Abstract
Three topics affecting cobalamin, folate, and homocysteine that have generated interest, activity, and advances in recent years are discussed. These are: (I) the application of an expanded variety of tools to the diagnosis of cobalamin deficiency, and how these affect and are affected by our current understanding of deficiency; (II) the nature of the interaction between homocysteine and vascular disease, and how the relationship is affected by vitamins; and (III) the improved understanding of relevant genetic disorders and common genetic polymorphisms, and how these interact with environmental influences.
The diagnostic approach to cobalamin deficiency now allows better diagnosis of difficult and atypical cases and more confident rejection of the diagnosis when deficiency does not exist. However, the process has also become a complex and sometimes vexing undertaking. Part of the difficulty derives from the lack of a diagnostic gold standard among the many available tests, part from the overwhelming numerical preponderance of patients with subclinical deficiency (in which isolated biochemical findings exist without clinical signs or symptoms) among the cobalamin deficiency states, and part from the decreased availability of reliable tests to identify the causes of a patient’s cobalamin deficiency and thus a growing deemphasis of that important part of the diagnostic process. In Section I, Dr. Carmel discusses the tests, the diagnostic issues, and possible approaches to the clinical evaluation. It is suggested no single algorithm fits all cases, some of which require more biochemical proof than others, and that differentiating between subclinical and clinical deficiency, despite their overlap, may be a helpful and practical point of departure in the evaluation of patients encountered in clinical practice. The arguments for and against a suggested expansion of the cobalamin reference range are also weighed.
The epidemiologic data suggest that homocysteine elevation is a risk factor for vascular and thrombotic disease. In Section II, Dr. Green notes that the interactions of metabolism and clinical risk are not well understood and a causative relationship remains unproven despite new reports that lowering homocysteine levels may reduce vascular complications. Genetic and acquired influences may interact in important ways that are still being sorted out. The use of vitamins, especially folate, often reduces homocysteine levels but also carries potential disadvantages and even risks. Folate fortification of the diet and supplement use have also markedly reduced the frequency of folate deficiency, and cobalamin deficiency is now the more common deficiency state, especially among the elderly.
Although genetic disorders are rare, they illuminate important metabolic mechanisms and pose diagnostic challenges, especially when clinical presentation occurs later in life. In Section III, Drs. Rosenblatt and Watkins use selected disorders to illustrate the subject. Imerslund-Gräsbeck syndrome, a hereditary disorder of cobalamin absorption at the ileal level, demonstrates genetic heterogeneity. Finnish patients show mutation of the gene for cubilin, the multiligand receptor for intrinsic factor. Surprisingly, Norwegian and other patients have been found recently to have mutations of the AMN (amnionless) gene, mutations that are lethal in mice at the embryonic stage. Two disorders of cobalamin metabolism, cblG and cblE, are now known to arise from mutations of the methionine synthase and methionine synthase reductase genes, respectively. These disorders feature megaloblastic anemia and neurologic manifestations. The folate disorder selected for illustration, methylenetetrahydrofolate reductase (MTHFR) deficiency, paradoxically causes neurological problems but no megaloblastic anemia. This rare deficiency is the most common inborn error of folate metabolism. It is distinct from the very common MTHFR gene polymorphisms, mutations that cause mild to moderate reductions in MTHFR activity but no direct clinical manifestations. The MTHFR polymorphisms, especially the 677C→T mutation, may contribute to vascular and birth defect risks, while reducing the risk of certain malignancies, such as colon cancer. These polymorphisms and those of genes for other enzymes and proteins related to cobalamin, folate, and homocysteine metabolism may be important role players in frequent interactions between genes and the environment.
I. Current Diagnostic Tests in Cobalamin Deficiency: A User’s Guide
Ralph Carmel, MD*
New York Methodist Hospital, 506 Sixth Street, Brooklyn NY 11215
The sensitive metabolic tests that are now available have put the diagnosis of difficult and atypical cases of cobalamin deficiency on a firmer footing and also permit more confident rejection of the diagnosis when deficiency does not exist. As important, they helped expand the definition of deficiency by facilitating the recognition of subclinical cobalamin deficiency.1 These advances and the issues as well as the inevitable uncertainties that they have raised will be discussed from the perspective of clinicians faced with patients who pose problems of cobalamin status.
The discussion must begin by defining “subclinical” cobalamin deficiency, an asymptomatic state in which metabolic insufficiency is demonstrable in patients and in seemingly healthy nonpatients who do not have megaloblastic anemia, neurological signs and symptoms, or other clinical manifestations of cobalamin deficiency.1Table 1 compares it with clinical deficiency. Despite its limited clinical impact and therefore still uncertain management, several features of subclinical deficiency make it worthy of clinicians’ attention: (a) Its frequency is at least 10-fold that of clinically expressed cobalamin deficiency, and it affects millions of people. (b) Occasional patients, although asymptomatic, nevertheless have subtle electrophysiologic findings that suggest that neurologic changes sometimes lurk under the asymptomatic veneer.2,3 (c) An unknown but probably small proportion of cases may eventually progress to overt clinical manifestations; the proportion is probably highest in that minority who have underlying classical malabsorptive disease, such as pernicious anemia (lack of intrinsic factor).
The sometimes unstated and often undifferentiated focus on subclinical deficiency in many large studies of cobalamin status in healthy populations has helped blur the distinctions between subclinical and clinical cobalamin deficiency and foster the impression that conclusions derived from the former automatically extend to the latter. Without minimizing the potential importance of subclinical deficiency, its medical impact remains poorly understood. However, because it is encountered so frequently nowadays, physicians faced with the potentially cobalamin-deficient patient may benefit from distinguishing between the 2 deficiency states, which are part of a continuum but often differ in kind as much as extent and pose diverse diagnostic, clinical, and management characteristics, problems, and goals (Table 1 ).
Although it is daunting and probably unnecessary to actively seek out new asymptomatic cases by screening, it is axiomatic that any patient identified during a medical encounter must always be investigated. An increasingly common clinical challenge is how to respond to a patient without the typical manifestations of cobalamin deficiency or one in whom an inexplicably abnormal cobalamin-related test result is found. Possible approaches to the array of patient types and deficiency states will follow a brief, practical review of the available diagnostic armamentarium.
Tests of Cobalamin Status
Serum cobalamin
Long familiarity, wide availability, and low cost have contributed to the favored role of cobalamin as the diagnostic mainstay for assessing cobalamin status. Despite limitations of specificity (Table 2 ) and considerable controversy about sensitivity, which is actually 97% for clinical cobalamin deficiency,4 no universally accepted replacement has emerged. Recommendations have emerged for broadening the cobalamin level criteria for deficiency. This issue has important ramifications and will be discussed later.
Paradoxically, modern technology and economics in clinical chemistry have inhibited assessment of the new generation of cobalamin assays. Whereas dozens of independent studies had established and scrutinized the older microbiologic and radioisotopic assays, the literature is virtually silent on the commercially protected, immunologically based chemiluminescence assays that have displaced the older methods. As a result, we have limited understanding of how well cobalamin assays perform in clinical laboratories today. An additional potential source of confusion is the literature’s expression of cobalamin values in pmol/L, whereas all clinical laboratories use ng/L (ng × 0.738 = pmol, so that 200 ng/L = 148 pmol/L).
