Abstract
Allogeneic stem cell transplantation is an accepted treatment modality for selected malignant and non-malignant diseases. However, the ability to identify suitably matched related or unrelated donors can be difficult in some patients. Alternative sources of stem cells such as cord blood provide a readily available graft for such patients. Data accumulated over the past several years have demonstrated that the use of cord blood is an accepted source of stem cells for pediatric patients. Since the cell numbers of hematopoietic progenitors in cord blood is limited and the collection can occur only in a single occasion, its use in adult patients can be more problematic. Here, new developments in the use of cord blood for adults and studies aimed at expansion of cord blood cells and immune reconstitution are described.
In Section I, Dr. Nelson Chao describes the early data in cord blood transplantation in adult patients. The patient outcomes are reviewed and analyzed for various factors such as cell dose, HLA typing, and patient selection that could have contributed to the final outcome of these adult patients. Myeloablative as well as nonmyeloablative approaches are presented. Discussion of the various benefits and risks are presented. More recent data from multiple single institutions as well as larger registry data comparisons are also provided. Analyses of these studies suggest methods to improve on the outcome. These newer data should lead to a logical progression in the use of cord blood cells in adult patients.
In Section II, Dr. Stephen Emerson describes the historical efforts associated with expansion of hematopoietic stem cells, specifically with cord blood cells. These efforts to expand cord blood cells continue with novel methods. Moreover, a better understanding of stem cell biology and signaling is critical if we are to be able to effectively expand these cells for clinical use. An alternative, more direct, approach to expanding stem cells could be achieved by specific genetic pathways known or believed to support primitive HSC proliferation such as Notch-1 receptor activation, Wnt/LEF-1 pathway induction, telomerase or the Homeobox (Hox) gene products. The clinical experience with the use of expanded cord blood cells is also discussed.
In Section III, Dr. Kenneth Weinberg describes immune reconstitution or lack thereof following cord blood transplantation. One of the hallmarks of successful hematopoietic stem cell transplantation is the ability to fully reconstitute the immune system of the recipient. Thus, the relationship between stem cell source and the development of T lymphocyte functions required for protection of the recipient from infection will be described, and cord blood recipients will be compared with those receiving other sources of stem cells. T cell development is described in detail, tracking from prethymic to postthymic lymphocytes with specific attention to umbilical cord blood as the source of stem cells. Moreover, a discussion of the placenta as a special microenvironment for umbilical cord blood is presented. Strategies to overcome the immunological defects are presented to improve the outcome of these recipients.
I. Cord Blood Transplants: How Close Are We to Using This in Adults?
Nelson J. Chao, MD*
Duke University, 2400 Pratt Street, Suite 1100, Durham NC 27705-3976
Umbilical cord blood transplantation (UCBT) has recently been explored in an increasing number of adult patients. The relative ease of procurement and the lower-than-anticipated risk of severe acute graft-versus-host disease (GVHD) has made UCBT an appealing alternative to bone-marrow–derived hematopoietic stem cells. The use of reduced-intensity or nonmyeloablative preparative regimens to allow engraftment of UCBT broadens the scope of patients who may benefit, including older and medically infirm patients without a matched donor. This section will summarize the available data on the use of UCB as alternative source of hematopoietic stem cell transplantation in adult patients.
The first known attempt at UCBT occurred 34 years ago when a young 16-year-old boy with acute lymphoblastic leukemia received cord blood units from 8 different donors. He was receiving 6-mercaptopurine and prednisone and did not receive a preparatory regimen. One unit engrafted as demonstrated by red cell antigens and the graft lasted for 38 days.1 However, it was the success of the allogeneic UCBT approximately 16 years ago in a child with Fanconi anemia that opened a new source of allogeneic hematopoietic stem cells.2 UCB is now accepted as an alternative source for hematopoietic stem cells for transplantation in children. Unrelated UCB offers many practical advantages as an alternative source of stem cells, including: (1) the relative ease of procurement and availability (given the ability to store fully tested and HLA-typed cord blood available for immediate use); (2) the absence of risk for mothers and donors; (3) the reduced likelihood of transmitting infections, particularly cytomegalovirus; (4) potential reduced risk of GVHD; (5) less stringent criteria for HLA matching for donor-recipient selection (with the potential of finding donors for minority populations); and (6) absence of donor attrition. UCB banks have been established for related and unrelated UCBT with about 100,000 units currently available.3– 6
While the clinical data are encouraging for pediatric patients, the limited number of hematopoietic stem cells in UCB has been a cause for caution for its use in adult patients. Moreover, the use of myeloablative preparatory regimens with their known toxicities together with the known delays in engraftment of all cell lineages and the enrollment of high-risk patients with advanced disease all contributed to this caution. Recently, older allogeneic recipients have been treated successfully following a variety of less intense nonmyeloablative conditioning regimens. On the basis of these encouraging observations, it has been hypothesized that a reduced-intensity preparative regimen would allow engraftment of the UCB stem cells. While the total numbers of mononuclear cells are limited, the progenitor content and the proliferative potential of cord blood cells are high. This nonmyeloablative approach could diminish some of those concerns raised with the use of ablative regimens. This review will summarize the available data on the use of UCB as an alternative source of hematopoietic stem cells for allogeneic transplantation for adult patients.
Myeloablative Preparative Regimens
In comparison to the published studies on pediatric patients, the clinical data on the use of UCBT in adult patients are relatively limited, although growing7–,14 (Table 1 ). As can be gleaned from the composite data, these patients received standard total body irradiation or busulfan-based myeloablative regimens. GVHD prophylaxis consisted of cyclosporine alone or in combination with steroids or methotrexate. The degree of HLA disparity was not associated with outcome in any of these studies, although the numbers in each were small. However, in a recent review update with 861 unrelated UCBT recipients from the Placental Blood program of the New York Blood Center, which includes 181 (21%) patients with age ≥ 18 years and 170 patients (20%) weighing ≥ 60 kg, Rubinstein et al15 have demonstrated in a multivariate analysis that HLA match is an independent predictor of event-free survival.
The median age varied considerably from a low of 29 to a high of 48 years with a range of 15 to 58 years. Age is an important factor in the outcome, with the younger patients generally doing better than the older patients. The median weight for these studies also varied from a low of 51 to 70 kg. As the cell dose of the UCB is given per recipient weight, the patients with lower body weights would have a higher chance of receiving a higher cell dose per cord blood unit. The range of mononuclear cells/kg recipient weight ranged from 1.5 to 2.43 × 107/kg. The total numbers of CD34+ cells has been correlated with both engraftment and the speed of engraftment.
The diseases for which these patients were transplanted consisted primarily of hematological malignancies. The differences in outcome can be partially attributed to the proportion with more favorable disease status at transplantation. For example, most of the data from Sanz et al8 or Ooi et al13 included recipients with chronic myeloid leukemia (CML) in first chronic phase or acute leukemia in first remission or de novo acute myeloid leukemia or untreated myelodysplastic syndrome. In contrast, the less favorable survival in the other studies reflects the poor-risk subjects enrolled into the study.
