Abstract
Advances in vascular biology and drug development, as well as improved interventional techniques, are yielding multiple new treatments for patients with venous and/or arterial thrombosis. Hematologists who are providing consultations for these patients often participate in a multidisciplinary approach to provide optimal care. New anticoagulants, simplified and validated tests for detecting vascular disease, and improved interventional procedures can all reduce the morbidity and mortality that result from venous and arterial thrombosis. In this chapter, different aspects of the diagnosis and treatment of these disorders are addressed by a hematologist, an expert in vascular medicine, and a vascular surgeon.
The key to the prevention and treatment of venous and arterial thrombosis is anticoagulant and antiplatelet therapy. In Section I, Dr. Charles Francis, a hematologist with expertise in thrombosis and hemostasis, describes the clinical trials that have resulted in the approval of newer anticoagulants such as fondaparinux and the thrombin- specific inhibitors. He also reviews the clinical trials that have shown the efficacy of the new oral anticoagulant ximelagatran. Although currently under study primarily for the prevention and treatment of venous thrombosis, these anticoagulants are likely to undergo evaluation for use in arterial thrombosis.
Peripheral arterial disease (PAD), which affects as many as 12% of individuals over the age of 65 years, provides a diagnostic and therapeutic challenge to physicians across multiple subspecialties. Dr. William Hiatt, a specialist in vascular medicine, discusses in Section II the epidemiology and manifestations of PAD, the best ways in which to diagnose this disorder and determine its severity, and the most appropriate pharmacologic treatment.
In Section III, Dr. Mark Jackson, a vascular surgeon, describes interventional procedures that have been developed or are under development to treat arterial thrombosis. He also reviews the status of inferior vena caval filters that are retrievable.
I. Newer Anticoagulant Agents for the Consultant’s Toolbox
Charles W. Francis, MD*
University of Rochester Medical Center, 601 Elmwood Avenue, Box 610, Rochester NY 14642-0001
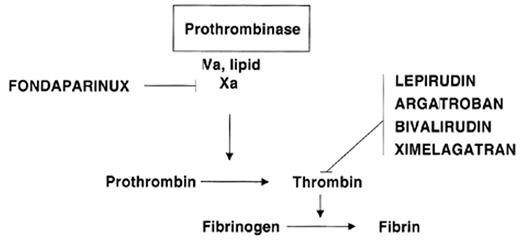
Clinical need and rapid scientific progress are the two powerful forces driving the rapid development of new anticoagulant drugs. Knowledge of the biochemistry of hemostasis has rapidly expanded in recent years with characterization of the structures and details of interaction of the proteins involved. This knowledge permits rational design of specifically targeted drugs and better prediction of anticoagulation effects. Drug development is also driven by the great burden of thrombotic disease, which represents the major cause of morbidity and mortality in developed countries. Before the introduction of low-molecular-weight heparins (LMWH), warfarin and heparin were the only two drugs available, but the introduction of new anticoagulant drugs has been rapid and is accelerating. The focus of this review is the several recently introduced specific factor Xa and thrombin inhibitors (Table 1 ). These differ fundamentally from previously available anticoagulants because they are synthetic and also are targeted to inhibit a specific enzyme in the coagulation system rather than to affect multiple factors (Figure 1 ). The hematologists’ “tool box” will certainly expand rapidly, as there are a variety of novel agents in development targeting these and other sites in the coagulation system. Broad application of these new agents requires that they meet important clinical needs. Thus, the widespread application of low-molecular-weight heparins (LMWH) resulted from their simplicity of dosing and subcutaneous (SC) administration in comparison with the difficulties with intravenous adjusted-dose heparin for treatment of venous thromboembolism (VTE). The two parenteral direct thrombin inhibitors, argatroban and lepirudin, have been successfully introduced to meet the need for treatment of heparin-induced thrombocytopenia (HIT).
The value of new anticoagulants is judged by their effectiveness, safety, convenience, and cost in relation to the seriousness of the clinical problem. Their overall use can be divided into short-term application for acute problems in hospitalized patients and long-term use in preventing venous thrombosis or systemic embolization. In this latter group, there is a large unmet need for an alternative oral anticoagulant for warfarin, a drug that has serious problems with monitoring, dose adjustment, and a narrow therapeutic margin. The second major problem is safety, as all available agents can either cause or exacerbate bleeding in doses within the therapeutic range, and a major challenge is to develop new anticoagulants with a greater margin of safety.
Expert management of anticoagulation is an important and growing area for the consulting hematologist, particularly in hospitalized patients who often develop difficult management problems due to concurrent bleeding risks and comorbidities. The basis for successful use of these newer anticoagulants is an understanding of their principles, pharmacology, and the clinical trial results that demonstrate their value.
Fondaparinux
Fondaparinux (Arixtra) is a recently-approved anticoagulant that is novel in two respects: it represents a totally synthetic polysaccharide and it has specificity for factor Xa. Fondaparinux is a pentasaccharide whose structure is based on the minimal saccharide sequence in heparin that interacts with antithrombin to accelerate its activity. Whereas heparin and LMWH are heterogeneous and derived from animal sources, fondaparinux is entirely synthetic with a uniform composition. Binding of fondaparinux to antithrombin accelerates inhibition of factor Xa activity approximately 300-fold, but the short chain length renders it incapable of inactivating thrombin, resulting in a specific Xa inhibitor with an activity of approximately 700 U/mg. This contrasts with unfractionated heparin and LMWH, which have varying amounts of both anti-Xa and antithrombin activity. The effectiveness of fondaparinux as an anticoagulant provides the first clear demonstration that specific inhibition of factor Xa alone can prevent thrombosis.
Fondaparinux is completely absorbed following SC administration with a peak concentration in 2 to 3 hours and a half-life of 17 to 21 hours, allowing once daily administration. There is no significant metabolism, and most drug is excreted unchanged through the kidneys. There is no appreciable interaction with platelets or plasma proteins, including platelet factor-4, suggesting that fondaparinux may be useful for patients with HIT. Fondaparinux does not induce release of tissue factor pathway inhibitor and does not affect platelet function or fibrinolysis. It is not neutralized by protamine sulfate, and there is no specific agent for reversal. It has minimal effects on the prothrombin time (PT) and activated partial thromboplastin time (aPTT); although not recommended routinely, plasma levels can be measured using an anti-Xa assay employing fondaparinux as the reference.
The clinical development of fondaparinux has focused primarily on prevention and treatment of VTE, and several large Phase III studies have been completed (Table 2 ). A dose of 2.5 mg SC once daily was very effective in prophylaxis of VTE following hip replacement, with total rates of 4.1% and 6.1%, which compare favorably with those in other large trials. The fondaparinux results in the Ephesus Study1 were significantly better than enoxaparin using the European regimen, in which enoxaparin was administered pre-operatively, but there was a nonsignificant difference in the groups in the Pentathalon Study2, which used enoxaparin twice daily beginning postoperatively, the regimen which is used in North America. The results with fondaparinux in hip fracture3 and knee replacement4 were superior to enoxaparin, and the Penthifra-Plus5 study of prolonged prophylaxis demonstrated a very low rate of 1.4% at 28 days. The surprising finding in that study was a high rate of 35% in the group that received fondaparinux for only 7 days. A meta-analysis of the 4 trials7 found a relative risk reduction for VTE of 55% compared with enoxaparin with a comparable effect across all types of surgery and subgroups, but major bleeding occurred more frequently in the fondaparinux groups. An important contribution to its high efficacy may be that fondaparinux was started 6 hours postoperatively, a time that optimizes effectiveness while minimizing bleeding complications.8 Enoxaparin was begun at least 12 hours before or after surgery in the comparison groups.
