Abstract
Much progress has been made during the past several decades in gaining understanding about the natural history of sickle cell disease and management approaches aimed at treating or even preventing certain disease complications. The characterization of the human genome now offers the opportunity to understand relationships regarding how gene polymorphisms as well as how environmental factors affect the sickle cell disease phenotype, i.e., the individual patient’s overall clinical severity as well as their specific organ function. This chapter explores some of these recent advances in knowledge.
In Section I, Dr. Michael DeBaun characterizes the problem of silent stroke in sickle cell disease, comparing and contrasting its clinical and neuroimaging features with overt stroke. Combined, these events affect virtually 40% of children with sickle cell anemia. New understanding of risk factors, associated clinical findings, and imaging technologies are impacting substantially on treatment options. The appreciable cognitive dysfunction and other sequelae of silent infarct demand more effective treatments and ultimate prevention.
In Section II, Dr. Charles Quinn addresses the conundrum of why some patients with sickle cell disease do well whereas others fare poorly. Some risk factors have been known for years, based upon careful study of hundreds of patients by the Cooperative Study for Sickle Cell Disease and investigators studying the Jamaican newborn cohort. Other prognostic measures have only recently been defined. Dr. Quinn devotes special attention to stroke and chest syndrome as organ-related complications but also describes attempts to measure overall disease severity and to predict survival. Recently, investigators have attempted to predict factors responsible for early mortality in children and following onset of pulmonary hypertension in adults.
In Section III, Dr. Martin Steinberg reviews pharmacologic approaches to sickle cell disease and the rationale for their use. In addition to the inhibition of hemoglobin S polymerization, newer targets have been defined during the past one to two decades. These include the erythrocyte membrane, changes in the red cell intracellular content (especially loss of water), endothelial injury, and free radical production. Hydroxyurea treatment attracted the greatest interest, but many uncertainties remain about its long-term benefits and toxicities. Newer “anti-sickling” agents such as decitabine and short-chain fatty acids also receive attention. Prevention of red cell dehydration, “anti-endothelial” therapy, and marshaling the potentially beneficial effects of nitric oxide are other new and exciting approaches.
I. Epidemiology and Treatment of Silent Cerebral Infarcts in Sickle Cell Anemia
Michael R. DeBaun, MD, MPH,* Robert Mckinstry, MD, PhD, Desiree White, PhD, and James F. Casella, MD
Washington University - St. Louis, 4444 Forest Park Blvd., Campus Box 8519, St. Louis MO 63108 Acknowledgments: We thank Rebecca Ichord, MD, for her helpful comments regarding the review. Funding made possible by a grant from the NIH-NINDS (1U01NS042804-01A1).
Silent cerebral infarcts (SCI) are the most common form of neurologic injury in children with sickle cell anemia (SCA) and are increasingly recognized as a major cause of school problems, lower intelligence quotient (IQ) and other neurocognitive deficits; thus, SCI represent a current and evolving concern for students with SCA, their family, teachers and practicing hematologists. SCI are defined as an abnormal magnetic resonance image (MRI)1 of the brain with increased signal intensity in multiple T2-weighted images and no history or physical findings of a focal neurologic deficit lasting more than 24 hours.2 SCI occur in 22% of children with SCA by 14 years of age who have not been identified as having a clinically evident stroke (overt stroke)3; these children are characterized by an increase in risk for further neurologic progression, including overt strokes,3 new or progressive MRI lesions,4 poor academic attainment,5 or lower IQ when compared to patients with SCA with normal MRI examinations1 or siblings without SCA.2 Despite evidence that SCI are prominent in SCA, associated with significant morbidity and a significant risk factor for progressive neurologic disease, the optimal evaluation and treatment remain vague.
Prevalence and Risk Factors for Silent Cerebral Infarcts
In children with SCA, SCI are prominent in infants and toddlers younger than 4 years of age, and cumulative prevalence continues to increase until at least 14 years of age. In an unselected group of 39 children with SCI less than 4 years of age, Wang et al demonstrated an incidence of 11% SCI.6 In the Cooperative Study for Sickle Cell Disease (CSSCD), an infant cohort of 266 was identified and followed for approximately 14 years. Each patient had at least one study-mandated brain MRI at age 6 years or older, and patients with overt strokes were excluded in the determination of the prevalence of SCI. Among the group of children with SCA, 22% had SCI. This prevalence is consistent with that reported in similar studies in Europe.4Table 1 describes the prevalence of SCI in three different cohorts of children with SCA. Children with hemoglobin SC disease and S Beta + thalassemia are also associated with an increased incidence of SCI, 6% (7 of 120) and 15% (3 of 20), respectively.4
In addition to a high incidence in SCA, SCI infarcts result in progressive neurologic morbidity, either new or progressive cerebral injury, compared to children without SCI. Using complete MRI histories from 266 children with SCA, participants with SCI had an increased incidence of new stroke (1.03/100 patient years) and new or more extensive SCI (7.07/100 patient years) relative to stroke incidence among all children without SCI (0.54/100 patient years).