The probability of myeloid engraftment ranged from 72% to 100% by day +60; however, some patients were censored to this event if they died prior to the set date for the definition of engraftment. The median times to engraftment of both neutrophils and platelets varied as well with approximately 1 month to reach 500 neutrophils/mL and 2–4 months to reach platelet counts > 20,000/μL. Again, the pace of recovery is likely to reflect the UCB stem cell dose as well as the amount of prior therapies a patient could have received. As compared to the pediatric patients, the incidence of severe grade III-IV acute GVHD was low (approximately 20%) given that the majority of the patients received HLA mismatched unrelated grafts. Of note, the HLA matching in general was done serologically for HLA-A and-B and molecularly for HLA-DR. Thus it is likely that the mismatching would have been greater than reported if molecular matching had been performed. Therapy-related mortality (TRM) was high, reaching approximately 50% in most series. The overall survival again varied considerably, reflecting the risk factors outlined above. Comparative studies of UCB transplantation versus unrelated bone marrow have been performed from two registry datasets. These two studies have demonstrated that if there is a cord blood unit that is at least a 4/6 HLA match with sufficient cells, the outcomes are similar following UCB transplantation to matched or one antigen mismatched unrelated donor bone marrow grafts.21,22
Thus, UCB contains sufficient numbers of hematopoietic stem cells to achieve engraftment in adult patients with lower than anticipated risk of severe acute GVHD, even when HLA-disparate grafts are infused. The use of UCB allows allografting to be offered to a greater proportion of patients, many of whom do not have a matched sibling or unrelated donor to allow allogeneic therapy as the only chance to cure the underlying disease. The results thus far suggest that UCBT can result in long-term disease-free survival in many of these patients. Similar to the pediatric series, clinical experience in the adult patients has also documented the importance of graft cell dose in determining engraftment and survival. The critical “threshold dose,” below which engraftment and survival become significantly inferior, remains to be defined in a larger study with a longer follow-up. Based on current results, it appears that a UCB graft that contains at least 2 × 107 nucleated cells/kg and 1.7 × 105 CD34+ cells/kg is acceptable for adult recipients.4– 6 It is hoped that the advantage of a lower GVHD without any apparent increase in relapse will offset the adverse impact of reduced cell dose on survival. As in the pediatric setting, TRM remains the main obstacle for success in adult recipients, although several investigators have demonstrated that the TRM is not different from that seen with matched unrelated donors. With the profound influence of UCB cell dose (both nucleated cell dose and CD34+ cell dose) on engraftment, survival and probably TRM in the adult setting, future research should also focus on increasing the cell dose of the UCB graft. Possible methods to increase cell dose include ex vivo expansion, infusion of multiple cord blood units or use of possible additional cells such as mesenchymal stem cells or improving HLA matching.
Nonmyeloablative Regimens
The nonmyeloablative stem cell transplantation (NST) regimen was proposed initially based on the rationale that the therapeutic benefit of an allogeneic transplantation is partially related to the crucial immune-mediated graft-versus-malignancy effect. The concept of graft-versus-malignancy as the pivotal therapeutic component of allogeneic transplantation is supported by the observation from clinical studies that (1) patients with acute and chronic GVHD have a reduced risk of relapse; (2) patients with syngeneic BMT and after T cell–depleted allotransplant have a higher incidence of relapse compared with other allogeneic donors; and (3) patients with a relapsed malignancy after allogeneic transplantation can be re-induced into complete remission without any chemotherapy by donor lymphocyte infusion (DLI). NST, with its reduced-intensity preparative regimen, makes allogeneic transplantation applicable to patients with relative contraindications to myeloablative regimens given its lower rate of TRM with fewer infections and less GVHD. However, the preparative regimen does not contribute significantly to the antimalignancy effect; thus, the risk of disease progression after transplantation remains higher compared with the myeloablative approach.
NST using UCB provides an opportunity for immunotherapy for older patients, sicker patients and also patients without suitable donors who are not eligible for this potentially curative approach. However, there is increased concern about graft rejection using this approach given that there are, on average, two logs fewer cells infused than would be considered for a standard matched sibling or unrelated donor transplant (Table 2 ). The clinical outcome of 2 patients with malignant lymphoma using this novel approach was first reported by investigators at Duke University.16,17 In that study, 2 patients with relapsed lymphoma who had no matched siblings, partially matched family members, or matched unrelated donors successfully underwent nonmyeloablative conditioning therapy followed by infusion of 4 and 6 matched, unrelated donor UCB cells, respectively, at the nucleated cell dose of 2.9 and 6.5 × 107/kg, respectively. The conditioning regimens consisted of fludarabine 30mg/m2 and cyclophosphamide 500 mg/m2 daily for 4 days with antithymocyte globulin 30 mg/kg per day for 3 days. Cyclosporine and prednisolone were given for acute GVHD prophylaxis. Both patients had 100% donor engraftment by the third month of transplant and remained in remission 6 and 12 months following transplantation. Extension of these data to 11 other patients confirms the low morbidity and the potential for robust engraftment using this approach. The favorable outcome demonstrates the feasibility of the mismatched unrelated UCB cells, even with the NST regimens.
The largest experience with transplantation using a reduced-intensity regimen was evaluated by investigators from the University of Minnesota on a cohort of high-risk patients with hematological malignancies.18 In their study, unrelated UCB graft with a median nucleated cell dose of 3.7 × 107 per kg (range, 1.6–6.0 × 107/kg) was infused into 43 patients (median age 49.5 years; range, 22–65 years), after receiving 2 types of conditioning regimens: fludarabine 200 mg/m2, total body irradiation (TBI) 200 cGy and busulfan 8 mg/kg (Flu/Bu/TBI) for the initial 21 subjects; fludarabine 200 mg/m2, TBI 200 cGy and cyclophosphamide 50 mg/kg (Flu/Cy/TBI) for the subsequent 22 subjects. All patients received GVHD prophylaxis with cyclosporine and mycophenolate mofetil. The median time to neutrophil recovery of more than 0.5 × 109/L was 26 days (range, 12–30 days) for the Flu/Bu/TBI recipients, but only 9.5 days (range, 5–28 days) for the Flu/Cy/TBI recipients. The cumulative incidence of engraftment for Flu/Bu/TBI and Flu/Cy/TBI recipients was 76% and 94%, respectively. Despite the use of 1–2 HLA-antigen mismatched graft in 93% of the recipients, the cumulative incidence of grade II–IV GVHD and grade III–IV GVHD for the entire cohort of patients were 44% and 9%, respectively. The disease-free survival of these high-risk subjects was also favorable: 24% at 1 year for Flu/Bu/TBI recipients and 41% at 1 year for Flu/Cy/TBI recipients. A similar approach and data have also been reported by investigators in University of Colorado Health Sciences Center.19
Immune recovery in 5 recipients of UCB transplant following the NST regimen were compared to recovery in adult recipients of UCB following a myeloablative regimen by investigators at Duke University.20 Recipients of NST regimens had a more rapid and robust recovery of myeloid and lymphoid cells. The T cell repertoire in UCB recipients treated with the NST regimen was markedly more diverse and robust compared with the repertoire in those receiving the myeloablative regimen at similar time points. T cell receptor excision circles (TRECs), which are generated within the thymus and identify new thymic emigrants and those that have not divided, were detected 12 months after transplantation in the NST recipients. This compared favorably to the delayed detection of TRECs at 18–24 months among recipients of myeloablative regimens. Thus, in adults receiving an NST preparatory regimen, the quantitative and qualitative recovery of T cells occurs through rapid peripheral expansion. The ability of patients receiving NST transplantation to recover within a few months suggests that the peripheral “niches” in which T cells can proliferate are preserved in these patients compared to those receiving myeloablative regimens. Moreover, the presence of TREC-positive cells within 1 year suggests that thymic recovery is likewise accelerated in NST compared with recipients of myeloablative regimens. The favorable results of T cell recovery following the NST suggest that it may be possible to have an excellent outcome with an unrelated mismatched UCB transplantation in adult patients. Patients have a rapid recovery of T cells with a complex diversity. The primary difference between the recipients of ablative and NST regimens was the extent of physiologic damage caused by the preparatory regimen. When the damage is relatively mild, as in nonmyeloablative regimens, the donor T cells are able to expand effectively in the periphery, and the development of new T cells through the thymus is also accelerated compared to the rate of development in those receiving ablative regimens. Alternatively, the lower incidence of GVHD in NST may also play an important role in the preservation of the peripheral and central niches for T cell development.