Studies of deep venous thrombosis (DVT) and pulmonary embolism (PE) treatment have been completed and presented in abstract form6 (Table 2 ). Both studies used a fondaparinux dose of 7.5 mg once daily but different comparators. In the Matisse PE trial, patients were initially treated with heparin followed by warfarin, whereas enoxaparin was used for initial treatment in the Matisse DVT trial. The rates of recurrence were comparable at 3.8% and 3.9%, respectively, close to the rates in the comparator groups and met statistical criteria for noninferiority. There was no significant difference in bleeding complications, which were low.
Because fondaparinux excretion is renal, increased drug levels can occur with renal impairment resulting in bleeding complications. Dose reduction and monitoring plasma levels may be needed in patients with renal impairment and in the elderly, even if renal impairment is not evident. Elevated drug levels can occur in patients of low body weight; fondaparinux should also be used with caution with neuraxial anesthesia.
Small clinical studies have also evaluated the use of fondaparinux in the management of coronary artery disease. The Pentalyse trial compared fondaparinux with unfractionated heparin as an adjunct to fibrinolysis in 333 patients with ST-elevation myocardial infarction in doses from 4 to 12 mg daily for 5 to 7 days with no significant difference in angiographic outcomes.9 Four different doses of fondaparinux were also investigated in the Pentua trial in 929 patients with acute coronary syndromes in comparison with enoxaparin. No clear dose response was found.10 Fondaparinux does not react with platelet factor-4; it may therefore be a useful alternative to heparin in the treatment of patients with HIT, but clinical trials have not been reported.
Direct thrombin inhibitors
Thrombin may be viewed as the central enzyme in hemostasis, and it has been a key target in development of new anticoagulant drugs. Although its major action is often viewed as conversion of fibrinogen to fibrin, thrombin has other prothrombotic actions through feedback amplification, activation of factor XIII, and platelet activation. Other important functions include an anticoagulant effect through activation of protein C, inhibition of fibrinolysis through activation of thrombin activatable fibrinolysis inhibitor, and a variety of effects on endothelial and vascular smooth muscle cells. Thrombin is inhibited physiologically through antithrombin, and this forms the basis for the pharmacological effectiveness of both heparin and LMWH.
Direct thrombin inhibitors block the enzymatic action of thrombin without the need for antithrombin or other cofactors. Detailed structural studies of thrombin including resolution of its crystallographic structure have led to the development of novel direct thrombin inhibitors for therapeutic use including lepirudin, bivalirudin, argatroban, melagatran, and ximelagatran. Direct thrombin inhibitors have several potential advantages over anticoagulants that act through antithrombin. They show little interaction with plasma proteins or blood cells, resulting in predictable pharmacokinetics and anticoagulant response, and their activity is not reduced when antithrombin levels are low. They are also able to inhibit thrombin bound to fibrin or fibrin degradation products because of their small size, whereas the large heparin-antithrombin complex does not completely inhibit thrombin bound to fibrin or to cells, thus allowing it to maintain coagulant activity.11
Hirudin
Hirudin is the native anticoagulant polypeptide in the leech, and the structure is slightly modified in the biosynthetic molecule lepirudin (Refludan), which binds tightly and nearly irreversibly to the catalytic site and exosite 1 of thrombin, resulting in prolonged anticoagulant action. In patients with normal renal function, lepirudin has a half-life of 1 to 3 hours and results in dose-dependent prolongation of the aPTT, PT, and thrombin time after intravenous administration. Therapy is typically monitored and the dose adjusted based on the aPTT. Formation of antilepirudin antibodies may occur and result in decreased drug clearance and an increased anticoagulant effect.12 No specific antidote is available to neutralize its activity, although case reports indicate that dialysis may be useful.13
Argatroban
Argatroban is a synthetic derivative of arginine that binds reversibly to the active site of thrombin. It is administered intravenously and has a short half-life of approximately 45 minutes with hepatic metabolism. Therapy is monitored and the dose is adjusted according to the aPTT. No specific antidote is available, but the drug is rapidly cleared except in patients with poor hepatic function.
Heparin-induced thrombocytopenia
The direct thrombin inhibitors show no immunologic cross-reactivity with the antibodies responsible for HIT, and both lepirudin and argatroban are approved by the FDA for treatment of HIT. Approval was based on small clinical studies using historical controls that showed a reduced rate of adverse clinical outcomes.14–,17 Argatroban is also approved for percutaneous coronary interventions in patients with HIT, based on a small study showing satisfactory outcomes.18 Both agents are effective, but argatroban is a better choice in patients with renal compromise, whereas lepirudin is a better choice in patients with hepatic dysfunction. Both drugs are given intravenously with doses titrated to achieve an aPTT 1.5 to 3 times the initial baseline. Because both prolong the PT as well as the aPTT, monitoring the conversion to warfarin can be problematic. Management of patients with HIT who require urgent surgery with cardiopulmonary bypass is difficult, although bivalirudin,19 recombinant hirudin,20 and argatroban21 have all been used with success.22
Bivalirudin
Bivalirudin (Angiomax) is a small peptide whose structure was engineered based on naturally occurring hirudin. The domain that inhibits the active site of thrombin is connected by 4 glycine residues to a dodecapeptide that interacts with a thrombin exosite.23 Following parenteral administration, the half-life is approximately 30 minutes with dose-dependent prolongation of the aPTT and activated clotting time (ACT), and elimination through both liver and kidney. In 2000, the FDA approved bivalirudin in combination with aspirin for patients with unstable angina who were undergoing percutaneous transluminal coronary angioplasty (PTCA); approval was based largely on trials performed several years earlier.24 The development of the direct thrombin inhibitors for coronary indications has been difficult, and early studies were equivocal or showed excess bleeding.25 The recently-reported REPLACE 2 trial evaluated bivalirudin in patients needing urgent or elective percutaneous coronary intervention and included the use of heparin and a GP IIbIIIa receptor inhibitor.26 In a composite primary end point of death, myocardial infarction, urgent repeat revascularization, or major bleeding by 30 days, the use of bivalirudin with provisional GP IIbIIIa receptor blockage was not statistically inferior to heparin plus planned GP IIbIIIa receptor blockage. Bleeding complications were somewhat less with bivalirudin, but this advantage was mostly related to a reduction in minor bleeding. Bivalirudin and other direct thrombin inhibitors need further evaluation in the setting of acute coronary syndromes.27
Ximelagatran
Ximelagatran (Exanta; H376/95) is an oral direct thrombin inhibitor, currently in Phase III trials, that shows promise in both VTE and atrial fibrillation. Ximelagatran is an oral “prodrug” formulation of melagatran, which is the active thrombin inhibitor. Melagatran is being developed in Europe as a parenteral direct thrombin inhibitor. It is a dipeptide whose structure mimics the sequence immediately N-terminal to the thrombin cleavage site on the Aα chain of fibrinogen. Melagatran is very selective for thrombin with minimal activity toward other relevant serine proteases. Because of its small size and high affinity, it inhibits clot-bound as well as free thrombin. Absorption is nearly complete after SC administration, reaching a maximum after 30 minutes with a half-life of approximately 1.6 hours. Clearance is primarily renal with over 80% excreted unchanged. Following oral administration, ximelagatran is rapidly absorbed and yields melagatran as its main metabolite with maximum plasma concentrations of melagatran between 1.5–2 hrs and a half-life of approximately 3 hours. Plasma concentrations of melagatran are linearly related to ximelagatran doses with low inter- and intra-individual variability. There appear to be no significant food or drug interactions. The thrombin time, PT, and aPTT are all prolonged after administration of ximelagatran and other direct thrombin inhibitors, but monitoring with the ecarin clotting time may also be useful, especially for elevated drug levels such as are needed during cardiopulmonary bypass surgery.28 Clinical studies have excluded patients with poor renal function because the drug is excreted renally.