Plasma total homocysteine (tHcy)
Elevation of tHcy is a very sensitive indicator of clinical cobalamin deficiency.4,5 Levels rise early in the course, preceding symptoms, progress as deficiency worsens, and do not recover until cobalamin therapy is given. However, it has the major flaw of poor specificity. Cobalamin deficiency accounts for only a minority of all high tHcy levels, common causes for which can be confused with it (e.g., folate deficiency), may be occult (e.g., alcohol abuse),6,7 or reflect personal and life style characteristics7 (Table 2 ). The reference interval is not universally agreed,8 and creatinine levels must always be taken into account when interpreting mildly elevated tHcy.
Assay methods are generally reliable, and cost has come down greatly. However, inappropriate sample collection and processing are major technical causes of slightly elevated levels.8 Anticoagulated plasma, not serum, must be used and centrifugation must be done within an hour (serum levels rise as much as 10% per hour’s delay).8 These requirements are not always met, a failure that often escapes the attention of the ordering physician.
Methylmalonic acid (MMA)
Elevation of MMA is at least as sensitive a marker for clinical cobalamin deficiency as tHcy5,9 and is more specific,5 which favors the diagnostic use of MMA. Nevertheless, an automatic assumption that an isolated elevation of MMA indicates cobalamin deficiency invokes circular reasoning and must be resisted. There are other, often poorly understood, causes for MMA elevation than cobalamin deficiency (Table 2 ); impaired renal status is an important influence.10,11 Serum MMA reference intervals, like those for tHcy, continue to be controversial12 and have shrunk sharply in recent years, necessitating caution when interpreting older data in publications and patients’ charts. The major limitations in MMA use are practical: assay complexity, high cost, limited availability, and often slow turnaround time. MMA can also be sensitively assayed in urine, but this approach is not used widely.
Holo-transcobalamin II
Most cobalamin in serum is carried on transcobalamin (TC) I, also called haptocorrin, which does not deliver its cobalamin to tissues. Therefore, measuring the smaller cobalamin fraction attached to TC II (i.e., holo-TC II) is theoretically attractive because it represents the cobalamin available to cells. However, initial claims that low holo-TC II is the earliest marker of cobalamin deficiency and a “surrogate Schilling test” were poorly documented. Specificity of low levels may also be limited,13 and other influences on holo-TC II are not well understood yet.14 The older crude methods have been replaced by immunoassays, one of which is available commercially15 but is not approved for clinical use in the US. It is too early to determine whether holo-TC II provides any practical advantage over the measurement of serum cobalamin.14
Response to cobalamin therapy
Therapeutic response can and should serve as the court of last resort, especially when the diagnosis remains in doubt. Conditions that ensure reliable assessment are the following: (a) monotherapy; (b) appropriate dose and route (e.g., small oral doses of cobalamin have no diagnostic value if malabsorption exists); (c) criteria for response must be appropriate as well as objective: originally normal clinical status cannot improve (exception: mean corpuscular volume (MCV) within normal range can decrease slightly after therapy sometimes), and metabolic changes must not be the sole criterion of improvement if clinical problems were present; and (d) the approximately 20% rate of reported improvement of mild metabolite abnormalities after placebo administration16– 18 illustrates that the phenomenon of regression to the mean, wherein abnormal results tend to drift toward normal upon retesting, spontaneous fluctuations, and the possibility of confounding events, must be borne in mind when assessing test result changes.
Tests of causes of cobalamin deficiency
Identifying what caused the cobalamin deficiency, although a process distinct from diagnosing the deficiency itself, serves many important purposes. However, the ability to diagnose cobalamin malabsorption has become impaired. The availability of Schilling tests has decreased sharply in recent years, and assay of intrinsic factor in gastric juice is rarely done. These gaps leave assays for antibody to intrinsic factor and gastrin or pentagastrin I levels as major diagnostic substitutes, but these blood tests identify only somewhat more than half of cases of pernicious anemia.9,19,20 The diagnosis of ileal malabsorption cannot be made without Schilling tests. The milder but more common defect of food-cobalamin malabsorption also is not tested clinically today, and neither blood tests nor gastric studies provide reliable substitutes.21,22 Approximately 15% of low cobalamin levels, especially those unaccompanied by evidence of cobalamin deficiency, appear to be associated with mild or severe TC I deficiency.23 Diagnosis requires TC I immunoassay. Suggestions that substandard elevation of serum cobalamin levels after oral cobalamin therapy helps diagnose either cobalamin malabsorption or TC I deficiency remain unproven.
The Diagnostic Approach to the Patient
No diagnostic gold standard exists today. All tests have disadvantages, including the purest candidates, clinical abnormalities or elevated MMA levels that are corrected by cobalamin therapy. Serum cobalamin is the most commonly used screening test. Despite its shortcomings, it has proved quite sensitive in clinically deficient patients, but it is less reliable in subclinical deficiency.
No single diagnostic algorithm fits everyone either, in part because requirements and goals vary with different patients. It may be more realistic to discuss options and adapting strategies to clinical contexts. Two not at all mutually exclusive strategies may be considered initially, especially when the clinical picture is unclear.
One strategy is to require abnormal results in a minimum of 2 tests in order to prove the diagnosis of cobalamin deficiency, especially in any patient in whom the diagnosis of cobalamin deficiency is doubtful after clinical assessment. However, the 2 tests must not share any common causes of falsely abnormal results (Table 2 ). For example, tHcy and MMA levels make an unsatisfactory pair, because both rise in the presence of renal insufficiency; cobalamin and holo-TC II are not a good pair because both reflect facets of circulating cobalamin. An acceptable combination might be cobalamin and MMA levels.
If the clinical dilemma is serious, the physician need not be limited to only 2 tests. Moreover, 2 tests do not suffice whenever an inborn error of metabolism is suspected. MMA, tHcy, and cobalamin are needed to screen such patients (see Section III).
A second strategy or modification of the first strategy is to tailor testing to the nature of the clinical problem being faced and its diagnostic requirements. Several representative patient encounters illustrate such nuances and how they can influence the diagnostic approach:
1. The patient with typical clinical signs and/or symptoms, whether mild or severe (e.g., megaloblastic anemia; myeloneuropathy typical for cobalamin deficiency). The main diagnostic goal here is to confirm the strong clinical impression that cobalamin deficiency is indeed responsible. For this purpose, 1 test usually suffices. The chief risk of relying on 1 test is that attempting additional tests, should that become necessary, often becomes unreliable after therapy is given. The most practical candidate may be cobalamin assay, which is cheap and readily available. Moreover, falsely normal levels occur in only 2.9% of patients who have clinically obvious deficiency.4 Cobalamin levels can be falsely low in many circumstances (Table 2 ) but, unless due to folate deficiency,19 those are unlikely to coincide with megaloblastic anemia; falsely low cobalamin levels might coincide on rare occasions with unrelated neurologic dysfunction, such as in multiple sclerosis24 or HIV infection.25
Diagnostic attention must be directed to the underlying cause as soon as the diagnosis of cobalamin deficiency is made. Malabsorption of free cobalamin explains > 90% of cases of clinical deficiency.19,26 Addressing the causal disorder, whether malabsorptive or other, will also serve to confirm or throw doubt on the diagnosis of deficiency made in these patients.