Future Directions for UCB Transplantation in Adults
While the use of NST signifies an advancement in the field of transplantation and immunobiolology, several unresolved questions remain:
What is the optimal UCB cell dose required for adult patients and can we increase it?
What is the optimal NST conditioning regimen and GVHD prophylaxis?
Is the incidence of GVHD similar in ablative and NST regimens?
Does the incidence of infection differ in ablative versus NST regimens and can one improve immune recovery?
What is the difference in overall efficacy between myeloablative and NST UCBT?
Conclusion
UCB is a viable alternative to bone marrow and peripheral blood as a source of stem cells capable of hematopoietic reconstitution for adults, when related or unrelated marrow donor is not available. UCBT following NST preparative regimen is an exciting new approach that provides an option for patients who are otherwise excluded from conventional hematopoietic stem cell transplantation, including elderly or medically infirm patients with no matched sibling donor. Preliminary results have shown that such an approach can be associated with timely engraftment with full donor chimerism. Comparison between myeloablative and NST approaches will be needed before this therapy can be considered for younger patients eligible for myeloablative transplant. At the moment, the use of NST UCBT cannot be encouraged outside of a clinical trials or selected patients. The future challenge will be to develop strategies to optimize the chance of early and durable engraftment, as well as to minimize the risk of GVHD and transplant-related death.
II. Expansion of Umbilical Cord Blood Cell Progenitors and Stem Cells: Biology and Application to Clinical Transplants
Stephen G. Emerson, MD, PhD*
Abramson Cancer Center; Univeristy of Pennsylvania, Departments of Medicine, Pathology, and Pediatrics, 510 Maloney Bldg., 36th & Spruce Streets, Philadelphia PA 19104-4283
The close correlation between preclinical assays and in vivo clinical biology that is observed with bone marrow stem cells and with hematopoietic growth factors such as erythropoietin (Epo), granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) has been very reassuring and has accelerated the development and improvement of clinical hematology practice. Clinical experience with UCB transplants, in contrast, has proved much more paradoxical and problematic. Early laboratory measurements showed that the density of both CD34+ cells and clonogenic progenitor cells was extremely high in UCB.1 Similarly, the proliferative capacity of progenitor cell differentiation in laboratory assays and of UCB stem cells transplanted into immunodeficient mice was measurably higher than for progenitors and stem cells from bone marrow (BM) or peripheral blood (PB).2,3 But when comparable numbers of UCB progenitors (1–5 × 106/kg) and stem cells are transplanted as are delivered to patients with standard BM or PB grafts, engraftment is far slower, and graft failure is more likely.4 Given that opportunistic infection and organ failure rise exponentially with time to engraftment, and that overall long-term survival correlates closely with platelet engraftment, this delay has proved to be a major barrier to adoption of what would otherwise be an overwhelmingly attractive stem cell source.
One of the striking lessons of BM and peripheral stem cell (PSC) transplant is that time to engraftment correlates inversely with progenitor and stem cell dose, with most graphed comparisons showing hyperbolic curves with asymptotic limits at 7–8 days for neutrophil engraftment and 10–12 days for platelet engraftments, both achieved with PSC stem and progenitor cell doses of > 5 × 106 CD34+ cells per kg. Providing lower doses of early hematopoietic cells results in increasingly longer nadirs, but providing higher doses does not further accelerate hematopoietic recovery. Based on these well-documented observations, the question has been raised whether one could significantly shorten UCB transplant nadirs by providing increased numbers of stem and progenitor cells to patients by expanding the numbers of hematopoietic stem cells (HSCs) and progenitor cells, in the laboratory, prior to transplant. This approach should succeed if the delay in engraftment is due to a paucity of HSC and progenitor numbers, but would fail if the delay were due to an uncharacterized-but-insurmountable qualitative defect in UCB stem and progenitor cells.
Stem Cells, Stem Cell Assays, and Engraftment
Attempts to characterize UCB expansion protocols have relied directly on the assays used to measure the cell populations that are targets for expansion. Over the past 20 years, these assays have become more sophisticated, and clinical investigators have progressively adjusted their clinical trial designs based on more recent, better assays. Pluripotent hematopoietic stem cells are very rare, representing only 1 cell in 2–10 × 106 BM cells, perhaps slightly more frequent in UCB. There is no phenotypic assay, to date, that directly identifies and measures these rare cells. Rather, all efforts to measure HSCs rely on functional assays. The easiest and most popular assays have been colony-forming cell (CFC) assays, which measure cells that are partially differentiated between HSCs and mature blood cells. These cells, which correlate closely but inexactly with CD34+ cell numbers, produce easily detectable blood cell colonies in 2 weeks in the laboratory. But careful cell fractionation studies have shown that the intermediate-maturity cells detected in these assays contribute only very slightly, and temporarily, to hematopoietic recovery after transplantation, since they don’t produce enough cells that survive long enough after transplantation; it is only the immature pluripotent stem cell population that contributes to sustained engraftment.5,6 So, although CD34+ cell numbers in an unmanipulated graft may correlate with clinical time to engraftment after transplantation, that is likely because the ratio of intermediate progenitor cells to very primitive stem cells is fairly constant between clinical samples, and therefore CD34+ cell numbers indirectly reflect primitive stem cell numbers transplanted. This assumption, however, may not be true following many ex vivo expansion procedures, in which progenitors may be greatly expanded but primitive stem cells may not.7
Better functional assays for primitive stem cells, developed in the late 1980s and 1990s, include the long-term culture initiating cell (LTCIC), cobblestone area forming cells (CAFC), and best of all the human to immunodeficient NOD/SCID mouse (NOD/SRC) repopulating cell assay.8 In evaluating UCB expansion protocols, it is important to determine what populations were measured and specifically whether LTCIC, CAFC or NOD/SRC were identified as being expanded.