Melagatran and ximelagatran are not approved for clinical use but are in advanced Phase III clinical trials (Table 3 ). Several large studies have been published or reported in prevention of VTE after orthopedic surgery with different approaches in Europe compared with North America. The European Express29 and Methro III30 studies used an initial dose of melagatran to provide rapid anticoagulation, and this treatment was followed by oral ximelagatran. In the Express study, 2 mg of melagatran was given SC immediately before surgery, another 3 mg on the evening of surgery, followed by ximelagatran 24 mg orally twice daily. This regimen had superior efficacy to the enoxaparin comparator, but there was also an increase in major bleeding defined as “excessive bleeding as judged by the investigator.” A modified regimen was used in Methro III, which started ximelagatran 3 mg SC 4 to12 hours after surgery followed by 24 mg of ximelagatran orally twice daily.30 This study showed comparable efficacy and similar bleeding results to enoxaparin. Notably, the European studies have combined hip and knee replacement subjects together for primary analysis.
The North American program in orthopedic prophylaxis has differed, with separate studies for knee and hip replacement and use of oral ximelagatran only. The initial dose of 24 mg twice daily was chosen after a Phase II study compared ximelagatran in several doses to enoxaparin after knee replacement in 594 patients.36 A dose-response effect on efficacy or safety was not established, but the 24 mg dose was comparable in effectiveness and safety to enoxaparin and was chosen for initial studies. The initial Phase III study in knee replacement31 compared ximelagatran with warfarin and showed comparable efficacy and safety. A study in hip replacement compared ximelagatran 24 mg twice daily with enoxaparin.33 Designed to show noninferiority, this study demonstrated that enoxaparin was better than ximelagatran at this dose with no difference in bleeding end points. Based on these 2 studies, the dosing was reevaluated, and a larger study in knee replacement was performed comparing 24 and 36 mg twice daily with warfarin after knee replacement. The results with 24 mg were similar to warfarin and comparable with that reported previously, whereas efficacy with ximelagatran 36 mg twice daily was significantly better than with warfarin with comparable bleeding.32 Overall, the results with the oral ximelagatran only program are mixed, and dependent on dosing, type of surgery, and choice of comparator. The knee replacement studies are encouraging and suggest that the lower, 24 mg dose, is comparable with warfarin, whereas the 36 mg dose is superior.
Studies with long-term ximelagatran treatment of established DVT include the Thrive II and V study that compared standard treatment with enoxaparin (followed by adjusted-dose warfarin) to a regimen of ximelagatran 36 mg twice daily as both initial and chronic therapy.37 The results have yet to be reported in detail. The Thrive III study examined 1233 patients with VTE who received 6 months of initial anticoagulation and then were randomized to receive either ximelagatran (24 mg twice daily) or placebo for another 18 months.35 The results showed a clear benefit for prolonged treatment with ximelagatran, with recurrence in significantly fewer patients than in those assigned to placebo (P < .0001).
Treatment of patients with atrial fibrillation to prevent stroke is a common indication for long-term warfarin anticoagulation and the subject of the large Sportif III study.34 Patients with atrial fibrillation and 1 or more additional risk factors for stroke were randomized in an open-label study with blinded adjudication to treatment with adjusted-dose warfarin or ximelagatran 36 mg twice daily to establish noninferiority. The intention-to-treat analysis of the primary efficacy end point of stroke and systemic embolism showed 40 events in the ximelagatran group (1.6%/yr) and 56 events (2.3%/yr) in the warfarin group, indicating noninferiority. An on-treatment analysis showed a significantly better outcome in the ximelagatran group. There were no differences in major bleeding or all-cause mortality.
The interpretation of studies of long-term therapy with ximelagatran must consider the problem of transaminase elevations. Elevations of greater than 3 times the upper limit of normal of alanine aminotransferase occurred in 6.4% of ximelagatran-treated patients in Thrive III and in 6.5% in Sportif III. The clinical implications are unclear, as levels may decline to normal with continued treatment. However, ongoing trials require discontinuation of ximelagatran in participants who develop marked or persistent transaminase elevations; this side effect may eventually result in the need for routine monitoring of liver function tests if ximelagatran treatment is continued for longer than 30 days.
The potential clinical application of ximelagatran and melagatran will depend on the results of additional studies and regulatory review. A new oral anticoagulant would be welcome, and ximelagatran appears to have comparable or better efficacy and safety than warfarin in short-term prophylaxis studies. Initial long-term studies with ximelagatran show promise, but limitations include the need for twice-daily administration and monitoring of liver function. Additionally, no specific agent to reverse the anticoagulant effect is available, as is also true with other direct thrombin inhibitors. Given the short half-life of the drug, this is unlikely to be a problem unless ximelagatran is administered to patients with poor renal function.
Future Directions
The development of fondaparinux and direct thrombin inhibitors has established that effective anticoagulation can be provided with a drug specifically targeted to a single enzyme in the coagulation system, representing a new therapeutic concept. The availability of multiple drugs targeting different enzymes raises the possibility of combined therapy using several agents in individualized doses, based on the characteristics of a patient’s hemostatic system or pattern of vascular disease and thrombosis. Many other new anticoagulant agents are in the early phases of clinical development and show promise. Idraparinux is a parenteral antithrombin-dependent specific anti-Xa inhibitor similar to fondaparinux but with a much longer half-life, allowing once-weekly administration. Heparin can be chemically derivatized to permit oral bioavailability and 1 such agent, SNAC-heparin, has shown promise in prophylaxis in orthopedic patients.38 NAPc2, an inhibitor of factor VIIa/tissue factor derived from the nematode, showed effectiveness in Phase II studies in thromboprophylaxis,39 and an oral anti-Xa agent has been in Phase II trials in orthopedic thromboprophylaxis. Other agents targeting factor VIIa and factor IXa are being developed, as are soluble thrombomodulin and other direct thrombin inhibitors.
Summary
The availability of new anticoagulants is expanding choices for prevention and treatment of thrombotic disease. The synthetic pentasaccharide, fondaparinux, provides excellent prophylaxis in orthopedic patients, and new studies suggest it may have a role in treating DVT and PE with once-daily injections. Either argatroban or lepirudin is an excellent choice for patients with HIT, with argatroban preferable in patients with renal compromise and lepirudin in those with liver dysfunction. Bivalirudin should be considered for patients undergoing percutaneous coronary intervention and may offer the advantage of reduced bleeding complications. Ximelagatran is not yet approved, but studies in atrial fibrillation, DVT treatment, and prophylaxis in orthopedic surgery suggest this new oral agent will be an important anticoagulant.