2. The patient with clinical findings for which cobalamin deficiency is an unlikely explanation but must be ruled out (e.g., anemia or neurologic problems of uncertain nature). The main diagnostic goal here is to ensure that if cobalamin deficiency exists, it is not missed. This can be a particularly common dilemma in patients with neuropsychiatric problems, in whom low cobalamin levels often may not represent deficiency.27 Full metabolic testing, including MMA and tHcy, is often advisable because atypical clinical findings provide no anchor. If deficiency is indeed responsible for the symptoms, the metabolic findings are often as striking as in category 1, in which metabolic abnormalities are rarely very mild.4,5 For reasons discussed in the preceding paragraph, the cause of deficiency must be sought next.
3. The asymptomatic patient with a condition known to cause cobalamin deficiency (e.g., ileal disease, gastric surgery, veganism). The main diagnostic goal is to determine whether cobalamin deficiency has developed yet in an asymptomatic patient known to be at risk. MMA assay is the test of choice because metabolic changes often precede low cobalamin levels in the evolution of the expected deficiency4,28,29; tHcy is acceptable as a cheaper alternative for this purpose. Metabolic abnormalities are typically mild to moderate in the asymptomatic state.
4. The asymptomatic patient accidentally found to have a low cobalamin level or other biochemical abnormality (e.g., a low cobalamin level found while screening because the patient is elderly; an elevated tHcy found during cardiovascular risk evaluation). The main diagnostic goal is to determine whether cobalamin deficiency exists; establishing whether it poses a continuing or progressive risk to the patient is an important subsequent goal, tends to depend on what caused the deficiency, and helps determine how long therapy will be needed. MMA is the best choice to diagnose deficiency because it is more specific than tHcy and also appears to be abnormal more frequently than tHcy.30 An elevated MMA that is not due to renal failure requires further diagnostic work, combining clinical reassessment, additional testing, evaluation for pernicious anemia or other causes of deficiency, and a monitored therapeutic trial. A normal MMA level tends to rule out cobalamin deficiency as the cause of the original finding.
Other Diagnostic Issues
Cobalamin level criteria for deficiency
The traditional cutpoint of 200 ng/L was derived from many studies in which unequivocally cobalamin-deficient patients, defined by clinical and hematologic criteria, were compared with healthy controls.19 Studies of clinically affected, unequivocally cobalamin-deficient patients confirm that cobalamin levels are almost always low.4,19 Normal levels were reported in only 2.9% of such cases,4 although a 5.2% rate within a subset of the latter cases has been more widely cited.
Widespread metabolic testing subsequently found mild MMA and/or tHcy abnormalities in a sizeable minority of seemingly healthy volunteers who had low-normal cobalamin levels (up to 300 or 350 ng/L).30–,34 The assumption that the mild, isolated metabolic abnormalities (most patients had no clinical evidence for cobalamin deficiency) represented subclinical deficiency was fortified by the frequent demonstration that the metabolic abnormalities disappeared after cobalamin therapy. The recommendation was made to raise the cobalamin cutpoint to 300 or 350 ng/L in order to capture those otherwise unrecognized cases of presumptive deficiency.31 That important study of Framingham residents found that 32% of those with cobalamin levels up to 350 ng/L had abnormal tHcy and/or MMA levels (14% had elevated tHcy alone and 28% elevated MMA alone). Other studies and surveys have adopted this cutpoint, as have some clinical laboratories and clinicians.
The validity of the strategy can be questioned, however, especially in the clinical setting.1 There is a great difference between being aware that cobalamin-deficient patients sometimes have low-normal rather than low cobalamin levels and the wholesale clinical redefinition, with all its complications, of an established test’s criteria in order to capture additional cases along with many “noncases.” The objections include the following: (a) The assumption that an isolated metabolite abnormality that often has alternative explanations constitutes adequate proof of deficiency can be invalid, especially when an independent gold standard is lacking. (b) Specificity of low cobalamin is a greater problem than sensitivity in patients with clinical deficiency (at 97%,4 the sensitivity is identical to that of MMA at 98% and tHcy at 96%)5; any advantage brought by raising the cutpoint to include low-normal levels is counterbalanced by greatly worsened cobalamin level specificity. (c) The gain in new cases will be almost entirely in subclinical cobalamin deficiency, which vastly predominates among persons with low-normal cobalamin levels (200 to 300 or 350 ng/L); relatively few additional cases of clinical deficiency will be found. (d) Metabolic abnormality suggestive of subclinical deficiency is also found in about 15% of all elderly persons with unquestionably normal cobalamin levels (> 350 ng/L),32,33 and the absolute number of such persons dwarfs those with low and low-normal levels combined; therefore, manipulating the cobalamin cutpoint will still not ensure detection of all or perhaps even most cases of subclinical deficiency. (e) The most serious argument against raising the cobalamin level cutpoint to 350 ng/L may be that whereas 58%–78% of persons with cobalamin levels < 200 ng/L have metabolic evidence for deficiency,32,33 the odds are reversed in persons with low-normal cobalamin levels, only 32%–35% of whom have such metabolic evidence.31,33 Most persons with low-normal levels, therefore, are not just asymptomatic but 65%–68% of them have normal cobalamin status by all metabolic criteria. (f) The resulting 65%–68% probability of mislabeling normal patients as deficient by moving the cobalamin cutpoint to 350 ng/L in order to identify the 32%–35% minority that has usually mild, subclinical deficiency has potentially great implications. The scope can be made explicit by estimating the absolute numbers. One third of elderly Americans, or ≈15 million, have low-normal cobalamin levels (as do 15% of younger adults, an even larger absolute number).31 All 15 million of these elderly people (and even larger numbers of nonelderly adults) would be labeled as cobalamin-deficient as a result, but 65%–68% or about 10 million of them (and even more nonelderly adults) will have entirely normal cobalamin status. (g) Many considerations must enter into setting screening strategies.35 The misidentification of those 10 million nondeficient elderly persons as deficient is justifiable only if the other 5 million (32%–35%) thereby clearly benefit clinically. However, it is undetermined if and how those 5 million persons, most of whom by far have subclinical, not clinical, deficiency, will benefit.1,36 This is obviously an important clinical issue, and its resolution requires more information and careful analysis than is currently available in order to arrive at a reasoned consensus.
Use of tests to monitor response
Monitoring response to therapy is important but often neglected. Clinical monitoring suffices in most patients with clinical deficiency, keeping in mind, however, that neurologic deficits are sometimes irreversible. Biochemical monitoring is usually reserved for patients who are asymptomatic or have equivocal clinical changes or other circumstances. Because serum cobalamin levels rise instantly regardless of cobalamin’s effectiveness, they have no monitoring value. MMA or the cheaper tHcy assay, however, do. Their levels begin to decline within a few days of effective therapy,17,37 which also creates a brief posttherapy window for reassessment, if needed. Responding to cobalamin but not folate therapy, the metabolite levels become normal within a week or two.