Clinically useful risk factors that will allow clinicians to predict what group of children will have a SCI have not been established. The CSSCD, study included 42 patients with SCI and patients without SCI. Low pain event rate, history of seizure, leukocyte count > 11.8 × 109/L, and the SEN globin gene haplotype were associated with an increased incidence of SCI7; however, the ability of these factors to predict a SCI is unreliable. Preliminary data suggest that genetic modifiers may influence the risk of SCI, but replication studies along with evidence for a mechanism for the association are needed.8
Other than the presence of an elevated transcranial Doppler (TCD) measurement, the greatest risk factor for overt stroke is the presence of an SCI. Again in the CSSCD, 5 (8.1%) of 62 patients with SCI had strokes for an incidence of 1.45 per 100 patient-years compared with 1 (0.5%) of 186 patients without prior SCI, for an incidence of 0.11 per 100 patient-years (P = .006).9 In an earlier study by CSSCD investigators, transient ischemic attack (TIA) was identified as a risk factor for strokes; however, this analysis did not account for the presence of SCI. When the same data set was later evaluated for the presence of SCI, it was determined that all patients with strokes and a prior TIA also had SCI, and the only patient who had a stroke without prior SCI had systemic lupus erythematosus.10 Taken together, these data reinforce the importance of SCI as a significant risk factor for an overt stroke.
Neurological Imaging of Silent Cerebral Infarcts
Radiologic definition of SCI
SCI is diagnosed on the basis of an abnormal MRI of the brain and a normal neurological examination, without a history or physical findings associated with an overt stroke. An early definition of SCI was established in the CSSCD1 and by Glauser et al.11 The only significant difference between the two definitions is the requirement of an evaluation by a pediatric neurologist in the study by Glauser et al as opposed to a clinical evaluation by a pediatric hematologist in the study by the CSSCD. The CSSCD investigators defined SCI as an area of abnormally increased signal intensity on the intermediate and T2-weighted pulse sequences. The area of abnormal signal had to have an appearance consistent with infarction in addition to the abnormal signal. This definition of SCI was refined in practice to include a focal 3-mm or larger area of abnormally increased signal intensity on the T2-weighted image in more than one view.
MRI mimics of silent cerebral infarcts
While the definition of the infarct-like lesion has been established, there are MRI signal abnormalities that mimic SCI but have distinct etiologies. Figure 1 illustrates this point. The T2 signal hyperintensities noted in the deep white matter are the result of acute disseminated encephalomyelitis, rather than SCA. However, the clinical setting would help distinguish these two entities. Other mimicry includes the sequelae of periventricular leukomalacia, dilated perivascular spaces, and delayed myelination in the terminal zones.
Other MRI observations in sickle cell disease
The relationship between SCI and other patterns of brain injury that can be seen in children with SCA is not well defined. In part, this is because SCI can only be identified with surveillance MRI evaluations, which have only recently become routine in some clinical centers. A common finding is cerebral atrophy. This is a non-specific finding that serves as a marker for disease severity in the brain. Another common finding is Moya-Moya, a description that comes from the Japanese for “puff of smoke” because of the angiographic appearance of secondary extensive collateral formation. Figure 2 is a time-of-flight MRI angiogram in a child with SCA and Moya-Moya. The arrows indicate the stenosis/occlusion of the distal internal carotid arteries. The child with SCA and Moya-Moya shown in Figure 3 has an extensive area of cortical infarction corresponding to the distribution of the posterior cerebral artery (arrows) and silent cerebral infarct in the frontal regions.
Transcranial Doppler measurement as a predictor of silent cerebral infarct
A National Heart, Lung, and Blood Institute (NHLBI) sponsored Stroke Prevention Trial in Sickle Cell Anemia (STOP) demonstrated that blood transfusion therapy in patients with increased TCD velocities of the middle cerebral artery or terminal portion of the internal carotid (> 200 cm/sec) could prevent overt strokes.12 Children were randomly assigned to an intervention regimen with transfusion therapy to maintain the Hgb S percentage below 30% or to standard care (observation).12 Of note, 7 of 10 patients in the observation arm who developed overt strokes had SCI at the start of the study, suggesting that the presence of both an elevated TCD velocity (> 200 cm/sec) and SCI may increase the risk of an overt stroke. Due to the design and size of the study, the investigators could not determine whether the presence of SCI alone or in combination with an elevated TCD measurement increased the risk of overt stroke. A post hoc analysis suggested that both the presence of SCI and elevated TCD measurement were important risk factors; however, the numbers were too small to determine whether the risk of overt stroke was different in patients with and without SCI.
No prospective study has been designed to assess the utility of TCD measurement for identifying SCI. In the best study to date addressing the association between TCD measurement and SCI, Wang et al6 compared the results of patients that participated in both the CSSCD natural history study and the STOP trial. A total of 78 children with SCD (mean age 11 years) were common to the natural history study and the STOP trial. Patients who experienced an overt stroke were excluded from the analysis. MRI findings were classified as normal or SCI. Results of TCD measurements were classified as normal, conditional or abnormal (> 200 cm/sec), based on the time-averaged maximum mean flow velocity in the proximal middle cerebral and distal internal carotid artery. Among 17 patients with SCI, only 1 had conditional and 4 had abnormal TCD velocities. In this small sample, no statistical association was found between TCD measurements and MRI findings. The study did not address whether the combination of a conditional TCD measurement (high normal > 170 cm/sec, < 200 cm/sec) and an SCI was a risk factor for future neurologic injury. Current National Institute of Health (NIH) recommendations are to identify children who are at greatest risk of developing overt strokes, not SCI (www.nlm.nih.gov/databases/alerts/sickle97.html).