UCB Expansion Ex Vivo: Cytokine Cocktails
When UCB progenitors were first detected, investigators noted that the progenitor cells formed colonies in agar more rapidly than did BM or PSC progenitors, and that a higher fraction of UCB colonies could be replated to form secondary colonies. These data on UCB progenitors, together with their much higher frequency in the UCB mononuclear cell fraction, suggested that they were more proliferative than their adult counterparts. In the early 1990s Moore and Hoskins applied the techniques of PB CD34+ cell isolation followed by culture in high concentrations of multiple hematopoietic growth factors first pioneered by Haylock et al to UCB, with prima facie very promising results. After removal of more mature CD34− cells, clonogenic progenitors could be expanded 50- to 100-fold, while slightly more primitive Δ cells could be multiplied 10–20X, all in 2 weeks.9 Subsequently, many investigators confirmed that UCB progenitor populations could be readily expanded, and some showed that LTCIC could also be expanded 10X, particularly if combinations of low-dose cytokines were combined with perfusion conditions that simultaneously removed the secreted products of maturing blood cells and supplied continuous fluxes of cytokines.10 Of note, however, no assays for primitive NOD/SRC repopulating cells were measured in these studies, so it is not known to what extent, if any, true HSC were expanded in these protocols. When Lewis et al assayed UCB NOD/SRC repopulating cells in parallel with CFC and LTCIC during ex vivo cytokine expansion cultures, they found that while CFC were increased 20–25X, LTCIC were only increased 40%, and NOD/SCR were maintained but not increased at all.11
True UCB NOD/SRC stem cell expansion may be achievable by very judicious combinations of HSC purification and cytokine selection. SDF-1 treatment increases the engraftment of UCB HSCs, but whether this effect reflects quantitative increases in HSCs or a qualitative improvement in their behavior is not certain.12 This may be the basis of the reported enhancement of UCB engraftment achieved by supplementing UCB grafts with mesenchymal stem cells. UCB HSCs are selectively responsive to interleukin (IL)-6 stimulation,13 and recent studies suggest that combining UCB CD34+ cell selection with IL-6/soluble IL-6 receptor/SCF/Flt-3l/Tpo stimulation induces NOD/SRC expansion 4–5X. Overall, it appears that UCB stem cell expansion may be possible with ex vivo cytokine cocktails, but conditions that can generate such HSC expansions, as opposed to the expansion of more mature progenitor populations, may have not yet been used in clinical trial settings.
Stem Cell Expansion: Hox Gene Products
Individual cytokines are known to trigger many intracellular signal transduction pathways, which presumably activate multiple gene transcription pathways. Thus, in retrospect, combinations of cytokines might well be expected to have multiple effects on HSCs that might not permit UCB stem cell expansion. An alternative, more direct, approach to expanding stem cells might focus on the specific genetic pathways known or believed to support primitive HSC proliferation. Over the past decade several such pathways and transcription factors have been proposed, including Notch-1 receptor activation, Wnt/LEF-1 pathway induction and telomerase. One of the most promising intracellular targets for HSC expansion are Homeobox (Hox) gene products. These transcription factors, which were first identified as master switch transcriptional regulators of early development, were discovered a decade ago to be expressed in the most primitive HSCs. When cloned and overexpressed in mouse HSCs, several of these Hox genes induce HSC proliferation, in addition to more or less disruption in differentiation.
One of these Hox genes, HoxB4, has proven to be the most interesting to date. HoxB4 is highly expressed in primitive HSCs, and its expression declines with differentiation. Overexpression of HoxB4 mRNA by retroviral infection results in HSCs that expand over 100X if transplanted in very low numbers (analogous to the clinical HSC scenario).14 Of even more interest, recent experiments suggest that the same effect can be achieved by treating purified HSCs with a modified form of soluble HoxB4 protein (TAT-HoxB4), which increases intracellular HoxB4 protein levels for several hours only.15 While this approach has thus far only been applied to murine HSCs, and not to human BM or UCB HSC expansion, the application to human UCB expansion could be very direct.
More recently, Zhu et al have discovered that the protein NF-Y is a normal transcriptional activator for multiple Hox genes, as well as telomerase, Notch-1 and LEF-1.16 When overexpressed in murine HSCs by as little as twofold, NF-Ya increases HSC numbers by 20X following transplantation. Application of TAT-NF-Y protein transduction to UCB HSC expansion thus offers the attractive possibility of activating multiple HSC expansion pathways simultaneously. The absolute and relative efficiencies of these protein treatments for expanding human UCB HSCs will need to be evaluated directly in NOD/SCID mouse xenotransplants, and then in appropriate Phase I clinical trials.
Similar approaches using other soluble, reversible, biochemical stimulators of stem cell transcription programs, including perhaps copper chelation, have also been proposed and are under preclinical and clinical trial.
Clinical Experience with Ex Vivo Expanded Hematopoietic Cells
Clinical trials of ex vivo culture UCB cells have begun to be reported, but their interpretation should be tempered by the knowledge that the conditions of culture used to date have not been shown to support USC amplification, only CFU expansion. At least three trials evaluating the combination of unmanipulated with ex vivo expanded UCB grafts have been published to date; in all three studies expanded cells were added on day 10–12 to supplement unmanipulated cells. One study involved the use of a static culture system in defined media while the other two used a perfusion culture expansion system. Although no toxicities were seen in these studies, no significant acceleration of neutrophil or platelet engraftment was seen.17– 19 Neutrophil engraftment to ANC > 500 was seen on day 24–33, and platelet engraftment to > 50,000 at approximately day 90.
These results, while disappointing, highlight the importance of building clinical trial design based upon the most precise and appropriate preclinical data. The cytokine cocktails employed in the three published studies included some but not all of the cytokines required to achieve UCB HSC expansion in vitro, as measured by our best NOD/SRC assays: One trial used SCF and a Tpo analogue, but not Flt3-l, and the other two used Flt3-l, but not Tpo or SCF, and neither included IL-6 or soluble IL-6 receptor. In addition, a lesser but possibly contributing variable was that in all three trials the ex vivo expanded cells were added 10–12 days later, thus mitigating any accelerating effect they might have otherwise have achieved.
It must be pointed out that these trial design limitations were known to the clinical investigators, but the lack of availability of clinical grade cytokines, and possibly also HSC selection devices, prevented the optimal cocktails and culture conditions from being employed. These difficulties would be surmounted if simpler expansion conditions, requiring few added reagents and/or devices while still supporting HSC expansion, could be devised and employed.
What if UCB HSC Numbers Are Not Rate Limiting? Exploring the Null Hypothesis
Another possibility that must be considered is that increasing UCB numbers will not, by itself, accelerate engraftment. Perhaps there is a qualitative “immaturity” defect that prevents rapid engraftment. If so, then is there a way to circumvent this defect: providing a maturation effect through some combination of in vivo cytokine treatment, or perhaps ex vivo cell activation targeted at maturation? Viewed through this perspective, one can envision manipulations of the non-HSC components of the UCB graft, including the mature hematopoietic cells, the immune cells, antigen-presenting cells, and mesenchymal stromal cell components of the graft. Interactions between HSC and non-HSCs could form the basis for potentially improved engraftment in mixed graft settings as well.20 Each of these manipulations could be evaluated by comparing engraftment in NOD/SCID mice, prior to clinical transplantation trials.