Identification of the cause of cobalamin deficiency
It has been argued that most cobalamin deficiency results from pernicious anemia (although this argument is now known to apply only to clinical,26 not subclinical,21,36 deficiency) and that other causes often do not alter the therapeutic approach. A more compelling argument is that testing for malabsorption with the Schilling test is onerous, subject to error, and, in any case, increasingly unavailable, that the logistics and cost of testing the many patients now being identified as cobalamin deficient are prohibitive, and that traditional gastroenterological tests are not helpful in most cases of subclinical deficiency.36 Nevertheless, differentiating between pernicious anemia, ileal disease, bacterial overgrowth, or inborn errors of metabolism in the cobalamin-deficient patient provides important clinical information. Direct therapy of some diseases is possible (e.g., tropical sprue, bacterial overgrowth); endoscopic screening for gastric malignancy is recommended in pernicious anemia but not other disorders38; and the diagnoses can modify not only prognosis and management but the cobalamin therapy itself. Finally, identifying the cause often affirms the diagnosis of cobalamin deficiency, and failure to find a cause warrants reexamination of the diagnosis of deficiency.
The presenting clinical picture can help guide the diagnostic approaches to the underlying cause. Anyone with clinical cobalamin deficiency has a > 90% likelihood of having gastrointestinal disease with malabsorption of free cobalamin.19,26 Because pernicious anemia is the most common of those diseases,26 any patient with clinical cobalamin deficiency can be tested immediately and cheaply for intrinsic factor antibody (parietal cell antibody is diagnostically unreliable)20 and serum gastrin. A positive intrinsic factor antibody (50%–70% sensitivity and near 100% specificity, as long as the sample is not obtained within a few days of cobalamin injection) can be taken as very suggestive of pernicious anemia, whereas lack of serum gastrin or pentagastrin I abnormality raises doubt about the diagnosis.20 The Schilling test can be sought in those with normal blood test results and will also help identify other gastrointestinal causes of deficiency.
The major uncertainties concern the approach to the much greater numbers of patients who have presumptive subclinical cobalamin deficiency.36 Because latent pernicious anemia may explain a small fraction of such cases and is potentially serious,39 the blood tests mentioned earlier are still recommended. However, very few other patients with subclinical deficiency will have malabsorption of free cobalamin, making the Schilling test advisable in only selected cases of subclinical deficiency rather than as a routine tool. Approximately 30%–40% of such patients will have food-cobalamin malabsorption,21 testing for which is clinically unavailable; no endoscopic or blood tests are reliable.22,40 This condition is generally stationary for many years but is occasionally a precursor of pernicious anemia;21 pernicious anemia probably always passes through a stage of food-cobalamin malabsorption but not all patients reach the end-stage of pernicious anemia. Other than in strict vegetarians, dietary cobalamin insufficiency is rare. However, even long-standing veganism usually causes only subclinical cobalamin deficiency,28,29 except in the severely symptomatic infants born to and breast-fed by vegetarian or otherwise cobalamin depleted mothers whose own deficiency is most often subclinical.41 TC I deficiency may account for 15% of unexplained low cobalamin levels, especially those with no metabolic evidence of cobalamin deficiency at all.23 The causes in the remaining patients with low cobalamin levels (i.e., about half of all cases), whether accompanied by metabolic abnormalities or not, have not been identified.
The impact of diagnosis on management
Clinical cobalamin deficiency is a medical disease, and any patient identified in a medical encounter must be approached and managed from that perspective. A distinction may be therapeutically helpful, too, between patients who have clinical cobalamin deficiency, usually a result of severe malabsorption affecting the intrinsic factor–mediated process, and those with subclinical deficiency, who usually have either only partial malabsorption (e.g., food-cobalamin malabsorption) or normal absorption. Different therapeutic doses, routes, and durations are needed for the various subgroups and further individualized for patients. Broad nutrition-based solutions for subclinical cobalamin deficiency are being proposed (e.g., routine cobalamin supplementation, food fortification), but many of the schemes may leave all or most persons with malabsorption unprotected. For this and other reasons, disorder-based considerations are needed even in formulating population-wide policies.
II. Homocysteine Today: The Folate-Cobalamin Connection
Ralph Green, MD*
Department of Pathology and Department of Internal Medicine, University of California at Davis, Davis Medical Center, 2315 Stockton Blvd., Sacramento CA 95817-2201
Vascular Disease and Homocysteine
Homocysteine is normally present in the plasma at low concentrations (5–15 μmol/L). There is now abundant evidence that a sustained elevation in plasma homocysteine (hyperhomocysteinemia) is an established independent risk factor for vascular disease, including thrombosis.1 It is not clear, however, whether this association is causal or merely serves as a surrogate marker of underlying conditions that cause both vascular disease and elevation of plasma homocysteine level.2–,4 Even if causally associated, whether hyperhomocysteinemia leads to or follows vascular disease has not been established definitively. Metabolism of homocysteine occurs along 2 major enzymatic pathways, either remethylation or transsulfuration, which require folate and vitamin B12 or vitamin B6 respectively (Figure 1; see Appendix, page 601). Disturbances of homocysteine metabolism leading to hyperhomocysteinemia can result from inherited or nutritional disorders1,5 as well as from various metabolic diseases, such as chronic renal disease6 and hypothyroidism.7 Even within the normal range for creatinine there is a correlation with homocysteine and the variation in homocysteine encountered in hypothyroidism is explained, at least in part, by the reduced creatinine clearance associated with hypothyroidism. Among the various causes of hyperhomocysteinemia only those caused by vitamin deficiencies are amenable to simple and relatively safe correction. Consequently, interventional approaches using vitamin supplementation provide a promising avenue through which to explore the possible amelioration of vascular disease risk or recurrence, through lowering homocysteine levels. Yet, the recent successful outcome of this approach8 still does not provide clear evidence for a direct role of homocysteine in vascular disease, thrombosis, and premature vascular disease. Reduction of cardiovascular disease risk with concomitant decrease in homocysteine levels does not connote a direct cause-and-effect relationship between elevated homocysteine and vascular damage.
Causes and Effects of Hyperhomocysteinemia
Severe hyperhomocysteinemia associated with homocystinuria due to inborn errors of metabolism is unquestionably associated with precocious atherosclerosis and both arterial and venous thrombosis.5,9 In these patients, the main cause of mortality and morbidity is thromboembolism, followed by cerebrovascular accident, peripheral arterial thrombosis, and myocardial infarction.1,9 Severe degrees of hyperhomocysteinemia (> 50 μmol/L) are most often the result of homozygosity for inborn errors of metabolism in children and in young adults. Among older adults, however, acquired disorders including chronic renal disease and vitamin B12 and other nutrient deficiencies are more likely causes of severe hyperhomocysteinemia. Mild (15–20 μmol/L) or moderate (25–50 μmol/L) degrees of hyperhomocysteinemia are generally the result of acquired disorders. The most frequent causes are deficiencies of 1 or more of the 3 vitamins that are required as cofactors or substrates for homocysteine metabolism. Serum homocysteine levels show a significant inverse correlation with serum B12, folate, and B6 and even individuals with low-normal B12, folate, or B6 concentrations may have elevated levels of homocysteine.10–,12 Certain populations also show a high frequency of heterozygosity for mutant forms of key enzymes in the homocysteine metabolic pathways that may be associated with hyperhomocysteinemia.13,14 Of several genetic mutations and disorders associated with hyperhomocysteinemia, those affecting methylenetetrahydrofolate reductase (MTHFR) have attracted the most attention. MTHFR is required for the generation of methyltetrahydrofolate, the required cosubstrate for remethylation of homocysteine to methionine (see Figure 1; see Appendix, page 601). Mutations in MTHFR can therefore result in varying degrees of hyperhomocysteinemia.1,14 There are a variety of uncommon mutations in the MTHFR gene resulting in deficient enzyme activity and disorders of varying clinical severity. These should be distinguished from the far more prevalent but clinically less severe polymorphisms of MTHFR. (Details of the heterogeneity and clinical spectrum of these genetic variations are provided in Section III.)