Cognitive Sequelae of Silent Cerebral Infarcts
Children with SCI have significant cognitive deficits that are intermediate in magnitude compared to children with overt strokes and those with normal MRI. Children with SCI have IQs in the low 80s, whereas children with overt strokes or normal MRI have IQs in the 70s and 90s, respectively.1 Schatz et al demonstrated that 80% of children with SCA and SCI were found to have clinically significant impairments in at least one neurocognitive domain, when compared to 30% of the children with SCA and a normal MRI of the brain.5
Other studies have shown that the majority of SCI related to SCA affect frontal brain regions.13,4 In addition, frontal lobe infarcts, whether overt or silent in children with SCA, have been associated with impairments in attention14,15 and various aspects of executive ability,14 such as working memory,16,17 and strategic long-term memory processing.16 These studies provide guidance for future research examining specific neurocognitive impairments in children with SCI.
Given the high prevalence of SCI coupled with significant cognitive impairment, cognitive remediation of brain injury is becoming an important area of research. In the first study assessing whether cognitive remediation is even feasible in children with SCD, 6 children were identified with cerebral infarcts in the frontal lobes (either overt or silent strokes) and placed into a structured 6-week cognitive remediation program.18 All of the children in the study were provided with general academic tutoring. Three of the children were provided with additional training using specific strategies to enhance working memory and long-term memory (silent rehearsal to facilitate working memory and semantic organization to facilitate long-term memory). In comparing memory performance pre- and post-intervention, the investigators found that the scores of children who received specific strategy training improved considerably, whereas the cognitive scores of children who received only academic tutoring remained relatively unchanged. Although the sample size in this study was quite small, these early findings optimistically suggest that, in addition to identifying cognitive impairments in SCI, interventional strategies may assist children in overcoming, circumventing, or compensating for their impairments.
Treatment for Silent Cerebral Infarcts
Blood transfusion therapy has been the mainstay of intervention for primary and secondary stroke prevention in children with SCD. Blood transfusion therapy to increase total hemoglobin while keeping the Hgb S percentage less than 30% has several physiologic benefits. Increasing the hemoglobin concentration with hemoglobin A improves the blood’s oxygen carrying capacity, lowers the fraction of cells that contain hemoglobin S by dilution, and reduces the erythropoietic drive that produces sickle hemoglobin. These physiologic factors combine to be an effective strategy for reducing the onset of stroke in patients who have an elevated TCD12 and in preventing second stroke.19 The relative risk reduction for the benefit of blood transfusion therapy for primary or secondary stroke prevention is approximately 85%, justifying the burdensome task of at least monthly blood transfusion therapy and deferoxamine administration 5 of 7 nights or more.
No therapy has been established for the treatment of SCI; however, preliminary evidence to support the possible benefits of blood transfusion therapy for SCI was recently published. As part of a post hoc analysis from the STOP trial, Pegelow et al identified individuals with SCA with both abnormal TCD measurement and SCI.20 Among this group of 47 patients, two groups were compared: those that were randomly allocated to receive (n = 18) or not receive (n = 29) blood transfusion therapy. None of the 18 patients receiving transfusion therapy who had a baseline lesion seen on MRI (SCI) developed new SCI lesions or overt strokes. In contrast, among the 29 individuals who had baseline SCI lesions who were not treated, 52% (15 of 29) of this group subsequently developed new or worse SCI lesions (n = 6) or suffered strokes (n = 9) over a 36-month period.20 Thus, in patients with both an elevated TCD velocity measurement and baseline SCI, blood transfusion therapy showed a 100% relative risk reduction for the frequency of new overt strokes or subsequent SCI. This study strongly supports the use of blood transfusion therapy in children with both SCI and an elevated TCD measurement. However, there are no data to support the use of blood transfusion therapy in children with SCI and TCD measurements with time-averaged maximum mean flow velocity measurements less than 200 cm/sec. Only a clinical trial can determine whether children with SCA and normal TCD measurements will benefit from blood transfusion therapy.
Available evidence would suggest that blood transfusion therapy may prevent further neurologic injury in patients with SCI. However, we do not know if these patients will benefit from this treatment. SCI lesions are typically smaller than those present in overt stroke and may represent microvascular pathology, as opposed to overt strokes, which are generally associated with larger ischemic cerebral areas and stenosis or occlusion of the internal carotid and medium size cerebral vessels. Further, we do not know if the benefit of blood transfusion therapy will outweigh the risks and inconveniences of monthly transfusion.
Given this background and the state of equipoise among pediatric hematologists as to whether blood transfusion therapy should be implemented, the Silent Cerebral Infarct Transfusion (SIT) Trial has been funded by the National Institutes of Health (NIH) and the National Institute of Neurologic Disease and Stroke. The primary hypothesis of this trial is that prophylactic blood transfusion therapy in children with SCI will result in at least an 86% reduction in the proportion of patients with clinically evident strokes or new or progressive SCI. Approximately 1800 children with SCA will be screened with an MRI of the brain to identify 200 children with SCI and TCD measurement less than 200 cm/sec. Among this group, 100 participants will be randomly allocated to either blood transfusion or observation for a total of 36 months. Secondary outcome measures will include cognitive assessment and a formal risk/benefit analysis. After the completion of the SIT trial, we should know whether blood transfusion therapy is effective and, if so, whether the benefits outweigh the adverse consequences.