II. Evaluation of Patients with Peripheral Vascular Disease
William R. Hiatt, MD*
University of Colorado Health Sciences Center, 4200 East 9th Avenue, Box 180, Denver CO 80262
Peripheral arterial disease (PAD) of the arterial circulation to the lower extremities is one of the major manifestations of systemic atherosclerosis. The disease is initially asymptomatic (patients are identified only by a reduced blood pressure in the ankle) but with progression will cause exertional symptoms of intermittent claudication. In rare cases, the disease progresses to severe chronic leg ischemia which can result in limb loss. All patients with PAD have an increased risk of cardiovascular morbidity and mortality. Thus, therapy is directed to both the limb and the systemic atherosclerotic disorder. Venous thromboembolic diseases also affect a large segment of the population, but have a different pathophysiology from arterial disease. However, recent reports describe an association between these conditions, suggesting that many patients with spontaneous venous thrombosis also have atherosclerosis in the peripheral arterial circulation.1 Patients with primary clotting disorders may therefore need assessment of their arterial circulation, with special attention to addressing their cardiovascular risk.
Epidemiology
In the Framingham study, both men and women had a similar incidence of intermittent claudication, which increased with age, and had a peak incidence in older individuals of approximately 60 new cases per 10,000 persons per year.2 When noninvasive screening instruments are used (the ankle/brachial index [ABI] described below), the prevalence of PAD is quite high, affecting 12% of the adult population and 20% of individuals over the age of 70.3,4 These figures extrapolate to approximately 8 million persons affected with PAD in the United States.
Patients with PAD are at high risk for concomitant coronary and carotid arterial diseases, resulting in increased morbidity and mortality. For example, in patients with PAD, the adjusted all-cause mortality risk is increased 3-fold and cardiovascular mortality is increased 6-fold.5 This risk is approximately the same for both men and women, and remains high even if the patient has had no prior evidence of cardiovascular disease.6,7 With increasing severity of PAD, as measured by the ABI, there is a concomitant increased risk of myocardial infarction, ischemic stroke, and vascular death.8,9 Thus, the ABI provides important information regarding the diagnosis and prognosis of PAD.
Clinical Evaluation and Differential Diagnosis
Chronic arterial insufficiency of the lower extremity causes two very characteristic types of pain: intermittent claudication and ischemic rest pain. Claudication discomfort most commonly involves the calf and is resolved within 10 minutes of rest. In contrast, patients with chronic critical limb ischemia typically present with rest pain in their distal foot that occurs at night and is relieved with dependency. These patients are at risk for developing painful ischemic ulcers at the distal points of the foot.
Palpation should be performed of the brachial, femoral, and pedal arteries. The location of the posterior tibial pulse is behind the medial malleolus, and the dorsal pedis pulse runs along the dorsum of the foot between the first and the second metatarsal bones. The grading of pulses can be described as normally present (easily palpable), diminished (difficult to palpate), or absent. Patients with a palpable femoral pulse, but absent pedal pulses, typically have arterial disease confined to the leg, whereas a diminished or absent femoral pulse indicates disease of the aorta or iliac arteries (the inflow).
The differential diagnosis in these patients includes diabetic sensory neuropathy that produces burning and numbness in both feet at rest. Severe neuropathy can lead to chronic diabetic malperforans ulcers (these ulcers are usually on the plantar surface of the foot at pressure points). Patients with diabetic neuropathy may have normal pedal pulses, but even diabetic ulcers are often associated with some degree of arterial occlusive disease. Reflex sympathetic dystrophy may be present after trauma to the limb, and leads to a painful, discolored, swollen extremity. Patients with arteritis may present with ischemic limbs, particularly those with Buerger’s disease (thromboangitis obliterans). Claudication-like symptoms may also arise from spinal stenosis, which is due to osteophytic narrowing of the lumbar neurospinal canal. These symptoms include numbness and weakness in the lower extremity that is produced by standing. Patients with arthritis of the knee or hip may also have pain in the joint with ambulation, but also pain at rest or with weight bearing. Finally, patients with prior venous thrombosis can develop venous claudication. This is characterized by venous engorgement in the affected leg with walking that causes swelling and discomfort. Relief requires leg elevation.
Vascular Testing
The noninvasive evaluation of patients with PAD begins in the physician’s office. Patients considered at risk for PAD are older, have cardiovascular risk factors of smoking or diabetes, or have exercise-induced leg symptoms. The initial evaluation includes a thorough vascular history and physical examination, followed by the measurement of an ABI.
Ankle/brachial index
Patients considered to be at risk for PAD should have an ABI. Patients considered for screening would be those between the ages of 50–69 years who have a history of smoking or diabetes, and all patients over the age of 70 years.10 In these patients, the prevalence of PAD can be as high as 29%. Patients with exertional symptoms in their legs or foot ulcers should also have an ABI to determine if their symptoms are due to vascular disease. Performing an ABI allows the physician to make the diagnosis of PAD, judge the severity of the leg arterial disease and risk of cardiovascular events, and identify patients with severe limitations in their exercise capacity. Arteriography is not necessary to make the diagnosis of PAD, but rather should be reserved for patients who are being considered for revascularization therapy.
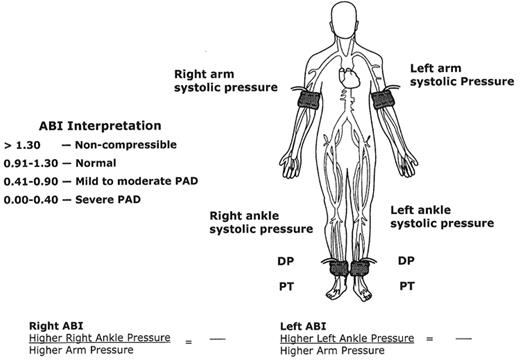
In order to perform an ABI, the physician needs a blood pressure cuff and a handheld Doppler. Figure 2 demonstrates the locations of the ankle and arm cuffs to obtain the ABI. The higher of the two systolic brachial pressures is used as the denominator of the ABI calculation. The higher of the two ankle pressures (either the dorsalis pedis or posterior tibial artery) is used as the numerator of the ABI calculation for each leg. A normal ABI is defined as a resting measurement ≥ 0.90. Any value < 0.90 represents the presence of PAD, with lower values indicating more severe disease. ABI values < 0.40 suggest the most advanced stages of PAD, which can result in ischemic rest pain, nonhealing ulcerations, and gangrene.
Vascular laboratory testing.
The ABI is generally sufficiently accurate to determine the presence and severity of PAD. However, if the ankle tibial vessels are calcified (patients with diabetes mellitus or end-stage renal disease), an accurate ankle pressure cannot be obtained. The cuff pressures are typically greater than 250 mmHg, or the ABI value is > 1.30, indicating noncompressible vessels under the cuff. If not recognized as artificially high, the physician may falsely conclude that arterial circulation is adequate, or even normal, in these patients. With high ABI values, other tests available in the vascular diagnostic laboratory are necessary to make the diagnosis of PAD, including toe pressures, arterial duplex ultrasonography, and transcutaneous oximetry (TcPO2) to assess wound healing potential.