In summary, experience with hematopoietic cell expansion to date supports the fundamental principle of experimental hematology, that the best clinical results follow from the most careful, comprehensive and creative preclinical trials in the proper in vitro and in vivo models. For the future, this experience suggests concentrating on ex vivo manipulations that can be shown to directly increase HSC numbers following NOD/SCID engraftment and/or accelerate the pace of engraftment in these same models.
III. Immune Reconstitution After Umbilical Cord Blood Cell Transplantation
Kenneth I. Weinberg, MD*
Children’s Hospital of Los Angeles, 4650 Sunset Boulevard, Box 62, Los Angeles CA 90027 Acknowledgments: Supported by NIH grants R01 HL54729, HL70005, AI50765; P50 HL54850; M01 RR00043. The thoughtful input of Gay Crooks, Bruce Blazar, Robertson Parkman, Nelson Chao, Wes Brown, Hisham Abdel-Azim and Dan Douek is greatly appreciated.
One of the events necessary for the success of hematopoietic stem cell transplantation (HSCT), regardless of the source of stem cells, is the development of a functional immune system from donor-derived cells. The production of adequate granulocytes, platelets, and red blood cells usually occurs rapidly after HSCT. In contrast, the ability to produce lymphocytes, especially T lymphocytes, is delayed. As a result, serious infection in the first 1–2 years after HSCT occurs in about 50% of uncomplicated transplants from histocompatible sibling donors, and up to 80%–90% of recipients of matched unrelated donor (MUD) marrow transplants or histocompatible recipients who developed GVHD.1 Infections attributable to poor lymphocyte function include viral pathogens such as the herpes group (cytomegalovirus [CMV], herpes simplex virus [HSV], varicella zoster virus [VZV], human herpes viruses [HHV] 6 and 8), respiratory viruses (respiratory syncytial virus [RSV], parainfluenza viruses), adenovirus, and enteroviruses (Coxsackie and ECHO viruses), as well as EBV-associated lymphoproliferative disorders. Other infections that may result from inadequate cellular immunity include fungal infections such as Pneumocystis carinii, Candida, and Cryptococcus. Increased susceptibility to Aspergillus infection may reflect defects in innate immunity. Susceptibility to some bacterial infections, notably those caused by encapsulated organisms (Staphylococcus pneumoniae, Haemophilus influenzae), is determined by the inability to produce antipolysaccharide antibodies.
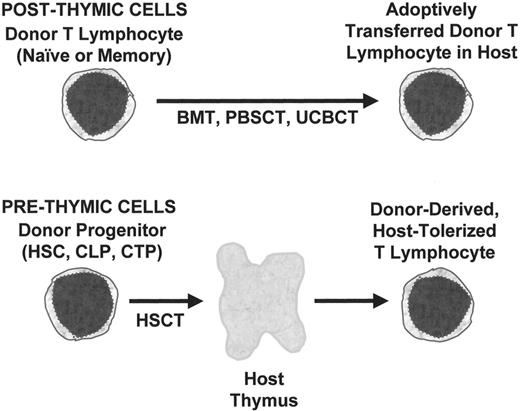
The emphasis of this review will be the relationship between stem cell source and the development of T lymphocyte functions required for protection of the HSCT recipient from infection. In particular, the unique aspects of UCBT, compared to either BMT or mobilized peripheral blood stem cell transplantation (PBSCT), will be described. Factors influencing the development of adequate immune function after HSCT include those related to the donor progenitor cell populations, pre-formed lymphocytes in the donor product, the host microenvironment, pharmacological interventions, and antigenic exposure. All clinically relevant stem cell sources include both progenitor cells and preformed lymphocytes (Figure 1 ). Prethymic progenitors may include HSC, common lymphoid progenitors (CLP), committed T progenitors (CTP), whose T lymphoid progeny depend on the host thymus for their development.2
Development of Thymic Progenitors
Upon entry to the thymus, progenitor cells undergo expansion; differentiation events including V(D)J recombination to generate functionally rearranged TCR genes; and both positive and negative selection events that cull potential T lymphocytes that would either be unresponsive to antigenic peptides presented by self-major histocompatibility complex (MHC) antigens or overly responsive to either MHC or self antigens. The ultimate fate of negatively selected cells is apoptosis. Since most thymocytes are fated to die, maintenance of thymic output depends on continual expansion and differentiation of the immature prothymocytes, which lack expression of the TCR invariant CD3 complex, and the co-receptors CD4 and CD8 (“triple negative,” TN).
The major signals for expansion of immature prothymocytes are two cytokines, interleukin-7 (IL-7) and c-kit ligand (KL), which are produced by thymic epithelial cells (TEC). Murine studies have demonstrated that pre-HSCT radiotherapy and chemotherapy kill TEC, resulting in defective intrathymic IL-7 production. Early studies demonstrated that the thymic microenvironment is a target of GVHD, and more recent studies have shown that TEC organization and function is abnormal in experimental GVHD models. In non-HSCT studies, aging also results in TEC damage and decreased intrathymic IL-7 production. Thus, thymic function may be adversely affected by several phenomena commonly associated with clinical HSCT-cytotoxic therapies given pre-HSCT, GVHD, and recipient aging. Clinical studies have demonstrated that decreased capacity for production of new T lymphocytes is associated with GVHD and increasing recipient age.3,4 The effects of pre-transplant conditioning on thymopoiesis has been more difficult to assess because of the complexity of the problem of dose intensity as well as the effects of previous therapy for malignancies. Any assessment of the effect of stem cell source, e.g., UCB, on immune reconstitution must take into account the function of the thymic microenvironment.
The biology of prethymic progenitors is also highly relevant to the outcome of HSCT. The lack of pre-existing TCR rearrangements means that donor-derived prethymic progenitors are unlikely to cause GVHD, as long as the recipient thymus maintains the ability to select out undesirable T cells. However, the development of an immune repertoire from prethymic progenitors may be delayed by ontogenic factors such as commitment to the T lymphoid lineage and the time normally required for development of large numbers of diverse T cells. Between 7 to 9 weeks of gestation, hematopoietically derived cells enter the thymic anlage, which forms from mesenchyme and endoderm of the III-IV pharyngeal pouches. Alloreactive T cells are present by week 14–15 and antigen-specific T lymphocytes appear later in the second trimester. Recapitulation of fetal ontogeny provides a framework for understanding the development of lymphoid cells after HSCT. The generation of T lymphocytes post-HSCT requires events similar to those observed in normal immune development, e.g., lymphoid commitment, thymic migration and entry, and thymic differentiation. It is likely that the time period required for such development is similar to the time normally required for prenatal T cell development. For example, in patients with severe combined immune deficiency (SCID) receiving CD34+ haploidentical HSCT, the appearance of functional mature T lymphocytes in the peripheral blood is 90–120 days after transplant, a time period similar to that required for normal prenatal immune ontogeny.