Other treatments
No treatment for SCI has been established, although several should be considered in practice. Probably the most often recommended therapy for SCI is hydroxyurea; however, significant caution must be exercised in its use, because no formal trial has been initiated to demonstrate efficacy. Further, such a trial may not be feasible, given the cost, total number of patients that would be required for identifying individuals with SCI, and the eventual size of the trial (presumably more than the 1800 participants in the SIT trial). Thus, only anecdotal experience and indirect evidence for support will justify using hydroxyurea for treatment of SCI. Another therapeutic option is a stem cell transplant from a matched related donor. If successful, this strategy is curative; however, only few patients have HLA–matched full siblings, making this option less practical for most children with SCA. Future studies using alternative stem cell choices in unrelated donors may be a viable option for the majority of children with SCA.
In summary, SCI in SCA represents a prevalent and morbid condition, with high risk for progression with either new SCI or subsequent strokes, and is associated with lower IQ and other neurocognitive impairments. Optimal treatments for SCI have not been established. Table 2 summarizes the similarities and differences between overt strokes and SCI. Past and ongoing clinical trials have contributed to our understanding of SCI, and promise to continue to do so. The role of transfusions for SCI is currently being explored in a large NIH-NINDS–funded clinical trial that should establish risk factors for SCI, the indications and risk/benefit ratio of blood therapy for this disorder.
II. An Update on Risk Factors and Prediction of Outcomes in Sickle Cell Anemia
Charles T. Quinn, MD*
University of Texas Southwestern Medical Center, Pediatric Hematology-Oncology, 5323 Harry Hines Blvd., Dallas TX 75390-9063
Sickle cell anemia (SS), despite its straightforward Mendelian inheritance, is a highly phenotypically variable disease. Some individuals with SS have frequent vaso-occlusive complications and die young, whereas others seem little affected by the disease and have a normal lifespan. Therefore, risk factors and prediction models are needed to best counsel patients and families and to formulate optimal therapeutic plans. This section briefly reviews classic studies of disease modifiers and focuses on investigations in the past 5 years that have elucidated new risk factors or defined early risk prediction models.
Past Studies
The CSSCD identified or confirmed many of the currently known risk factors, however incomplete, for common complications of SS (Table 3 ). For example, low Hgb F concentration and leukocytosis are associated with increased risk of early death, acute chest syndrome (ACS), and painful episodes. Patients with a low steady-state Hgb concentration, compared to those with higher Hgb concentration, are more likely to suffer early death and stroke but are less likely to have ACS and pain. Both avascular necrosis (AVN) and sickle retinopathy are associated with a higher steady-state Hgb concentration. Other known disease modifiers are the beta globin cluster haplotype and alpha thalassemia. These established risk factors are reviewed elsewhere.1
Recent Studies
Risk factors for stroke
Stroke prediction for individuals with SS was recently transformed when Adams et al demonstrated the utility of transcranial Doppler (TCD) ultrasonography in primary stroke prevention.2 TCD is used to measure flow velocities in the intracranial carotid and cerebral arteries. Stenosis of these vessels, which may predispose to stroke, produces locally increased flow velocities that are detectable by TCD. Thus, children at risk of stroke can be started on a regimen of chronic red blood cell transfusions to prevent stroke. Although clearly predictive, the utility and universal acceptance of TCD screening is limited by the inconvenience and morbidity of chronic transfusions. Also, despite abnormally increased TCD velocities, some children do not develop a stroke. Approximately 10 children will have to be chronically transfused to prevent 1 stroke in 1 child each year. Thus, other predictors of stroke are still needed.
Although a prior stroke is one of the strongest risk factors for stroke in SS, the prognostic significance of clinically “silent” strokes is now becoming clear. Children with SS in the CSSCD who had silent strokes identified at 6 years of age had a 14-fold increased incidence of subsequent new overt stroke (1.45 vs 0.11 per 100 patient-years, P = 0.006) during a mean follow-up of 5.2 years.3 A multivariate model showed that silent stroke was the strongest independent risk factor for overt stroke (hazard ratio = 7.2, P = 0.027).3 MRI of the brain to identify silent strokes might, therefore, prove to be another useful screening measure to identify young children with SS who have an increased risk of overt stroke.
The familial risk of stroke in SS was recently confirmed,4 but the molecular genetic basis of stroke risk has been investigated only recently. Styles et al found an association between specific HLA alleles and either increased or decreased risk of stroke,5 and similar findings were reported in an analysis of CSSCD participants.6 Polymorphisms of genes associated with adhesion, thrombophilia, inflammation, and regulation of blood pressure have also been associated with risk of stroke.7 Of interest, a polymorphism in VCAM-1 (G1238C) protected against stroke in patients with SS (odds ratio 0.35, 95% confidence interval [CI], 0.15– 0.83; P < 0.04).8
The hypothesis that nocturnal hypoxemia could predict central nervous system (CNS) events, including stroke, transient ischemic attacks, and seizure, in individuals with sickle cell disease has been tested.9 Mean overnight oxygen saturation was found to be independently associated with the time to a CNS event (hazard ratio 0.82 for every 1% increase in saturation; P = 0.003). Screening for and appropriate management of nocturnal hypoxemia might, therefore, be useful to predict stroke and possibly to prevent it.