Occasional patients with classic symptoms of claudication may have a normal physical examination and ABI at rest. This condition often reflects iliac artery stenosis with a normal distal circulation. In this case, exercise treadmill testing should be performed in the vascular diagnostic laboratory. Patients have resting pressures measured, and are then placed on a treadmill at a constant speed and constant grade of incline. The patient is asked to report initial symptoms of limb discomfort, and then terminate the exercise when the discomfort is limiting, or after 5 minutes. After exercise, pressures are again measured. A significant decrease in postexercise pressures confirms the diagnosis of PAD.
If the treatment plan includes consideration for revascularization, location of the arterial occlusive disease is required. These tests include segmental limb pressures, pulse volume recordings, and arterial duplex ultrasonography. The use of sequential limb blood pressure cuffs placed on the thigh, calf, and ankle provides a rough localization of the arterial segment(s) involved.11 Each vascular laboratory will have developed standards for determining significant changes in systolic blood pressure between cuffs. For example, the pressure obtained in the proximal thigh cuff is normally > 30 mmHg higher than the brachial pressure. Lower pressures suggest the presence of inflow aortoiliac artery disease. These patients typically respond well to angioplasty in terms of treatment success.
Pulse volume recordings (PVRs) are also obtained from cuffs placed on the thigh, calf, and ankle that describe arterial waveforms. In the normal setting, this waveform is similar in appearance to an intra-arterial pressure waveform. As the arterial circulation worsens, the PVR changes morphology and decreases its amplitude. This methodology is often used when the ABI is noncompressible, since the arterial waveforms are not affected by calcification.
Arterial duplex ultrasonography is a highly accurate examination, which may be performed from the aortic bifurcation to the ankles. Utilizing currently available Duplex ultrasound scanners, arteries are visualized, often with color imaging, and Doppler velocities are obtained. A doubling in the peak systolic velocity suggests a 50–99% stenosis. In patients who are in need of revascularization, this examination is as accurate as arteriography in predicting the optimal revascularization method.
Functional assessment
Patients with claudication have a severe limitation in exercise performance and walking ability. The severity of this functional limitation should be determined as a means to assess response to claudication therapies.12 Treadmill testing can be performed using a low-level protocol usually set at 2 mph and increasing the grade 2% every 2 minutes until patients reach their individual maximal claudication pain. In addition to treadmill testing, several questionnaire measures of functional status have been developed and validated in this patient population. The walking impairment questionnaire (WIQ) is a disease-specific instrument that asks a series of questions regarding the patient’s claudication severity and ability to walk defined distances, speeds, and stairs.12 This questionnaire is simple to administer, and has been shown to predict the response to claudication therapies.13
Medical Management of Ischemic Risk
Risk factor modification
Cigarette smoking is associated with a marked increased risk for peripheral atherosclerosis.4,14 The number of pack years is associated with disease severity, an increased risk of amputation, peripheral graft occlusion, and mortality.15,16 Given these associations, smoking cessation has been a cornerstone of the management of PAD. While advice to stop smoking is associated with modest quit rates, pharmacologic therapy can assist in smoking cessation, including nicotine replacement and antidepressant drug therapy.17,18
Current recommendations for the management of lipid disorders in PAD are to achieve an LDL cholesterol level to less than 100 mg/dL and to modulate the increased triglyceride and low HDL pattern.19 The data from the Heart Protection Study (HPS) provide the first primary data supporting the role of statin therapy in patients with PAD.20 Simvastatin was associated with a 12% reduction in total mortality, 17% reduction in vascular mortality, 24% reduction in CHD events, 27% reduction in all strokes, and a 16% reduction in noncoronary revascularizations. Similar results were obtained in the PAD subgroup, whether they had evidence of coronary disease at baseline or not. Thus, the HPS demonstrated that in patients with PAD (even in the absence of a prior myocardial infarction or stroke), aggressive LDL lowering was associated with a marked reduction in cardiovascular events (myocardial infarction, stroke, and vascular death).
Hypertension is an independent risk factor for PAD.4 Guidelines also support the aggressive treatment of blood pressure in patients with atherosclerosis, indicating that PAD is equivalent in risk to ischemic heart disease.21 Regarding specific drug choices, the angiotension-converting enzyme (ACE) inhibitor drugs have shown benefit beyond blood pressure lowering in high-risk groups. Results from the Heart Outcomes Prevention Evaluation study indicated that the ACE inhibitor ramipril was cardioprotective in patients at high risk, including those with PAD.22
Diabetes increases the risk of PAD approximately 3- to 4-fold.4 While diabetes is highly associated with peripheral atherosclerosis, the degree of glycemic control does not predict the severity of the peripheral atherosclerosis.23 These observations suggest that diabetes is a critical risk factor for PAD primarily in conjunction with other cardiovascular risk factors. Several studies in patients with type 1 and type 2 diabetes have shown that aggressive blood glucose control does not effectively prevent cardiovascular disease.24,25 Thus, it is increasingly important to control other cardiovascular risk factors in patients with diabetes, including optimal management of blood pressure and lipid disorders. A recent study demonstrated that in patients with diabetes and PAD, intensive lowering of blood pressure with an ACE inhibitor or calcium channel blocker was associated with a large reduction in the risk of cardiovascular events (myocardial infarction, ischemic stroke, and vascular death).26
Antiplatelet drug therapy
Aspirin is a well-recognized antiplatelet drug that reduces the risk of cardiovascular events 25% in patients with cardiovascular diseases.27 What is remarkable is that specific studies in the PAD population using aspirin have not shown a statistically significant reduction in cardiovascular events.28 Thus, while antiplatelet drugs are clearly indicated in the overall management of PAD, aspirin does not have FDA approval in this patient population.29
In addition to aspirin, the thienopyridienes are an important class of antiplatelet agents that have been well studied in patients with cardiovascular disease. Ticlopidene has been evaluated in several trials in patients with PAD, and has benefit in reducing the risk of myocardial infarction, stroke and vascular death, but with an unacceptable side effect profile.30 In contrast, clopidogrel has been shown to be highly effective in the PAD population.31 Thus, current consensus documents would recommend clopidogrel as an important agent for patients with PAD and intermittent claudication; clopidogrel may in fact be more effective than aspirin alone.32
Treatment of Limb Symptoms
Exercise rehabilitation for claudication
The use of a formal exercise program to treat claudication is the best-studied and most effective nonsurgical therapy for claudication. This topic has been extensively reviewed.33 The most successful training regimens are supervised in a cardiac rehabilitation environment and employ repeated treadmill-walking exercise. The initial evaluation should establish the baseline treadmill walking time as described above. A typical supervised exercise program is 60 minutes in duration and is monitored by a skilled nurse or technician. Patients should be encouraged to walk primarily on a treadmill, since this most closely reproduces walking in the community setting. The initial workload of the treadmill is set to a speed and grade that brings on claudication pain within 3–5 minutes. Patients walk at this work rate until they achieve claudication of moderate severity. They then rest until the claudication abates, and then resume exercise. Patients should be reassessed clinically on a weekly basis as they are able to walk further and further at their chosen workload. The typical duration of an exercise program is 3–6 months. This intervention will allow patients to walk 100% to 150% farther and improve their quality of life.