The most common polymorphism in MTHFR is a 677C→T substitution that is associated with reduced enzyme activity.13 Homozygotes for this mutation frequently also have low plasma and red cell folate.15 In some studies, an increased risk of vascular disease has been reported in individuals homozygous, as well as heterozygous, for the 677T allele. Whereas many studies have been negative for such an association, from a meta-analysis it was concluded that the combined odds ratio for coronary heart disease associated with homozygosity for the 677T allele was significantly increased in the setting of low folate status.16 In a recent case-control multicenter study on patients with vascular disease, although the frequency of the TT genotype in cases was not different from the controls, adjustment for traditional risk factors resulted in a significant odds ratio for risk of vascular disease in the TT group.17 Because this risk was attenuated by adjustment for plasma folate and homocysteine, the authors concluded that the modest risk increase was attributable mainly to increased homocysteine and low plasma folate in individuals with this genotype. Furthermore, heterozygosity for the C677T mutation has been reported to carry an increased risk for thrombosis when it coexists with hetererozygosity for the factor V Leiden mutation, a genetic defect that results in activated protein C resistance and a hypercoagulable state.18
Moderate or mild hyperhomocysteinemia, caused either by heterozygosity or homozygosity for genetic defects14 or by nutritional deficiency of the vitamins involved in homocysteine metabolism, is an independent risk factor for stroke, coronary, or peripheral artery disease.1,12,19–,22 For each 5 μmol/L increment in total plasma homocysteine (approximately 1 standard deviation of the mean for the normal population), there is a corresponding increase of about 40% in the relative risk of developing coronary artery disease.19 This increase is comparable to the risk associated with the same proportional increment in cholesterol and equates homocysteine and cholesterol as risk factors for ischemic heart disease. The relative risk/odds ratio for cerebrovascular and peripheral arterial disease associated with elevated homocysteine appears even greater than it is for coronary artery disease. The European Concerted Action Project on Hyperhomocysteinemia found that subjects in the top one fifth of the population for plasma homocysteine had a relative risk for vascular disease of 2.2 compared with the lower four fifths, and plasma homocysteine was shown to confer an independent risk of vascular disease similar to that of smoking or hyperlipidemia.20
The mechanism by which homocysteine might cause thrombosis and vascular damage is not known. The pathological changes attributed to homocysteine that are seen in association with severe hyperhomocysteinemia are indicative of a profound disruption of normal endothelial cell function or blood coagulation, but even in this setting, the mechanism whereby homocysteine produces these effects remains obscure. Homocysteine in various concentrations has been shown to affect platelets, monocytes, endothelial cells, vascular smooth muscle cells, cytokines, and adhesion molecules. Homocysteine has also been shown to inhibit anticoagulant, as well as to promote procoagulant pathways, in vitro. This subject has been extensively reviewed.23– 26
Identification, Investigation, and Management of Hyperhomocysteinemia
The identification of hyperhomocysteinemia usually takes place through simple measurement of plasma levels of the compound. Even when basal homocysteine levels are within the normal range, derangements in homocysteine metabolism may be present and may be uncovered by determining peak plasma homocysteine levels following administration of an oral methionine load.1,20 In general, impairment of the remethylation pathway is more likely to result in increased fasting levels of homocysteine, whereas transsulfuration pathway defects are more often associated with abnormal responses to methionine loading (Figure 1; see Appendix, page 601).
There is no general agreement on whether plasma homocysteine should be measured as part of any assessment of a patient with a precocious or unexplained vascular occlusive event. It is not clear how often an elevated level of homocysteine is the only identifiable risk factor associated with the occurrence of a precocious vascular event. Finding hyperhomocysteinemia merits further investigation of the possible cause through measurement of relevant B-vitamin, thyroid, and renal functional status. This serves both to identify the possible presence of a previously unrecognized underlying disorder and to institute appropriate treatment to correct the hyperhomocysteinemia as well as its cause, where possible. The systematic workup of patients found to have hyperhomocysteinemia is important because of the need to uncover its proximal cause (vitamin deficiency or metabolic disorder) as well as the underlying disease process. Lowering of homocysteine through administration of appropriate vitamin supplements is generally safe, efficacious and inexpensive. Although the results of some long-term interventional studies have not yet appeared, there is mounting evidence that vitamin supplements may ameliorate cardiovascular disease complications among at-risk patient groups8,27 as will be discussed below.
There is clear evidence that homocysteine levels can be lowered by the administration of supplemental folate, with or without pyridoxine.28,29 However, the need for supplementation of B vitamin intake in the population at large remains controversial. The conclusion reached in a meta-analysis of randomized trials of homocysteine-lowering B vitamin supplements was that 0.5- to 5-mg folic acid supplements lower homocysteine levels on average by 25%. The addition of vitamin B12 (0.5 mg) produces a further reduction in homocysteine of approximately 7%, whereas vitamin B6 (16.5 mg) had no significant effect.30 It is not yet established whether lowering of homocysteine per se by increased intake of these vitamins results in a diminution of cardiovascular risk. Recently published data strongly support a protective role for high folate and vitamin B6 intake against ischemic heart disease.31 In studies on at-risk groups, the administration of a folate, B6, and B12 supplement reduced the restenosis rate following coronary artery bypass surgery,8 as well as the progression of carotid atherosclerosis in hyperhomocysteinemic renal transplant recipients.27 The prospect of modifying risk of cardiovascular disease in the general population by simple nutritional intervention is appealing and, if substantiated, could constitute a substantial advance in the control of a major cause of morbidity and mortality worldwide.
Is Homocysteine Merely a Marker for Preexisting Underlying Cardiovascular Disease?
It remains possible that homocysteine does not, itself, directly cause vascular damage. Rather, it may be merely a biochemical marker of some related process, perhaps even an acute phase reactant, indicative of underlying inflammation in the process of atherogenesis. Conclusions drawn from an extensive meta-analysis of 43 studies published through 1998, were that although most cross-sectional and case-control studies found higher mean homocysteine levels or a greater frequency of high homocysteine levels in persons with cardiovascular disease, the results of most prospective studies indicated a smaller or no association. This supports the possibility that homocysteine may be a marker of atherogenesis or an indicator of factors more directly linked to cardiovascular disease risk.32 The associations as well as the known or presumed cause and effect relationships between homocysteine, deficiencies of B vitamins, metabolic disorders and vascular disease are illustrated in Figure 2 .