Wierenga et al reported an increased risk of cerebrovascular accidents among individuals with SS who had aplastic crises due to parvovirus B19 infection.10 From this retrospective analysis, the crude risk of cerebrovascular episodes was 58-fold greater in the 5-week interval after parvovirus infection. Many of the episodes were coincident with the acute anemia, suggesting a mechanism, but several individuals had seizures and one had cortical blindness in the 2–5 weeks following infection. The authors suggest that the development of a parvovirus vaccine for humans might decrease the risk of stroke. Furthermore, an increased awareness of potential neurologic complications of parvovirus infection is needed.
Risk factors for acute chest syndrome
ACS is a descriptive name for a variety of acute pulmonary illnesses in individuals with sickle cell disease (SCD). ACS often occurs as a complication of another SCD-related illness, such as a painful crisis, and may not be apparent at the time of admission to the hospital. The prediction of impending ACS would permit preventive therapeutic interventions, such as red blood cell transfusions, to decrease morbidity and mortality. Secretory phospholipase A2 (sPLA2) is an enzyme that cleaves fatty acids, including arachidonic acid, from triglycerides, and this enzyme may be a biomarker for impending ACS. The release of free fatty acids and the pro-inflammatory arachidonic acid in the lungs likely contributes to the pathogenesis of ACS, especially in cases of pulmonary fat embolism. Styles et al showed that the serum concentration of sPLA2 increases before ACS is clinically apparent, peaks at the clinical onset of ACS, and declines during its resolution.11 These data are promising, but the published evidence for a predictive role of sPLA2 for ACS is limited to an analysis of about 20 patients, so further prospective study of this predictor is needed.
Risk factors for severe disease or early death
Miller et al tested many putative risk factors in the CSSCD infant cohort.12 To this end, they defined four adverse outcomes: death, stroke, recurrent pain episodes (greater than 2 per year on average over the course of follow-up) and recurrent ACS (greater than 1 per year over the course of follow-up). Infants with SS or sickle-β0-thalassemia (Sβ0) who had a Hgb concentration below 7 g/dL in steady-state during the second year of life, those with an increased WBC count, and those who had dactylitis before 1 year of age were at significantly increased risk of an adverse outcome. This model is notable because it uses easily identifiable predictors in early life, but its clinical utility is limited because only 3% of the cohort had a combination of factors that predicted a 2-fold or greater risk of an adverse outcome. Also, it is unclear if this model is still valid, because the second most common adverse outcome, childhood death from SS, is becoming uncommon. For example, most of the deaths (56%) in this study were from infection, which is now a rare event because of universal newborn screening, prophylactic penicillin, and the conjugated pneumococcal vaccine.13
Considering that leukocytosis predisposes to more severe disease in SS, Okpala et al hypothesized that the biological basis could be that the adherence of leukocytes to vascular endothelium, mediated by adhesion molecules, facilitates vaso-occlusion.14 Compared to those without, patients who had complications of SCD showed high expression of αMβ2 integrin by neutrophils and high expression of L-selectin by lymphocytes and neutrophils (P < 0.03). The β2 integrin, CD18, was highly expressed by neutrophils in patients with sickle nephropathy (P = 0.018), and L-selectin was highly expressed by lymphocytes in those with stroke (P = 0.03). Monocyte L-selectin increased in sickle cell crisis relative to steady state (P = 0.04). They also found that hydroxyurea therapy decreased the expression of certain adhesion molecules coincident with clinical improvement and before significant induction of Hgb F. Whether polymorphisms in adhesion molecules or differences in their expression can be used to predict a severe clinical course remains to be determined.
Gladwin et al studied the prevalence and effect on survival of pulmonary hypertension among individuals with SCD.15 Using echocardiographic measurement of tricuspid regurgitant jet velocity, the investigators found that 32% of their subjects had pulmonary hypertension. A tricuspid regurgitant jet velocity of ≥ 2.5 m/s, as compared with a velocity of < 2.5 m/s, was strongly associated with an increased risk of death (rate ratio, 10.1; 95% CI, 2.2–47.0; P < 0.001). Thus, echocardiographic screening might be warranted for all adults with SCD to identify a high-risk group that might benefit from intervention. Interestingly, hydroxyurea therapy was not associated with a decreased tricuspid regurgitant jet velocity, and there was no significant association between the Hgb F or the WBC count, independent risk factors for death in SCD, and tricuspid regurgitant jet velocity.
Summary
The explanations for the phenotypic heterogeneity of SS remain incomplete, but the answers will likely be found among the multitude of genes that are co-inherited with the sickle (βS) mutation. That is, to understand a monogenic disease like SS, one must consider the molecular genetic context of the causative mutation in each individual.16 Indeed, to understand phenotypes, the paradigm of a single gene disorder does not hold. Toward this end, genomic and proteomic profiling methods are promising, but they are still in their infancy. Until newer, comprehensive methods are available, we must continue to rely upon simple tools that include individual clinical features, laboratory values, and a small number of genetic polymorphisms.
III. Drug Treatment for Sickle Cell Disease: The Old and the New
Martin H. Steinberg, MD*
Boston University, 88 East Newton Street, E-211, Boston MA 02118
Treatment of Sickle Cell Disease Is Based on Pathophysiology
HbS polymerization
Deoxygenation causes HbS (α2β2S) to polymerize—the proximate cause of sickle cell disease and a necessary but insufficient precursor of the disease phenotype. HbS and its polymer induce a panoply of cellular and tissue injuries. Neither the fetal hemoglobin (HbF) tetramer (α2γ2) nor the α2βSγ hybrid tetramer is incorporated into the HbS polymer, providing the rationale for treatments aimed at increasing HbF concentration.