Drug therapy for claudication
Pentoxifylline has been approved for the treatment of claudication since 1984. However, a meta-analysis concluded that although the drug produced modest increases in treadmill walking distance over placebo, the overall clinical benefits were questionable.34 Cilostazol is currently the most effective drug for claudication. The primary action of cilostazol is to inhibit phosphodiesterase type 3, which results in vasodilation and inhibition of platelet aggregation, arterial thromboses, and vascular smooth muscle proliferation. A recent meta-analysis demonstrated that the drug significantly improved both treadmill exercise performance and questionnaire-assessed quality of life.13 Cilostazol should not be given to patients with claudication who also have heart failure. This is based on previous concerns of a mortality risk with use of the phosphodiesterase inhibitor class of drugs in patients with heart failure.
Additional drugs for treating claudication are being studied in clinical trials. Propionyl-l-carnitine is a metabolic agent that has been shown to improve treadmill performance and quality of life in patients with claudication.35 Prostaglandins have been extensively studied in patients with PAD, but with mixed results. Therapeutic angiogenesis is also being studied with initial positive results.36 However, these therapies are in their infancy.
III. Interventional Approaches in the Management of Thrombosis
Mark R. Jackson, MD*
Greenville Hospital System, 701 Grove Road, Greenville SC 29605
Arterial thrombosis is a major contributor to limb loss and amputation. Approximately 60,000 major limb amputations are performed annually in the US. Peripheral arterial occlusive disease and arterial embolic events are the primary underlying etiologies. While open vascular surgery is the traditional gold standard for major limb revascularization, the development of new endoluminal devices has resulted in rapid advances in minimally invasive techniques for limb revascularization, most of which are performed percutaneously. Similar devices and techniques have also allowed “less invasive” variations of standard open surgical techniques of limb revascularization. This chapter will summarize these recent advances.
Just as newer interventional devices are being developed for arterial thrombosis, new developments are also occurring in the design of devices, such as inferior vena cava filters, to prevent pulmonary emboli in well-defined, high-risk patients. Examples of these improvements will also be provided.
Arterial Interventions
Thrombolytic therapy
Thrombolytic therapy has been evaluated in numerous clinical trials involving patients with thrombotic or embolic occlusions of lower extremity arteries. With the initial use of systemic thrombolytic therapy (intravenous) in the early 1970s, partial or substantial lysis was observed in approximately 40% of patients, and no discernible lysis was observed in the remaining 60%.1 Results were influenced by the duration of occlusion, and serious bleeding complications were observed in approximately one third of the patients.
Since the mid-1970s, regional or intra-arterial administration of thrombolytic agent directly into the site of clot has been the preferred approach.2 The rate of successful reperfusion (50% to 85%) appears higher than with systemic thrombolytic therapy. Other advantages of the selective approach are that it provides useful diagnostic information (determination of embolic versus thrombotic occlusion) and allows simultaneous angiographic definition of the nature of vessel wall abnormalities that would lead to rethrombosis if not corrected by surgery or balloon angioplasty. A major drawback is that arterial catheterization is required for prolonged periods (hours to days), leading to major bleeding and thromboembolic complications in 6% to 20% of patients3,4 and added costs, as this therapy requires ICU observation for signs or symptoms of bleeding during thrombolysis. Despite this, intra-arterial thrombolytic therapy appears superior to systemic treatment. Tissue plasminogen activator (tPA) and urokinase have proven to be superior to streptokinase for peripheral arterial thrombolysis.5– 7
Randomized trials have compared surgical thrombectomy and thrombolytic therapy in patients with acute lower extremity arterial ischemia. A larger, multicenter trial compared intra-arterial thrombolytic therapy with urokinase or tPA with surgery in patients presenting with acute lower extremity ischemia.8 The study was stopped prematurely when an interim analysis showed that patients randomized to surgery did significantly better than those given thrombolytic therapy. However, in patients presenting with symptoms of greater than 2 weeks’ duration, surgery was superior; in patients presenting with symptoms of less than 2 weeks’ duration, amputation rates were lower with thrombolytic therapy. There was no difference in efficacy or safety between tPA and urokinase.
In a multicenter trial of thrombolysis or peripheral arterial surgery (TOPAS), the role of thrombolytic therapy and surgical intervention in the setting of acute ischemia of the lower extremity was evaluated.9 In this clinical trial, 213 patients were studied who had lower extremity ischemia for up to 14 days. No difference was observed in 1-year mortality or amputation-free survival in the urokinase-treated patients or those undergoing surgery. Open surgery was avoided in 45.8% of patients randomized to urokinase. The TOPAS investigators recently published their follow-up study of a total of 548 patients.10 Amputation-free survival at 6 months was the same for both groups (urokinase, 71.8%; surgery, 74.8%). There was a significant increase in the rate of major hemorrhage in the urokinase group compared with the surgery group; and 4 patients treated with urokinase sustained intracranial hemorrhage, one of which was fatal. The only apparent benefit of urokinase was that fewer patients required standard surgical procedures.
From these studies and subsequent clinical experience, thrombolytic therapy appears most useful for distal thromboembolic occlusions in surgically inaccessible small arteries of the leg, and foot, or in patients who are too ill to undergo conventional open surgery. Thrombolytic therapy can also be applied for acute embolic occlusions not readily amenable to surgical embolectomy. Thrombolytic therapy is also used to “buy time” in high-risk patients while underlying medical problems are treated prior to conventional surgical revascularization.
Until recently, rt-PA was the only available thrombolytic agent after urokinase was removed from the market in 1998. Urokinase has been recently reintroduced, but most interventionalists are now very comfortable and familiar with rt-PA and retavase, a nonglycosylated deletion mutation of rt-PA. The existing literature would appear to support the use of rt-PA as a substitute for urokinase for peripheral arterial thrombolysis. In the STILE study, rt-PA was as effective and as safe as urokinase.8
Catheter thrombectomy
Introduction of the Fogarty balloon catheter in 1963 dramatically altered the management of peripheral emboli. It reduced mortality from this disorder by nearly 50% and decreased the incidence of amputation by approximately 35%.11 The Fogarty balloon catheter is the standard in surgical thrombectomy, although variations of the design are used. These devices consist of a small caliber (2F–6F) catheter with a central port for inflation of a soft, compliant latex balloon (Figure 3 ). The catheter is advanced through an open artery, and then passed through the region containing thrombus or embolism. The balloon is gently inflated while the catheter is withdrawn, removing the thrombotic material from the opening in the artery. Since the inflated balloon is at least as large as the diameter of the treated artery, such devices are not well suited to percutaneous use through an introducer sheath. Several passages are generally required to remove all of the thrombus.
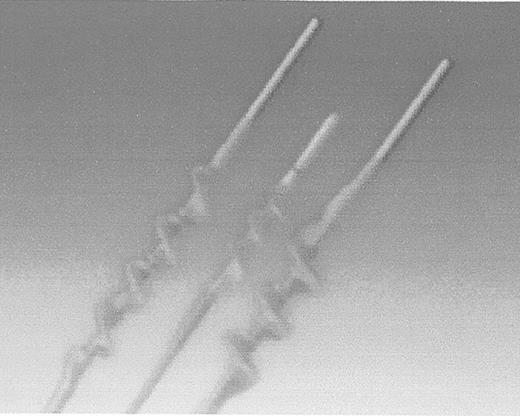
More recently, variations on this design have been introduced by Fogarty. There are two newer catheters—one that removes adherent clots within an artery, and another that removes clots adherent to the lumen of a prosthetic bypass graft. Older, chronic clots are not easily removed with the compliant latex balloon catheters—the balloon simply deforms, leaving much of the more tenacious clot within the artery or graft. The Adherent Clot Catheter (Edward Lifesciences, Irvine, CA) works like a latex-covered corkscrew (Figure 4 ). The catheter has a deployment trigger that winds the inner wire loop, which is covered with an outer layer of protective latex to minimize the prospect of arterial injury, in a cork screw configuration. The author has used this device with success in arteries that cannot be adequately treated by a balloon catheter alone.