Is Homocysteine Merely a Marker for Folate Deficiency? Evidence for an Association Between Folate Deficiency and Cardiovascular Disease
Folate deficiency is associated with raised homocysteine, and raised homocysteine is associated with increased risk of cardiovascular disease. In a few studies, folate nutritional status (either intake or blood levels) is correlated directly with cardiovascular disease risk. In the Nutrition Canada Survey involving over 5000 men and women a statistically significant association between serum folate levels and risk of fatal coronary heart disease was found. In the same study there was a 69% increase in relative risk of fatal coronary heart disease among the population third with the lowest serum folate compared with the third that had the highest serum folate.33
A prospective cohort study involving over 80,000 women as part of the Nurses’ Health Study noted a significant inverse correlation between the dietary intake of folate (and vitamin B6) and mortality and morbidity from cardiovascular disease over a 14-year follow-up period. There was a 31% reduction in relative risk of coronary artery disease among women in the highest compared with the lowest quintile of folate intake. The authors of this study also calculated that each 100 μg/day increase in folate intake was associated with a 5.8% lower risk of coronary heart disease.31 This figure is in close agreement with that derived by Boushey and coworkers,19 who estimated that increasing folate intake by 100 μg/day would reduce homocysteine levels by 6% and the risk of coronary heart disease by 5%. Folate consumption was shown to correlate inversely with both homocysteine level and carotid artery thickening.
It is not yet known whether lowering homocysteine by increased intake of folate results in a diminution of cardiovascular risk, even though evidence is accumulating that this might be the case. This evidence includes the studies, cited above, that found lower blood folate levels are predictive for coronary artery disease. There is, therefore, a distinct possibility that cardiovascular disease incidence might be reduced in the general population by improvement of folate nutrition. Already there is clear evidence that folate status in the general population has been markedly improved in the postfortification era.10,34 From the prospective Nurses’ Health Study it was predicted that an increase in folate intake would have a favorable impact on coronary heart disease rates with a maximum benefit achieved at a folate intake of at least 400 μg/day.31 Ward and associates29 administered 3 graded doses of folic acid (100 μg, 200 μg and 400 μg daily) to groups of subjects separated into tertiles according to plasma homocysteine levels. The group with the highest homocysteine showed a progressive fall in homocysteine at each escalated dose of folic acid. A large-scale longitudinal intervention trial will be required to directly address the question of whether improvement in folate nutrition through increased intake will lower cardiovascular disease risk in the general population. Such a study would need to be conducted in a country where folate fortification of the food supply has not been undertaken.
Folate fortification of the food supply was introduced in the US in 1998 as a means to reduce the prevalence of neural tube defect pregnancies. The nutritional modification of the population-at-large could potentially have a beneficial effect on the prevalence of ischemic vascular disease. Despite the reasonable anticipation that homocysteine levels will fall in the population as a whole, the overall effect of this change in nutritional status on the prevalence of cardiovascular disease-related deaths and disease will need to be evaluated. Even if a change in cardiovascular death rates occurs, it will be difficult to attribute this to a cause-and-effect relationship with either homocysteine levels or folate status.35 Also, considerable genetic heterogeneity exists with respect to homocysteine metabolism, in particular because of MTHFR polymorphisms. Folate requirements may, therefore, vary considerably between individuals and different levels of intake may be required to control hyperhomocysteinemia and modify its potential effects throughout the population.15,36
The Contribution of Vitamin B12 Deficiency to Hyperhomocysteinemia
Vitamin B12 deficiency is common and B12 deficiency is a significant public health problem, particularly among the elderly. In the US there are 37 million people over age 65 and conservative estimates indicate that 2%–3% of this population has or will develop pernicious anemia caused by failure of gastric intrinsic factor production and consequent B12 malabsorption.37,38 If undetected, this can result in progressive B12 deficiency with its consequent effects on the blood and nervous system. Additionally, there is evidence that the prevalence among the elderly population of borderline and suboptimal B12 status leading ultimately, in some cases, to frank B12 deficiency may be as high as 20%–30% among the elderly. The most common cause is believed to be food B12 malabsorption caused by chronic gastritis, gastric atrophy, and the use of proton pump inhibitors.39 Recently, a high prevalence of B12 deficiency has also been observed in both children and young adults in diverse locations, such as Guatemala, India, and Israel.40– 42 The causes of B12 deficiency in these populations are unclear, but may be related to a combination of low intake and unrecognized malabsorption.
The classic pathophysiological manifestations of B12 deficiency include megaloblastic anemia and neurological complications that run a gamut from peripheral neuropathy to depression, cognitive disturbances, and dementia.43,44 More recent evidence suggests that B12 deficiency may contribute to the risk of vascular disease (through its association with elevated levels of homocysteine in the blood). Since the introduction of folic acid fortification of the US diet, there has been a dramatic reduction in the prevalence rates of folate deficiency as well as hyperhomocysteinemia. This has resulted in a substantial change in the proportional risk of raised levels of homocysteine attributable to either folate or B12 deficiency. B12 deficiency now ranks well above folate deficiency as the primary modifiable cause of hyperhomocysteinemia.45,46
To further complicate this interrelationship between folate and vitamin B12, it is possible that the risks of B12 deficiency may be accentuated by folic acid fortification. The importance of recognizing B12 deficiency and conditions that cause B12 malabsorption is therefore magnified by the addition of folic acid to the US food supply as well as the widespread use of nutritional supplements containing folic acid. A recent national advisory committee on B vitamin intake has cautioned on the need for increased vigilance to detect B12 deficiency and its causes, particularly among the elderly in the wake of the folic acid fortification initiative.47 This is based on the observations that folic acid can reverse megaloblastic anemia caused by B12 deficiency while neurological degeneration may progress unabated and that B12 deficiency in the elderly is often not suspected until anemia develops. It is therefore unknown whether the supplemented level of folic acid in the food supply will increase the incidence of neurological damage due to undiagnosed B12 deficiency. Several authoritative sources have cautioned that there is no known safe dose of supplemental folic acid in patients with untreated B12 deficiency.37,47 Because of the precipitous introduction of folic acid fortification in the United States, there had been no opportunity to properly evaluate this risk. A recently published study attempts to address this issue by comparing the prevalence of anemia among patients with low B12 concentrations for 9 years spanning the period before and after the folic acid fortification era. Since the proportion without anemia did not increase significantly over this time, the authors concluded that food fortification has not masked the hematological manifestations of vitamin B12 deficiency.48 Theoretically, it is also possible that the masking of B12 deficiency by increased levels of folate intake may obscure the presence and delay the correction of the B12 deficiency, allowing the persistence and progression of hyperhomocysteinemia among the elderly, a population already at risk for vascular disease. Assuming an agonistic role for hyperhomocysteinemia in vascular damage, then the institution of folic acid fortification necessitates increased consideration of B12 status and vigilance for B12 deficiency, particularly in older adults. Elucidation of this question will need to come from properly conducted clinical trials in countries that have not mandated folic acid fortification.
III. Genetic Diseases and Polymorphisms
David S. Rosenblatt, MD,* and David Watkins, PhD
Department of Human Genetics, Royal Victoria Hospital, H5-63, 687 Pine Ave West, Montreal Quebec H3A 1A1, Canada
The inborn errors of folate and cobalamin absorption and metabolism, reviewed comprehensively elsewhere,1 usually come to clinical attention because of hematological or neurological manifestations. The most common cause of low serum cobalamin and anemia in an infant is nutritional, usually seen in the breast-fed infant of either a vegetarian mother, or of a mother with sub-clinical pernicious anemia.2 Because this disorder is acquired, it will not be discussed here.