Sickle erythrocyte damage and dehydration
A distinguishing feature of sickle cell disease is the presence of dense, dehydrated erythrocytes and abnormal reticulocytes. Since polymerization of HbS is uniquely dependent on the cellular concentration of HbS, the increased cellular hemoglobin concentration of dehydrated sickle erythrocytes markedly enhances the tendency for HbS polymerization and cell sickling. Four pathways have been implicated in the dehydration of sickle erythrocytes, and modulation of these pathways, especially the Gardos channel and K+-Cl− co-transport, is a potential means of treatment.
Endothelial damage
Cellular damage enables adhesive interactions between sickle cells, endothelial cells and leukocytes. As endothelium is perturbed, vasoconstriction may be favored as nitric oxide (NO) production is impaired. Several agents directed at the endothelial receptors for sickle erythrocytes or leukocytes may interrupt cell-cell interactions.
Inflammation, reperfusion injury, oxidant radical production
Neutrophils mediate inflammation and tissue damage. Neutrophil numbers are increased and they may be abnormally activated and adherent. An “oxidant” environment may also be present in the sickle erythrocyte and endothelium. Novel ways of countering oxidant-induced injury have been proposed.
HbS Polymerization
Several classes of drug, when titrated optimally, can increase levels of HbF in most patients with sickle cell anemia. Only one is approved for treating sickle cell anemia.
Hydroxyurea
Hydroxyurea, the sole US Food and Drug Administration (FDA)–approved drug for treating sickle cell anemia, should be used in all adults where indications for this treatment are present.1 Unfortunately, for complex reasons, only a fraction of patients who might benefit from treatment receive it. Hydroxyurea increases HbF in sickle cell anemia because its cytotoxicity causes erythroid regeneration and perhaps because its metabolism leads to NO–related increases in soluble guanylate cyclase (sGC) with an increase of cGMP that augments γ-globin gene expression.2 A multicenter trial of hydroxyurea in adults with sickle cell anemia, where the drug was given at sub-toxic doses, showed that hydroxyurea reduced the incidence of pain and acute chest syndrome by nearly half, with little risk seen during more than 9 years of observation. Cumulative mortality was reduced nearly 40%, and a favorable result was related to the ability of the drug to increase HbF and reduce painful episodes and acute chest syndrome.3 No relationship between decrements in neutrophil counts and mortality was found. In infants, children and adolescents with sickle cell anemia, the HbF response to hydroxyurea is more robust than in adults. In a study of more than 100 children who received maximal drug doses, HbF increased to almost 20% and the treatment effects were sustained for 7 years without clinically important toxicity.4,5 HbF levels achieved during treatment were associated with baseline HbF level, hemoglobin level, reticulocyte count, and leukocyte count and with compliance to treatment. According to some experts, pushing the drug dose to near toxic levels is not necessary for a clinically beneficial result. Some have proposed using hydroxuyrea for secondary prevention of stroke in children.6 Hydroxyurea may also conserve resting energy expenditure by curbing the hypermetabolic state observed in children with sickle cell disease.7
Hydroxyurea is potentially mutagenic and carcinogenic.5 Cancer and leukemia have been reported in hydroxyurea-treated sickle cell disease patients, but whether the incidence is higher than in the general population is not known.3 The relative risk of leukemia in hydroxyurea-treated sickle cell anemia is much less than the observed risk in myeloproliferative disorders. To put this into perspective, the risk of death from the complications of adult sickle cell disease appears to be at least 10-times greater than the possible incidence of leukemia in hydroxyurea-treated sickle cell anemia patients.
Questions remain about the clinical benefits of hydroxyurea in HbSC disease. The pathophysiology of HbSC differs from that of sickle cell anemia in some aspects so one might expect that the response to hydroxyurea treatment may also differ. While cell density was reduced, mean corpuscular volume increased and hemolysis apparently reduced, HbF increases were inconsistent.8,9 No randomized clinical trials have studied the effectiveness of hydroxyurea in HbSC disease so that a decision to treat patients with this genotype is a matter of clinical judgment.
Decitibine
A less-toxic analog of 5-azacytidine, 5-aza-2′-deoxycytidine (decitibine), may affect HbF levels by causing hypomethylation of the γ-globin genes. In 8 symptomatic sickle cell anemia patients who failed to respond to hydroxyurea, decitibine treatment (0.2 mg/kg subcutaneously 1–3 times per week for 2 6-week cycles) led to an increase in HbF from 6.5% to 20.4%, with an increase in hemoglobin concentration from 7.6 to 9.6 g/dL and a fall in reticulocytes from 231 to 163 × 109/L.10 Clinical trials of this agent are planned in patients with sickle cell anemia and β thalassemia.
Short chain fatty acids
By acting as inhibitors of histone deacetylase (HDAC) and causing histone hyperacetylation and changes in chromatin structure, short-chain fatty acids, their derivatives, and other compounds with HDAC activity can enhance γ-globin gene expression in erythroid cells of patients with sickle cell anemia and β thalassemia. Very low concentrations of one HDAC inhibitor, an analog of trichostatin A, induced γ-globin gene expression in an erythroleukemia cell line transfected with a reporter construct and in erythroid colonies of normal adults.11
In the most advanced clinical trials of HDAC inhibitors, still only Phase II studies, arginine butyrate given by infusion once or twice a month was associated with a mean increase in HbF from 7% to 21% in 11 of 15 patients with sickle cell anemia. In some individuals, this level was maintained for 1–2 years.12 No HDAC inhibitor of any class other than butyrate and phenylbutyrate has yet been used clinically.