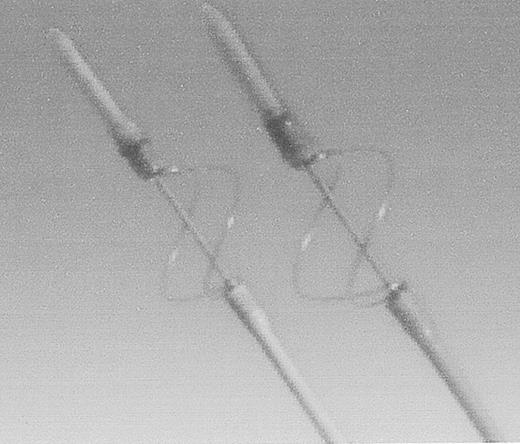
The other Fogarty design catheter is used to remove chronic thrombi from occluded prosthetic grafts. It consists of a wire loop that deploys around the catheter in a cloverleaf configuration (Figure 5 ). In the author’s experience, this catheter will remove any occlusive material if the occlusion can be traversed. The wire loops are robust, and therefore should not be used in a native artery.
Rheolytic thrombectomy catheters
Rheolytic thrombectomy achieves removal of intra-arterial thrombus using a suction type of catheter. A number of such catheters are used for thrombectomy of thrombosed arteriovenous access dialysis grafts, but one catheter is also approved by the FDA for use in peripheral arteries. The Possis AngioJet system uses a high velocity stream of saline within the catheter to produce a low-pressure region (Bernoulli effect) within the catheter tip. This low pressure results in extraction of clot material through a lumen in the catheter, and is thereby removed from the body. Potential advantages of this system include reduced time to achieve thrombectomy compared to many hours or even days with thrombolysis. Rheolytic thrombectomy is particularly attractive for patients with contraindications to thrombolytic therapy.
An initial report on the use of the Possis device for peripheral arterial use in cases of acute, limb-threatening ischemia was reported by Silva and associates in 1998.12 In their study, 21 patients with acute limb ischemia (presentation within 2 weeks of symptom onset) were treated. Contraindications to thrombolytic therapy were present in 52% of the patients. The authors reported an initial technical success rate of 91%, with a 6-month limb salvage rate of 89%. In a more recent study of 86 patients undergoing rheolytic thrombectomy using the AngioJet device, investigators from the Cleveland Clinic reported successful arterial recanalization in 61%, partial success in 23%, and failure to recanalize the occluded artery in 16% of cases.13 Interestingly, adjunctive thrombolytic therapy was used in 58% of cases, highlighting the potential to use multiple modalities in cases of acute arterial ischemia of the leg. An underlying arterial stenosis was found in 53 cases, 51 of which were successfully treated. This experience illustrates how thrombolysis, rheolytic thrombectomy, and percutaneous balloon angioplasty can be used in combination to effect a minimally invasive revascularization approach to selected patients with acute limb ischemia.
Laser-assisted angioplasty
Laser-assisted balloon angioplasty is an endovascular technique that allows recanalization of long segment arterial occlusions that are too long to be successfully crossed with a guidewire and conventional techniques alone. Most short segment arterial occlusions can be crossed using standard guidewires with hydrophilic coatings that enable the wire to be “slipped” through an obstructed area. In some long segment lesions, however, this is not possible. The excimer laser device (Spectranetics, Colorado Springs, CO), on the other hand, is advanced in small increments along with the guidewire, while the laser energy recanalizes the occluded arterial segment. Once the diseased segment is crossed, a standard guidewire is placed through the area and it is treated with balloon angioplasty as with other arterial stenotic lesions (Figure 6 ). These devices come in a range of diameters (0.9–2.5 mm) and can be used for coronary and peripheral arterial lesions.
These newer peripheral laser catheters are not in widespread use, and there is little published clinical data in the peripheral arterial system. There are two ongoing clinical trials, one for long segment (> 10 cm) occlusions of the superficial femoral artery, and the other for patients with critical ischemia of the leg who are poor-risk candidates for conventional surgical revascularization. In a pilot study of the feasibility of this device for treating patients with critical ischemia of the legs, Gray and associates reported the use of the excimer laser and balloon angioplasty in 23 patients with ischemic ulcers or gangrene of the legs.14 In 88% of cases, crossing of the lesion with successful angioplasty was accomplished. Over a 6-month follow-up, 3 patients required major limb amputation, and 4 others eventually required surgical bypass to revascularize the leg. Patency data were not described. While these results appear to be inferior to that generally reported with conventional surgical revascularization, these were high-risk patients, many of whom likely would not have been offered surgery. For such patients, catheter-based interventions such as laser-assisted balloon angioplasty remain an attractive alternative. In our practice, laser-assisted balloon angioplasty of the tibial arteries is reserved for medical high-risk patients and those in whom autologous vein conduit for bypass graft is not available.
Tibial artery balloon angioplasty
Balloon angioplasty has emerged as an effective and well-accepted treatment for occlusive disease of the iliac arteries. However, clinical success and long-term patency rates have generally not been as good when applied to the smaller arteries of the lower leg, particularly the tibial arteries. Nonetheless, several reports have been published that indicate at least a reasonable degree of effectiveness. This, along with advances in small diameter angioplasty systems developed for the coronary circulation, has led to an increased use of these techniques, particularly in those patients who are medically unfit for standard surgical bypass. For the most part, clinical applicability of balloon angioplasty of tibial artery occlusive disease is much the same as summarized above for laser-assisted balloon angioplasty. In fact, the techniques and indications are largely the same, except that the laser device is used to recanalize long segment occlusions that cannot be crossed with a guidewire alone.
Much of the reported experience with tibial angioplasty is from the work of Amman Bolia, in Leicester, England.15 Bolia and colleagues reported a summary of their experience of angioplasty of isolated infragenicular arteries in 2002.16 In this study of 67 patients treated from 1997 to 2000, they report an initial technical success of 86%, and a 3-year limb salvage rate of 94%. Reflecting the advanced, end-stage nature of this pattern of peripheral vascular disease, 51% of the patients had died by 3 years. Although patency rates were not reported in this study, it can be assumed that many of these arteries did not remain patent at the same 94%, 3-year rate as was the case with limb salvage. However, if the offending ischemic lesion heals, and then the treated artery reoccludes later, this can still result in a clinically stable situation in which limb salvage is achieved.
In a recent report of peripheral angioplasty in 221 diabetics with ischemic foot ulcers, the authors were so encouraged by the good clinical outcomes as to recommend balloon angioplasty as the first line treatment for such patients, in preference to surgical bypass.17 In this retrospective study, balloon angioplasty of tibial artery occlusive disease was performed at the time of the original angiogram regardless of lesion length or any anatomic criteria of the arterial occlusive disease. In 191 (85.3%) cases, balloon angioplasty was performed. At a mean follow-up of 14.7 months only 10 patients (5.2%) required a major limb amputation (amputation at or above the ankle joint). Clinical recurrence was noted in 14 patients, 10 of whom underwent repeat balloon angioplasty.