Post-Thymic T Lymphocytes
In contrast to prethymic progenitors, post-thymic T cells have already undergone maturation and have antigenic and functional specificities that were conferred by the donor’s thymus. Donor-derived post-thymic T cells express a fixed TCR and thereby are programmed to respond to a set of antigenic determinants. Although adoptive transfer of mature T lymphocytes had long been thought to be a relatively static process, recent studies have indicated that mature T lymphocyte populations are dynamic and regulated by interactions with other T lymphocytes as well as the host microenvironment. Mature T lymphocytes may undergo activation if they encounter a stimulating antigen and the proper set of co-stimulatory signals. Activation of T lymphocytes leads to acquisition of functional properties such as cytolytic capacity or cytokine secretion. Activated T cells may proliferate and mature into memory cells, undergo apoptosis (activation-induced cell death [AICD]), or be anergized. Expansion of activated T lymphocytes is dependent on production of IL-2 by helper T lymphocytes and expression of high affinity IL-2 receptors (IL-2R) by the responsive helper and cytotoxic cells.
Recent studies have demonstrated that naive T lymphocytes in a lymphopenic environment can undergo proliferation while maintaining a mainly naive phenotype.5 Such homeostatic proliferation differs from activation-induced proliferation in which naive T cells recognize foreign antigens and proliferate in response to IL-2. In homeostatic proliferation, the stimuli recognized by the TCR are the same self antigens that normally induce positive selection in the thymus. Like thymopoiesis, homeostatic proliferation also requires IL-7, although the source is probably extrathymic, not TEC. Homeostatic proliferation has also been described for memory T lymphocytes with evidence that IL-15 is critical for the expansion of these cells.
The appearance of T lymphocytes after HSCT is conventionally seen as evidence of de novo generation of T lymphocytes from progenitor cells. However, as previously noted, transplantation of stem cell products that have been extensively depleted of mature T lymphocytes in order to prevent GVHD results in significant delays in immune reconstitution. Therefore, it is likely that much of what has been attributed to T cell generation in the first few months after transplant is the result of adoptive transfer of post-thymic cells. Such cells may undergo homeostatic proliferation in the lymphopenic environment of the host, or IL-2 driven proliferation if activated by exposure to alloantigens or nominal antigens. Analyses of the length of time required for de novo generation of T lymphocytes after HSCT are best performed in patients receiving highly purified progenitors are depleted of all post-thymic T lymphocytes.
UCBC HSC
HSC derived from UCBC differ from those of adult marrow or PBSCT in several ways. The most notable difference is quantitative—the cell dose in UCBT is significantly less than the HSC dose in adult sources such as PBSC or marrow. While low cell dose has been associated with failure of hematopoietic engraftment, HSC dose may also influence immune reconstitution. In a murine congenic transplant model, increasing the dose of phenotypic HSC administered resulted in greater thymic cellularity post-transplant, as well as greater numbers of mature T lymphocytes derived from the transplanted cells.6 Thus, one of the limiting factors for T lymphocyte reconstitution after HSCT, besides microenvironmental defects, may be the number of progenitor cells entering the thymus and contributing to thymopoiesis. Limited numbers of HSC in UCBC compared to marrow or PBSC products may delay or decrease thymic regeneration.
There are also qualitative differences between UCBC and other HSC sources (Table 5). Comparisons of CD34+ CD38− phenotypic HSC derived from UCBC versus adult marrow have shown that UCBC contain a higher frequency of LTCICs and a higher percentage of cycling cells.7 UCBC-derived HSC also had greater cloning efficiency and generative capacity. Thus, the HSC in UCBC may be both more primitive and more capable of regenerating hematopoiesis in the recipient. The contrasting biological properties may represent intrinsic or environmental differences in the UCBC and adult marrow HSC. The most likely intrinsic difference may be the age of the HSC—a few months for UCBC versus decades for adult HSC. There have been some studies of the aging process in hematopoietic progenitors, but there is little direct evidence that HSC from adult donors have decreased capacity to contribute to lymphocyte development compared to other progenitors.
The Placenta as Unique Microenvironment That Influences UCBT
Besides intrinsic differences, HSC in UCBC have had a different set of microenvironmental exposures compared to those of adult marrow or PBSC. All HSC sources are influenced by the microenvironment from which they are derived. An example of differences between sources are some of the observed changes in HSC cell cycle status, gene expression and adhesive and invasive properties induced by mobilization procedures used to generate PBSC, e.g., G-CSF.
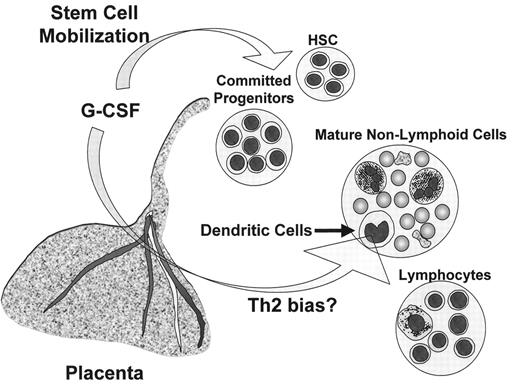
The placenta is a complex organ that regulates maternal-fetal interactions. Many cytokines that can influence lymphohematopoietic development, e.g., G-CSF, c-kit ligand (stem cell factor [SCF]), GM-CSF, IL-15, and others, are produced by the placenta. Production of G-CSF by the placenta may be especially relevant to UCBT (Figure 2 ). G-CSF is produced both by the maternal decidua and the fetal chorionic villi8 and enters the fetal circulation by a process that does not require a functional G-CSF receptor. G-CSF from the mother probably does not enter the fetal circulation as administration of recombinant human G-CSF (rhG-CSF) to pregnant macaques did not result in detectable rhG-CSF in the fetuses. The function of placental G-CSF production is unknown; however, it may serve as an immunoregulator that protects the mother and fetus from each other’s allogeneic immune systems. G-CSF inhibits the ability of placental mononuclear cells to mediate cytotoxicity against allogeneic targets including choriocarcinoma cells. The production of G-CSF and other cytokines capable of HSC mobilization, e.g., SCF, is likely to explain the high percentage of HSC in UCBC and their rapid disappearance from the neonate’s circulation after birth. In contrast to mobilization induced by a short period of monotherapy with G-CSF before PBSCT, the HSC in UCBC have been exposed continually to a complex mixture of cytokines that likely affect their behavior, e.g., induce a high rate of cell cycling,8 or alter the homing and invasive properties needed for entry of progenitors into the thymus.
UCBC and Lymphoid Progenitors
Little is known about the numbers and functional properties of circulating lymphoid progenitors present in UCBC compared to other sources. Commitment to lymphoid differentiation by donor HSC could be a rate-limiting step in the ability to repopulate the recipient immune system after HSCT. At present, there is some controversy regarding the nature of intermediate lymphoid progenitors. The murine common lymphoid progenitor (CLP) has been described as a phenotypic population in the marrow that can contribute to T, NK, and B lymphopoiesis, but it is not clear if CLP generate all thymocytes. There is some evidence that early thymic progenitors (ETP) in the thymus can arise in a CLP-independent manner. At present, CLP are the only lymphoid progenitor that are relevant to transplantation; besides the logistical problem of transplanting the intrathymic ETP population, it is unclear if such cells could home and engraft after transplantation. Murine CLP can be transplanted into irradiated hosts and contribute to post-transplant lymphopoiesis. Co-transplantation of CLP with HSC is able to more rapidly restore immune function than transplantation of HSC alone, and can result in increased protection of mice from experimental challenge with murine CMV.9 Although human cells with CLP-like features can be found abundantly in UCBC (personal communication, Gay Crooks), rigorous comparison of the numbers and functional properties of CLP in UCBC versus marrow or PBSC still need to be performed.