Sickle Erythrocyte Dehydration
Inhibition of K+-Cl−co-transport
Oral magnesium supplementation inhibits erythrocyte K+-Cl− co-transport in vivo. Following studies that showed a beneficial effect on the erythrocyte membrane of transgenic sickle mice, a 6-month clinical trial of oral Mg pidolate improved erythrocyte hydration and was associated with a reduction in the number of painful days.13 Studies to evaluate the effects of long-term magnesium supplementation in adult and pediatric patients with sickle cell anemia are ongoing, and additional studies are planned in HbSC disease patients where activated K+-Cl− co-transport and cell dehydration are likely to play a major pathophysiological role.
Inhibition of Gardos channel
Clotrimazole is an inhibitor of the human red cell Gardos channel but its use was associated with dysuria and reversible hepatocellular toxicity. ICA 17043, a clotrimazole derivative lacking the toxic imidazole residue, was a 10-fold more potent blocker of the Gardos channel than the native drug. In a recently completed Phase II trial of this agent in patients with sickle cell disease, cell density and hemolysis were decreased while hemoglobin concentration was increased. To see whether clinical benefit accrues from these erythrocyte and hematological changes will require a Phase III clinical trial.
Inhibition of other channels
Movement of K+ via the Gardos channel requires the parallel movement of Cl− anions to maintain electroneutrality. High-affinity blockers of Cl− conductance can reversibly block human erythrocyte chloride conductance in vitro without directly affecting the Gardos channel or the K+-Cl− co-transport. In sickle mice treated with a Cl− conductance inhibitor, packed cell volume (PCV) increased and mean corpuscular hemoglobin concentration (MCHC) decreased with an increase in cell K+. A selective loss of the most dense erythrocytes, with a shift from sickled to well-hydrated discoid erythrocytes, was seen. Clinical trials of this class of agent have not been done.
Anti-Adherence Therapy
Anti-adherence therapy for sickle cell disease targets the abnormal interactions among erythrocytes, endothelial cells, leukocytes and platelets that are part of the pathophysiology of the disease process. Potential anti-adherence agents have been studied in acute painful events, where, through poorly understood mechanisms, they restore microvascular circulation and improve tissue ischemia. In Phase II studies of a non-ionic surfactant copolymer, poloxamer 188, this agent reduced the duration and increased the resolution of acute painful episodes, an effect especially notable in children less than 15 years old and in patients receiving hydroxyurea.14 Whether poloxamer 188 exerts its effects by modifying interactions of sickle cells or other blood cells to endothelium is not known. Its clinical effectiveness as a single agent to treat or prevent vasoocclusive complications is, at best, modest, and currently no additional trials of this agent are ongoing.
Endothelium-dependent vasodilation is disturbed in sickle cell disease.15 Superoxide generation induces chronic inflammation that may be inhibited by apolipoprotein (apo) A-1 mimetics. L-4F, an apoA-1 mimetic, inhibited superoxide production and improved vasodilation in sickle mice.16 While a mechanism of action is not yet totally clear, this agent may preserve endothelial function and endothelial nitric oxide synthase (eNOS) activity. This type of drug may have widespread application in vascular diseases including sickle cell anemia.17 Most agents (Table 4 ) that might disrupt the adhesive interactions and inflammation hypothesized to presage sickle vasoocclusion have not been studied clinically.
Nitric Oxide
A potent vasodilator, NO is an important regulator of vascular tone and, because of its interaction with hemoglobin, blood vessels and blood cells, has been hypothesized to have several advantageous effects in sickle cell disease. Generated from l-arginine by NO synthases, NO activates sGC, to produce the second messenger, cGMP. NO inhibits the adhesive interactions among platelets, leukocytes and sickle erythrocytes and decreases VCAM-1 expression in endothelial cells.
NO metabolites were decreased in severe sickle cell vasoocclusive crises. Plasma l-arginine and serum l-arginine levels were normal in children in the steady-state and fell during vasoocclusive crisis while NO levels were normal at presentation but decreased during hospitalization. Three young patients with acute chest syndrome showed an increase in PaO2 and a decrease in pulmonary artery pressure after inhaling NO. However, in each case, standard treatment for this complication was also given making the results difficult to interpret. While a biologically sound rationale exists for using NO in the acute chest syndrome and pulmonary hypertension of sickle cell disease, a controlled trial of this treatment has not been reported. It should be remembered that this approach could be deleterious as NO can be metabolized to damaging oxidants like nitrite (NO2−) and peroxynitrite (ONOO−). In a clinical trial, inhaled NO was associated with a small reduction in pain score and opioid use in children with acute painful episodes.18
Future Goals of Drug Treatment
Our growing knowledge of the human genome and its variation among populations and individuals raises the possibility of being able to know very early—even antenatally—the genetic susceptibility to developing one or another of the many subphenotypes of sickle cell disease and intervening to forestall anticipated complications. Understanding the genetic predispositions to a phenotype many also help the design of innovative treatments. Pharmacogenomics may further help tailoring a treatment to a patient. Preliminary work has associated several genetic polymorphisms in a diverse set of genes to selected complications of sickle cell disease (Table 5 ).