In spite of these encouraging results, the mainstay of treatment for peripheral arterial occlusive disease of the tibial arteries continues to be surgical revascularization, primarily due to better long-term patency rates. Nonetheless, for many of these patients, particularly those with advanced comorbidities and age, tibial artery balloon angioplasty is an attractive alternative to surgical revascularization. Despite lower reported patency rates compared with surgical bypass, even limited primary patency of several months can be sufficient to allow healing of ischemic lesions of the foot. The exact role of tibial artery angioplasty will remain a matter of controversy until the appropriate clinical trials can be performed and published.
Remote Endarterectomy
Occlusion of the superficial femoral artery is a common anatomic pattern in patients suffering from intermittent claudication. When combined with additional anatomic levels of atherosclerotic occlusive disease, such as the aortoiliac segment or tibial arteries, it can cause critical, or limb-threatening ischemia. Occlusion of the superficial femoral artery generally involves the entire length of the artery rendering it relatively unsuitable for balloon angioplasty. Surgical bypass is most commonly utilized to revascularize these occlusions. Another option is that of endarterectomy, where the plaque is physically removed from the artery. Endarterectomy procedures are typically done with conventional open surgical technique, but such long segment endarterectomies have generally fared poorly in terms of patency rates, and require a long incision on the thigh to expose the entire superficial femoral artery.
With the advent of catheter-based techniques, it is possible to perform a remote endarterectomy of an occluded superficial femoral artery utilizing only a single small incision in the groin area. The procedure is termed “remote” because the devices used to perform the endartectomy are manipulated under fluoroscopy, from the “remote” location of the proximal aspect of the superficial femoral artery while the entire length of the artery is internally stripped of the occluding plaque.
The use of the remote endarterectomy was championed by Moll and associates18 and has only recently received more attention in the United States as the cutting device has only recently become available. Recently reported clinical results with the technique have been mixed. In a report of 60 patients undergoing remote endarterectomy of the superficial femoral artery, Rosenthal and associates reported a 3-year primary patency rate of 61%, with a primary assisted patency rate of 83%.19 Only 1 patient required major amputation. A more sobering analysis of the remote endarterectomy technique has been reported by Nelson and associates.20 In their study of 17 patients, the initial technical success was only 65%, and at 1 year the primary patency was 26%. No patients, however, required amputation. Given the disparate results from these 2 centers, one might infer that a significant procedural learning curve exists with this technique.
Summary
Thrombolytic therapy is the mainstay of catheter-based treatment of acute arterial occlusion. Rheolytic thrombectomy allows for treatment of acute arterial occlusions without the bleeding risks associated with thrombolytic therapy. Balloon thrombectomy catheters can be used during arterial revascularization in a form of “hybrid” procedure that requires some open surgery and the use of Fogarty balloon catheters. Chronic arterial occlusions are increasingly being treated with endovascular techniques such as balloon angioplasty, laser-assisted angioplasty, and remote endarterectomy as minimally invasive alternatives to conventional open surgery. From a clinical practice standpoint it is clear that these techniques are here to stay and that further device refinement will only expand their applicability to the treatment of peripheral arterial occlusive disorders.
Update on Vena Cava Filters
Indications for placement of vena cava filters are not clearly established; the situations in which their use is most justified are in patients with venous thromboembolism who have an absolute contraindication for anticoagulation (e.g., cerebral or gastrointestinal bleeding) or a complication of anticoagulation therapy and in patients who have sustained massive trauma, which includes brain or spinal cord injuries or fracture of the pelvis and long bones of the extremities.21,22
Although vena cava filters may be efficacious in preventing fatal pulmonary embolism initially,23 with time their efficacy is counterbalanced by complications in recipients such as deep venous thrombosis (6%–32%), inferior vena cava thrombosis (3.6%–11.2%), and thrombosis at the insertion site (23%–36%).21
Recently, a newer filter, the TrapEase filter (Cordis Europa, NV, LJ Roden, The Netherlands), has undergone approval by the FDA. The advantage of this filter is that it can be introduced through a small catheter (6F), allowing delivery through an antecubital, jugular, or femoral vein approach.22 The filter, which is compatible with magnetic resonance imaging (MRI), is symmetrical, so that orientation of the filter is not an issue. A prospective study in 65 patients showed protection from pulmonary embolism at 6 months, with 2 episodes of thrombosis at the site of the filter. This filter design has been modified so that it can be manipulated or potentially removed (OptEase), although the safety of removal has not been specifically studied.24
An ideal vena cava filter is one that could be placed on a temporary basis. The Gunther Tulip Retrievable Vena Cava Filter is approved in the US for permanent placement, with trials of retrieval currently in progress. In Canada, the filter has been approved for both permanent placement and for retrieval since March 1998. The Canadian Interventional Radiology Association has established a registry for the patients who have received the Gunther Tulip Filter.25 In this registry of 90 patients who have received the filter between 1998 and 2000, retrieval was successful in 51 of the 52 patients in whom it was attempted. Implantation times at retrieval ranged from 2 to 25 days (mean, 9 days). The manufacturer recommends the filter be removed after a period of 2 weeks.
Recently, the Nitinol Recovery Filter has been approved for permanent implantation. This filter can be removed several weeks to months after implantation.26,27
The availability of newer vena cava filters with improved characteristics as well as additional studies on the success of retrieval of temporary filters will increase the range of options for patients who are candidates for temporary or permanent vena cava filter placement.
Sites of action of new anticoagulants that inhibit specific single enzymes in the coagulation system rather than acting at multiple sites as do heparin, low-molecular-weight heparin and warfarin.
Sites of action of new anticoagulants that inhibit specific single enzymes in the coagulation system rather than acting at multiple sites as do heparin, low-molecular-weight heparin and warfarin.
Edwards Lifesciences Fogarty Adherent Clot Catheter.
Inner wire loop is protected by outer latex covering. Catheter is used for arterial and graft occlusions.
Edwards Lifesciences Fogarty Adherent Clot Catheter.
Inner wire loop is protected by outer latex covering. Catheter is used for arterial and graft occlusions.
Fogarty Graft Thrombectomy Catheter.
Bare metal loops remove tenacious, prosthetic graft-bound thrombus. Not to be used in native arteries.
Fogarty Graft Thrombectomy Catheter.
Bare metal loops remove tenacious, prosthetic graft-bound thrombus. Not to be used in native arteries.
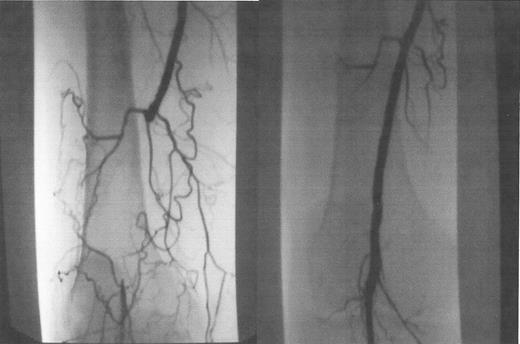
Occluded popliteal artery, before (A) and after (B) laser-assisted balloon angioplasty.
Occluded popliteal artery, before (A) and after (B) laser-assisted balloon angioplasty.