UCBC Post-Thymic T Cells
Studies over the last 20 years have demonstrated differences between neonatal T lymphocytes and those of adults (Table 5 ). The major notable difference between the T lymphocytes in UCBC and those derived from adult marrow or mobilized PBSC is the maturational status. Because the fetus is exposed to few foreign antigens, the T lymphocytes in UCBC are almost exclusively naive. Naive T lymphocytes express a phenotype that is identifiable as CD45RA+ CD45R0− and CD62L+. As individuals age, there is an increase in the frequency of T cells that have differentiated into a memory phenotype, CD45RA− CD45R0+ and CD62L−/low as a result of antigenic exposure. Memory cells are more readily activated by antigen than naive T cells and may be able to respond to weaker co-stimulatory signals through the B7-CD28 pathway. The predominant naive phenotype of T lymphocytes in UCBC may contribute to the reduced alloreactivity observed after UCBT.
Investigations of neonatal T cell function have been driven by interest in the increased susceptibility to severe viral infections in neonates. For example, newborns infected with HSV have higher rates of dissemination (sepsis, meningoencephalitis) than adults with primary infection, and increased severity of some viral infections such as enteroviruses is observed until at least several months of age. Investigations of neonatal helper T lymphocytes have demonstrated decreased production of Th1 cytokines needed for cytolytic and anti-viral responses, compared to adult T lymphocytes.10 Specifically, the activation of the interferon-γ (IFN-γ) gene is markedly reduced in neonatal T lymphocytes, because of decreased induction of transcription factors, e.g., NF-AT, which are required for activation of the IFN-γ gene after TCR stimulation. A central question is whether such differences are intrinsic to UCBC versus adult T lymphocytes. Interpretation of the differences has been confused by comparison of signaling properties of UCBC T lymphocytes to unfractionated adult T lymphocytes. Since adult T lymphocytes are comprised of both naive and memory T cells, while UCBC are almost exclusively naive T cells, ascribed differences between them may simply reflect differences between the stringent activation requirements of naive helper T lymphocytes and the more easily activated memory cells. Only comparisons between UCBC and purified naive T lymphocytes from adult blood can be used to evaluate whether UCBC T cells have unique properties different from adult peripheral blood naive T lymphocytes. Regardless of the reason, T lymphocytes from UCBC appear to be less capable of mediating Th1 cytotoxic responses than those derived from adult sources.
The decreased capacity for Th1 responses has both positive and negative implications for HSCT. The decreased absolute numbers and decreased responsiveness of UCBC T lymphocytes contribute to the decreased alloreactivity and greater tolerance for MHC disparity observed in clinical UBCBT. However, the decreased responsiveness probably means that there is also less adoptive immunity derived from UCBC T lymphocytes. There are fewer T cells overall, and the number of memory cells capable of mediating recall responses to antigen is extremely limited. Any adoptive immunity must be derived from the naive T lymphocytes present in the UCBC product. As discussed above, naive T lymphocytes are difficult to activate, rendering them less capable of responses to pathogens. These properties of the mature T lymphocytes in UCBC would predict that antiviral and fungal immunity may be decreased after UCBT than after BMT or PBSCT.
UCBC Dendritic Cells
Besides intrinsic differences in T lymphocytes, there may be differences in antigen presenting cells (APC) derived from UCBC versus adult sources (Table 5 ). Analyses of dendritic cells (DC) in UCBC have shown that both so-called myeloid and plasmacytoid DC are present, although there may be a decreased proportion of myeloid DC compared to adult peripheral blood. The myeloid dendritic cells are thought to promote Th1 responses by producing IL-12. Studies of the DC derived from UCBC have indicated that there may be intrinsic defects in production of IL-12 as well as other functions of DC. Recent data on DC generation after HSCT suggests that G-CSF administration significantly decreases the ability to produce IL-12.11 The decrease in IL-12 production after post-HSCT G-CSF administration may be relevant to UCBT. As discussed above, cells in the fetal circulation are probably constantly exposed to placental G-CSF. Furthermore, many clinical UCBT protocols have used post-transplant G-CSF administration to accelerate the development of granulocytes. The routine administration of G-CSF, combined with intrinsic defects in DC function, may decrease the generation of Th1 responses after UCBT. The expected consequences of such an effect would be decreased capacity to control opportunistic viral and fungal infections.
Anticipated and Empiric Properties of UCBT
Based on the progenitor and immunological properties of UCBC, certain predictions can be made regarding the expected immune reconstitution after UCBT. Compared to the detailed information available about the recovery of marrow function and the decreased risks of severe GVHD, there are few systematic data on lymphoid recovery, and even less on such properties as antigenic repertoire and effector function. As discussed above, immune function after HSCT can be derived from either the adoptive transfer of preformed mature T lymphocytes in the stem cell product or from the development in the host of donor-derived lymphocytes from the donor HSC or other prethymic populations, e.g., CLP.2 The adoptively transferred T cells would be expected to represent most of the lymphocytes seen in the first few months after transplant, but are more likely to be both impermanent and narrow in repertoire. Adoptively transferred T cells after UCBT are largely naive cells which will be less responsive to specific antigen than the memory cells in adult sources and probably less able to mediate Th1 effector functions than marrow or PBSC. The HSC and pre-thymic progenitors are expected to produce a long-lasting, broad immune repertoire but would be slow to develop. The increased generative capacity of UCBC HSC might predict more rapid engraftment kinetics, but would be expected to be offset by the decreased dose of transplanted HSC in the average UCBT. Analyses of UCBT recipients have demonstrated slow de novo generation of T lymphocytes in the first year after transplant; however, by 2 years post-UCBT recipients have evidence of both a broad repertoire and thymic function.3,12,13 It is unclear if there are differences in the kinetics of post-transplant lymphopoiesis in UCBT versus BMT. More rapid recovery in children versus adults and nonmyeloablative than myeloablative conditioning suggests that damage to the thymic microenvironment as a result of aging or cytotoxic therapies may be important determinants of post-HSCT immune reconstitution.12
Although several studies have demonstrated the importance of UCBC CD34+ cell dose for likelihood of hematopoietic engraftment and engraftment kinetics, analyses of the engraftment kinetics of lymphoid populations are more limited. In a study from Case Western University, unrelated donor UCBT was compared to the matched unrelated donor marrow transplantation.14 UCBT led to decreased rate of appearance of lymphoid populations in the first 3 months after transplantation. In the subsequent year, the absolute lymphocyte count of the UCBT recipients exceeded that of the MUD recipients. Indeed, the mean ALC in the UCBT group was normal by day 200 post-transplant but remained abnormally low in the MUD recipients during the entire first year. Since the T lymphocytes seen in the first 100 days post-transplant are likely to be derived from adoptively transferred mature T cells, the decreased lymphocyte count in the first 3 months after UCBT would be expected as a result of lower amount of such cells in UCBC products. The increased numbers of lymphocytes observed later could reflect greater capacity of the UCBC progenitors to produce lymphocytes de novo. However, this is uncertain: an alternative explanation could be that the increased GVHD observed in MUD recipients led to more severe lymphopenia than that seen after UCBT.