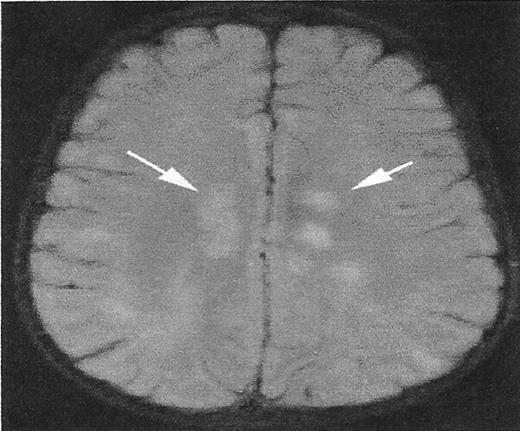
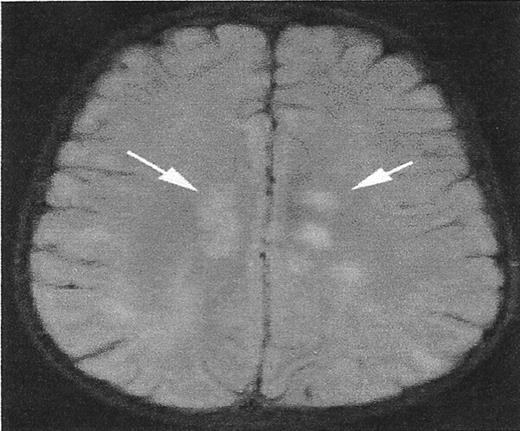
A lesion that mimics silent cerebral infarct (SCI).
High signal intensity lesions in the central white matter of both cerebral hemispheres (white arrows) in a child with acute disseminated encephalomyelitis. SCI often have a similar appearance.
A lesion that mimics silent cerebral infarct (SCI).
High signal intensity lesions in the central white matter of both cerebral hemispheres (white arrows) in a child with acute disseminated encephalomyelitis. SCI often have a similar appearance.
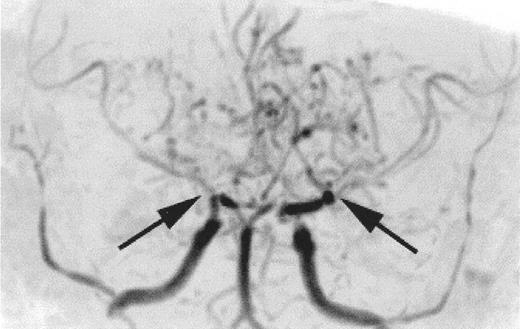
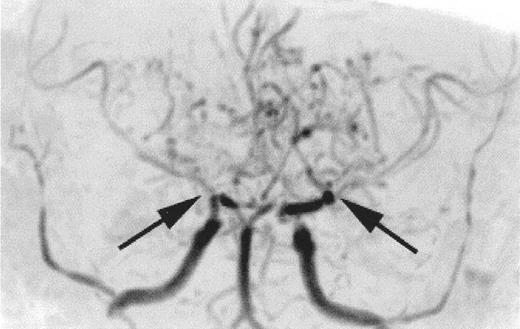
Time-of-flight MR angiogram from a child with sickle cell anemia (SCA) and Moya-Moya.
Severe narrowing of the distal internal carotid arteries and proximal middle cerebral arteries (black arrows) is typical of the intracranial vasculopathy seen with SCA. Prominent lenticulostriate collateral vessels produce the Moya-Moya (“puff of smoke”) pattern seen above the stenoses.
Time-of-flight MR angiogram from a child with sickle cell anemia (SCA) and Moya-Moya.
Severe narrowing of the distal internal carotid arteries and proximal middle cerebral arteries (black arrows) is typical of the intracranial vasculopathy seen with SCA. Prominent lenticulostriate collateral vessels produce the Moya-Moya (“puff of smoke”) pattern seen above the stenoses.
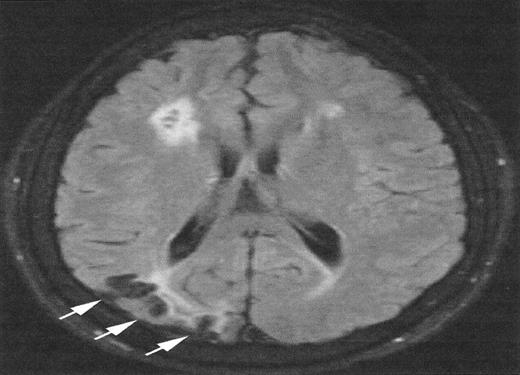
Magnetic resonance imaging (MRI) from a child with sickle cell anemia (SCA) and Moya-Moya.
The severe hemodynamic compromise associated with the Moya-Moya vasculopathy is associated with overt stroke secondary to a right parietal cortical infarction (white arrows). The signal hyperintensities in the frontal regions correspond to silent cerebral infarctions.
Magnetic resonance imaging (MRI) from a child with sickle cell anemia (SCA) and Moya-Moya.
The severe hemodynamic compromise associated with the Moya-Moya vasculopathy is associated with overt stroke secondary to a right parietal cortical infarction (white arrows). The signal hyperintensities in the frontal regions correspond to silent cerebral infarctions.