Recent advances in genetics and genomics have resulted both in the discovery of mutations in genes involved in rare inborn errors of metabolism and in the discovery of polymorphisms in these genes. These polymorphisms have been promoted as role players in the interaction between genes and environment that underlie many common diseases. The present section will first give an overview of the known inborn errors of folate and cobalamin absorption and metabolism. It will then focus on four rare disorders, one involving cobalamin absorption (Imerslund-Gräsbeck syndrome) and three involving folate and cobalamin metabolism: deficiencies of methionine synthase (cblG), methionine synthase reductase (cblE), and MTHFR (Table 3 ). It will also examine the 677 C→T polymorphism in the MTHFR gene, as a model for the study of the relationships between polymorphisms and common disease. These disorders illustrate how advances in genetics have increased our understanding of the biology underlying disease states and the challenge of integrating this information into clinical practice.
Overview
Patients with inherited disorders of cobalamin transport are not able to deliver cobalamin to cells. These rare diseases are important because they are more easily treated than some of the disorders of intracellular metabolism, and they must be considered in the differential diagnosis of anemia, neurological problems, and even infection. The defect may be at the level of gastric intrinsic factor synthesis, intestinal transport (Imerslund-Gräsbeck syndrome) or at the level of a circulating cobalamin binding protein (TC I or TC II). Except for those with TC II deficiency, patients will have a low serum cobalamin level, and except for those with TC I deficiency, the Schilling test will be abnormal. Elevated levels of homocysteine and methylmalonic acid may be found in these transport disorders, but they tend not to be as high as in the disorders of intracellular metabolism. It should be noted that TC I deficiency is not associated with clinical cobalamin deficiency, or with elevated levels of these metabolites. It is extremely important to consider the diagnosis of TC II deficiency in the anemic infant because the disease is relatively easily treatable. Since serum cobalamin levels are often normal (reflecting cobalamin bound to TC I), direct measurement of total TC II levels must be requested.
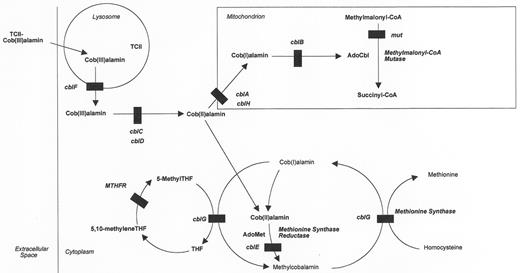
The critical laboratory findings among patients with inborn errors of intracellular cobalamin metabolism (Figure 3 ) are hyperhomocysteinemia and methylmalonic acidemia, either alone or in combination. It is reasonable to test for these metabolites in infants, children or adults with developmental delay or regression, anemia, thrombosis, metabolic acidosis, and a wide variety of neurological and psychiatric manifestations. Usually the serum cobalamin level is within the reference range. Hyperhomocysteinemia results from the abnormal function of the enzyme methionine synthase (cblG) or as a result of the inability to produce its cofactor, methylcobalamin (MeCbl) (cblE). Methylmalonic acidemia occurs because of the abnormal function of methylmalonyl coenzyme A (CoA) mutase (mut) or an inability to produce its cofactor adenosylcobalamin (AdoCbl) (cblA, cblB, cblH). Those patients who cannot produce either MeCbl or AdoCbl have both hyperhomocysteinemia and methylmalonic acidemia (cblC, cblD, cblF). Only those patients with impaired methionine synthase or MeCbl synthesis will have megaloblastic anemia (cblC, cblD, cblE, cblF, cblG). Whereas most patients present in infancy or childhood, some can present in adolescence or adulthood.3– 5
These inborn errors of intracellular metabolism have been classified by complementation analysis in cultured human fibroblasts into complementation groups: mut and cblA-cblH.6,7 In this test, the patient’s fibroblasts and a fibroblast line having a known defect are fused by exposure to polyethylene glycol. In general, if the defects in the two cell lines affect different loci, there will be a correction toward normal cobalamin metabolism in fused cultures as compared to nonfused cultures. Correction is not seen if the defects in the two cell lines affect the same genetic locus.8 The genes for some of these complementation groups are known: mut (MUT),9cblA (MMAA),10cblB (MMAB),11cblE (MTRR),12 and cblG (MTR).13– 15
In contrast to the inborn errors of cobalamin transport and metabolism, there are only three validated inherited disorders affecting folate. Hereditary folate malabsorption presents early in life with severe anemia and developmental delay. Serum folate levels are low. Treatment with high levels of reduced folate is required not only to correct the anemia, but also to get folate across the blood brain barrier. Glutamate formiminotransferase deficiency has a variable phenotype. Although the original patients had megaloblastic anemia and developmental delay, some later patients had only large amounts of formiminoglutamate excretion with little else. The first mutations in an affected patient have been described in the FTCD gene on chromosome 21.16 MTHFR deficiency is the most common inborn error of folate metabolism and will be discussed below in more detail.
Imerslund-Gräsbeck Syndrome (MGA1)
Patients with this syndrome usually present with megaloblastic anemia between 1 and 5 years of age, although some patients have been much older.8 Many patients have proteinuria of the tubular type, as well as decreased levels of serum cobalamin in the presence of normal levels of gastric intrinsic factor. The abnormal pattern in the Schilling test result is characteristic of the inability of the enterocyte to absorb the intrinsic factor-cobalamin complex. More than 250 patients have been published. Whereas the original patients were described from Finland and Norway, numbers of patients have been described from the Middle East. There have been interesting developments in our understanding of the molecular basis of this disorder, which has locus heterogeneity. The first of these was the finding that in all affected Finnish families studied, the disease is caused by mutations in the cubilin (CUBN) gene on chromosome 10p12.1 that encodes for a receptor for the intrinsic factor-cobalamin complex.17 The investigators who discovered this gene also found that the frequency of the disease appeared to be decreasing in recent generations, and postulated that some environmental alteration such as diet may affect expression of the disease. Although the reason for the proteinuria is not known, cubilin is highly expressed in the kidney.
Another observation in this disease is that mutations in CUBN were not found in Norwegian patients showing the same phenotype. Through linkage studies, a candidate gene was located on the long arm of chromosome 14; mutations were found in the AMN gene in the Norwegian patients.18 This gene emerged as a candidate because of an expression pattern similar to that of CUBN, with high levels of expression in both small intestine and kidney. What was of particular interest is that inactivation of the mouse AMN gene results in an embryonic lethal condition in which the embryos have no amnion, whereas mutations in humans result only in the mild MGA1 phenotype. Thus this rare inherited disorder gives evidence for genetic/environment interaction, genetic heterogeneity, and “moonlighting proteins” (proteins that have multiple roles). The function of such proteins can vary with cell type, cellular localization, oligomeric state, differential binding sites, or the cellular concentration of a ligand, substrate, cofactor, or product.19 In the case of AMN, it is postulated that the 5′ end of the gene product is needed for cobalamin absorption, whereas the 3′ end is needed for embryonic development.18
Methionine Synthase [cblG, MTR gene product] Deficiency and Methionine Synthase Reductase [cblE, MTRR gene product] Deficiency
In the cell, cobalamin is converted to AdoCbl in the mitochondrion and to MeCbl in the cytoplasm. Synthesis of MeCbl occurs following binding of cobalamin to the target enzyme, methionine synthase.8 Patients in the cblG and cblE complementation groups have hyperhomocysteinemia and homocystinuria but low levels of methionine and no methylmalonic aciduria. Clinically, it is not possible to differentiate these diseases and classification is usually done by means of somatic cell complementation analysis. Megaloblastic anemia is common, but often neurological and even psychiatric symptoms are more prominent features. These include developmental delay, cerebral atrophy, alterations in muscle tone, ataxia, seizures and EEG abnormalities, nystagmus, and blindness. Although presentation is typically in the first years of life, some patients have only presented as adults, including a 21-year-old woman who had an initial diagnosis of multiple sclerosis.3 It is therefore important for the adult as well as the pediatric hematologist to be aware of these diseases when faced with a patient with megaloblastic anemia and neurological findings. Treatment of cblG and cblE is with systemic hydroxycobalamin, and usually results in a rapid hematological response, although the neurological changes may not be reversible.
