at low doses (dashed line) and at higher doses (solid line).
The probability of an exchange aberration is proportional to dose (D) and the square of the dose (D2), respectively. At low doses, a secondary electron resulting from absorption of an x-ray photon induces breaks in each of the chromosomes. At high doses, secondary electrons from multiple atoms induce these chromosome breaks. The net effect of a break is interchange of chromosomal material, resulting in the formation of a dicentric and an acentric fragment.
Reprinted with permission from
Frequency of chromosome aberrations (dicentrics and rings) at low doses (dashed line) and at higher doses (solid line).
The probability of an exchange aberration is proportional to dose (D) and the square of the dose (D2), respectively. At low doses, a secondary electron resulting from absorption of an x-ray photon induces breaks in each of the chromosomes. At high doses, secondary electrons from multiple atoms induce these chromosome breaks. The net effect of a break is interchange of chromosomal material, resulting in the formation of a dicentric and an acentric fragment.
Reprinted with permission from
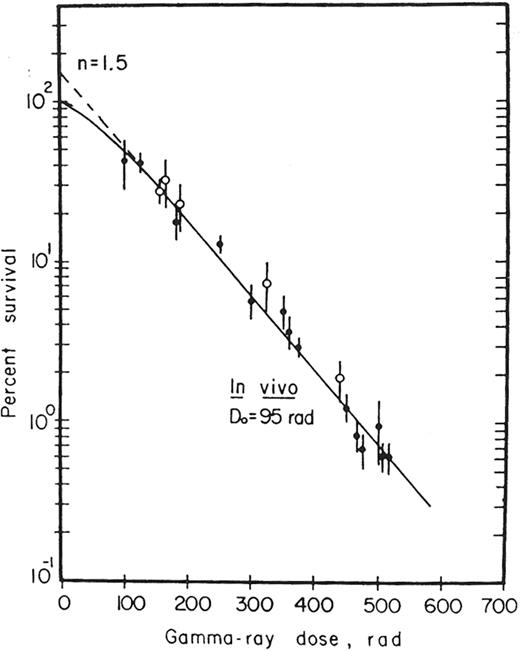
K-ray survival curve for CFU-S.
The surviving fraction for a dose D = colonies counted/cells inoculated × plating efficiency.
Reprinted with permission from
K-ray survival curve for CFU-S.
The surviving fraction for a dose D = colonies counted/cells inoculated × plating efficiency.
Reprinted with permission from
Survival curve for CFU-S.
Following an initial small shoulder, a single exponential curve is evident between doses of 0.8 Gy to 2.0 Gy. This is followed by greater than expected survival at 4.0 and 6.0 Gy. The results are consistent with a multiphasic, concave model.
Reprinted from Experimental Hematology, Vol. 23, Inoue T, Hirabayashai Y, Mitsui H, et al. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction, p. 23, 1995, with permission from The International Society for Experimental Hematology.51
Survival curve for CFU-S.
Following an initial small shoulder, a single exponential curve is evident between doses of 0.8 Gy to 2.0 Gy. This is followed by greater than expected survival at 4.0 and 6.0 Gy. The results are consistent with a multiphasic, concave model.
Reprinted from Experimental Hematology, Vol. 23, Inoue T, Hirabayashai Y, Mitsui H, et al. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction, p. 23, 1995, with permission from The International Society for Experimental Hematology.51
General approach to triage and therapy of radiation incident victims.
A numerical degree of severity is assigned for the cutaneous, gastrointestinal, neurovascular, and hematopoietic systems, as defined in Tables 4 and 5. Thehighest degree in any system indicates the overall “response category” (i.e., 1, 2, 3or 4). *Victims with significant injury to a nonhematopoietic system are poorcandidates for transplantation. The presence of G4, C4, and/or N4 degree indicatesprobable death. Supportive therapy alone is indicated (fluids, blood components,antibiotics, pain Rx, counseling). Modified from N. Dainiak.48
General approach to triage and therapy of radiation incident victims.
A numerical degree of severity is assigned for the cutaneous, gastrointestinal, neurovascular, and hematopoietic systems, as defined in Tables 4 and 5. Thehighest degree in any system indicates the overall “response category” (i.e., 1, 2, 3or 4). *Victims with significant injury to a nonhematopoietic system are poorcandidates for transplantation. The presence of G4, C4, and/or N4 degree indicatesprobable death. Supportive therapy alone is indicated (fluids, blood components,antibiotics, pain Rx, counseling). Modified from N. Dainiak.48
Leukocyte counts based on exposure dose in patients exposed to radiation in Chernobyl.5
The abortive rise (transient increase before the fall) in leukocytes, which are primarily composed of granulocytes, in doses < 500 rad (< 5 Gy). The onset of neutropenia may not occur for weeks especially with lower exposures and the duration of neutropenia may be weeks.
Reprinted with permission from
Leukocyte counts based on exposure dose in patients exposed to radiation in Chernobyl.5
The abortive rise (transient increase before the fall) in leukocytes, which are primarily composed of granulocytes, in doses < 500 rad (< 5 Gy). The onset of neutropenia may not occur for weeks especially with lower exposures and the duration of neutropenia may be weeks.
Reprinted with permission from