The genes responsible for both cblG and cblE have been cloned. The MTR gene, which encodes methionine synthase, is localized on chromosome 1q4313–,15 and causal mutations were identified in cblG patients, including a common P1173 mutation.20 The MTRR gene on chromosome 5p15.2–15.3 encodes a member of the flavodoxin NADP+ reductase family and causal mutations have been found in cblE patients.12,21 Before the description of the cblE patients, the existence of this function was not known in mammalian cells.
Methylenetetrahydrofolate Reductase Deficiency
MTHFR catalyzes the reduction of methylenetetrahydrofolate, the single carbon donor for thymidine synthesis, to methyltetrahydrofolate, a methyl donor for the remethylation of homocysteine to methionine (Figure 3 ).1,22 Because the reaction catalyzed by MTHFR is not reversible, folates can return to the pool of functional reduced folates, which are needed for both purine and pyrimidine metabolism, only through the cobalamin-dependent methionine synthase reaction.
Severe MTHFR deficiency is the most common inborn error of folate metabolism with more than 85 published cases.1,23 The major laboratory findings are hyperhomocysteinemia and hypomethioninemia. Because there is no deficiency of methylenetetrahydrofolate, the substrate for MTHFR and thymidylate synthase, patients with severe MTHFR deficiency do not have megaloblastic anemia. This helps differentiate them from patients with cblE and cblG. Clinical presentation can occur at any age from the neonatal period to adulthood, although most cases have been described in infants. The predominant findings are neurological, with apnea, developmental delay and seizures being common, and are probably related to the low levels of methionine in the brain. Another common feature is thrombosis, probably mediated through the elevated levels of homocysteine. Some adults have been completely without symptoms and only identified in a family after diagnosis of the proband. The gene on chromosome 1p36.3 has been cloned24 and to date 33 mutations have been found in 32 patients.23 Mutations causing severe deficiency are different from the polymorphisms described below. Treatment is very difficult in severe MTHFR deficiency, with betaine being the most useful therapy.
MTHFR Polymorphisms
Dr. Green introduced the relevance of polymorphisms in the previous section. In early studies of patients with severe MTHFR deficiency, we showed in confluent cultured fibroblasts, that the residual specific enzyme activity in some patients was thermolabile.25 In subsequent studies in the general population, Kang et al reported individuals with a thermolabile MTHFR and postulated an increased incidence of thermolabile MTHFR among patients with heart disease.26 After the MTHFR gene was cloned,27 the cause of the thermolabile enzyme in the general population,28 as well as in the severely affected patients with a thermolabile enzyme,29 was shown to be a common polymorphism, 677C→T, that results in the change of an alanine to a valine. This polymorphism in the catalytic domain of MTHFR results in a 50% to 60% decrease in the specific activity of MTHFR when it is present in the homozygous state. The gene frequency for 677C→T varies among ethnic groups with the T allele having a frequency of around 30% in Europeans and Japanese but only a frequency of around 11% in African Americans.30 A second polymorphism, 1298A→C, changes a glutamate to an alanine in the C-terminal regulatory domain of MTHFR and is associated with an approximately 35% decrease in MTHFR activity, but not with thermolability.31,32 The C allele frequency is about 18% among Asian populations, and about 30% in Western Europe.33
It is now known that some of the effects of the 677C→T polymorphism on folate and homocysteine levels can be altered by folate intake,34 and that studies on the effect of this polymorphism can be confounded by the fortification of cereal grains with folic acid.35
After the discovery that thermolability of MTHFR in the general population was associated with the 677C→T alteration, it was relatively easy to perform large studies of this common polymorphism using small amounts of DNA. The types of diseases that have been studied, and for which a correlation with the polymorphism has been attempted, include neural tube defects, Alzheimer’s disease, colon cancer, leukemia, cardiovascular disease, diabetes mellitus, Down syndrome, and complications of pregnancy.36– 41
There is good evidence that hyperhomocysteinemia is an independent risk factor for vascular disease,37 but there is less evidence that lowering homocysteine levels reduces the risk. The results of prospective trials of vitamin therapy should clarify this issue in the next few years. Early studies showing an effect of 677C→T on heart disease were difficult to interpret because the polymorphism was not always associated with elevated homocysteine levels. Recent studies have clarified that the polymorphism is only associated with elevated homocysteine levels in patients with low folate intake.34,42 It is thus possible that the effect of the polymorphism depends on the nutritional status of the individual.22
There are reports that 677C→T may be protective for colon cancer38 and leukemia.33,41 Early studies suggest that the T allele may be protective for lymphocytic leukemia, but not for myeloid leukemia.33 Studies are beginning to look at the 1298A→C polymorphism in MTHFR43 as well as at polymorphisms in a number of other genes involved in folate and cobalamin metabolism such as TC II, 776C→G, MTR, 2756A→G, and MTRR, 66A→G.43– 45 This list is only a fraction of the number of polymorphisms affecting genes in this pathway.
It is difficult to predict the usefulness of the increasing number of these polymorphisms in clinical practice. Because each may interact with others, it will require large population studies to sort out both the genetic and environmental interactions. It seems reasonable to have data on these polymorphisms in the clinical record as part of the investigation of, for example, recurrent neural tube defects or of hyperhomocysteinemia. At this point, the presence or absence of a particular polymorphism in a gene of the folate or cobalamin pathway is not a complete explanation of an individual phenotype for one of the common multifactorial diseases such as cancer or heart disease.
Possible interrelationship between hyperhomocysteinemia and vascular disease.
Cobalamin metabolism in mammalian cells.The pathways by which vitamin B12 (cobalamin) is taken up by cells and converted to its active coenzyme derivatives are shown. The steps affected by inborn errors of cobalamin metabolism (cblA-cblH, mut), as well as MTHFR deficiency are shown. The methionine synthase reaction (cblG) is shown twice in order to separate folate and homocysteine metabolism.
Abbreviations: AdoCbl, Adenosylcobalamin; AdoMet, S-adenosylmethionine; 5-Methyl-THF, methyltetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; TCII, transcobalamin II.
Cobalamin metabolism in mammalian cells.The pathways by which vitamin B12 (cobalamin) is taken up by cells and converted to its active coenzyme derivatives are shown. The steps affected by inborn errors of cobalamin metabolism (cblA-cblH, mut), as well as MTHFR deficiency are shown. The methionine synthase reaction (cblG) is shown twice in order to separate folate and homocysteine metabolism.
Abbreviations: AdoCbl, Adenosylcobalamin; AdoMet, S-adenosylmethionine; 5-Methyl-THF, methyltetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; TCII, transcobalamin II.