The differences in UCBC T cells from adult sources would be expected to translate into UCBT recipients having less GVHD but also less protection from viral infections such as CMV or EBV-LPD. Overall, the risk of infectious death in studies of UCBT may be higher than that of transplant from other sources, but large, multi-institutional, randomized studies have not been done, and there is a bias toward use of UCBT in sicker patients who cannot await a MUD BMT.15–,19 In a non-randomized study of children with ALL, the incidence of viral infections was greater after UCBT than after unrelated BMT.16 Complicating the analysis of actual infectious risk for some infections like herpes viruses is the difference in potential source of virus between UCBT and either BMT or PBSCT. CMV- or EBV-infected B cells can be derived from either the donor or recipient in BMT or PBSCT. In UCBT, CMV- or EBV-infected cells would be expected to exclusively arise from the recipient. Assuming that CMV-negative blood products are administered to CMV-negative recipients, then only CMV-positive recipients of UCBT are at significant risk of CMV disease, while CMV-negative BMT or PBSCT recipients could be infected through their seropositive donor. Therefore, comparisons of herpes virus infections after UCBT versus BMT or PBSCT need to analyze at risk populations, not the total number of recipients. For several infections, notably CMV and HHV-6, there is evidence that at-risk recipients may have greater rate of progression to symptomatic infection, with decreased evidence of viral control, although this is not a universal finding.17–,19 In the comparison of UCBT with MUD transplants at Case Western University, the overall risk of infection, including bacterial infection, correlated with the degree of lymphopenia.17 The risk of early bacterial infection in UCBT recipients may reflect differences in duration of neutropenia, not just lymphocyte numbers or function.16
The available information about immune reconstitution after clinical UCBT is quite limited. In order to better understand both the kinetics and quality of immune reconstitution after UCBT, analyses of specific lymphoid populations, immunological repertoire, mitogen and antigen-driven proliferation and function, and responses to test neo-antigens, e.g., keyhole limpet hemocyanin, are needed. Ideally, such studies would be multi-institutional but use similar degrees of matching, CD34+ cell dose, pretransplant conditioning, supportive care and post-transplant immune suppression to control for the variables of GVHD, conditioning related microenvironmental damage and progenitor cell dose that likely have an impact on immune reconstitution. At this time, the lack of direct comparisons of immune reconstitution hinders the ability to make decisions regarding the optimal source of HSC for transplantation. In a patient with an active viral infection, e.g., CMV or a history of Epstein-Barr virus lymphoproliferative disease (EBV-LPD), unrelated marrow from an immune donor may be better than UCBT. However, potential HSCT recipients may have limited choices regarding potential HSC sources, and frequently the ability to rapidly perform transplant may trump any immunological disadvantages of UCBT.
Strategies to Overcome the Problems of Immune Reconstitution After UCBT
A paradox of UCBT is that some of the associated immunological problems are causally related to the properties that make UCBT attractive in the first place. For example, the decreased alloreactivity that has been clinically observed in UCBT reflects the quantitative and functional abnormalities that make adoptive transfer of antiviral immunity so unlikely immediately after UCBT. Efforts to improve immune reconstitution after any form of HSCT have been directed at two somewhat different questions—accelerating the rate of development of functional immunity, especially that required to control certain early infections, e.g., CMV; or altering the long-term qualitative and functional defects such as hypogammaglobulinemia that contribute to morbidity and mortality over the first decade after transplant. The first goal has been more pressing because of the high impact of opportunistic infection on transplant-related mortality. Strategies in BMT or PBSCT designed to increase the adoptive transfer of antigen-specific T cells directed against important pathogens such as CMV or EBV have used co-transplantation of cloned T cells that have been expanded in vitro. The application of such strategies to UCBT would require either cloned cells that are derived from the host or a third party, or techniques for in vitro priming of the naive T cells in the UCBC graft. Nonspecific activation of the T cells in the UCBC product would likely increase the risk of GVHD.
Besides the manipulation of the preformed T cells in a graft, other strategies that have been proposed for accelerating immune reconstitution have been aimed at increasing the rate at which prethymic cells contribute to the mature T lymphocyte compartment, either by increasing the number of transplanted lymphoid progenitors or by accelerating their development in vivo. One potential strategy that has been demonstrated in murine models is the co-transplantation of committed progenitors, such as CLP.9 Administration of 3000 CLP in addition to 500 HSC in lethally irradiated mice provided significant protection from experimental infection with murine CMV, compared to HSC alone. Impressively, small numbers of CLP provide levels of protection from MCMV that are comparable to that of millions of post-thymic cells. The technical difficulty for UCBT would be obtaining sufficient CLP to affect reconstitution, e.g., via ex vivo expansion. The use of ligands for the Notch receptors that regulate both proliferation and commitment to lymphoid lineages might be useful for in vitro generation of prethymic progenitors that could be used to augment those present in the UCBC product.20 Based on the data of Chen et al, expansion techniques to increase the number of transplanted HSC might be expected to increase thymic output.8
Other strategies have focused on ways to increase the in vivo development of T lymphocytes in the recipient after transplantation. These studies have been driven by the hypothesis that the thymic microenvironment of HSCT recipients lacks sufficient capacity to efficiently produce new T lymphocytes from prethymic progenitors. Our group has emphasized that defects in TEC numbers or function caused by aging, cytotoxic therapies, or GVHD lead to a relative lack of intrathymic IL-7 needed for thymopoiesis. Systemic administration of IL-7 in murine models can dramatically improve thymic output after transplantation, but the effects have not been reproducibly observed in larger animal models and IL-7 has additional effects on mature T cells that could limit its usefulness in allogeneic HSCT. Besides promoting the development of thymocytes, IL-7 treatment also increases the expansion of mature T lymphocytes by promoting their proliferation and survival. These effects have led to increased GVHD in some preclinical experiments but have not been observed by others. Experimental usage of IL-7 in clinical allogeneic transplants should probably be initially restricted to T cell–depleted grafts, not UCBT, until more is known about both efficacy and promotion of GVHD.
Another strategy to address the problem of microenvironmental damage has been to decrease the toxicity of the conditioning regimen. Besides decreasing the intensity of conditioning, another approach is the administration of a biological response modifier, recombinant keratinocyte growth factor (KGF). KGF is a mesenchymally derived member of the fibroblastic growth factor family which interacts with an epithelial-specific receptor. Administration of KGF in both preclinical experimental models and clinical trials has demonstrated decreased regimen-related toxicities such as oral mucositis, but also less GVHD. KGF protects TEC in murine transplant and aging models, thereby increasing thymopoietic capacity and improving both the immediate rate of lymphocyte recovery and medium-term (3–4 months) immune reconstitution. The clinical relevance of protection of murine thymopoiesis by KGF needs to be determined.
Summary
Immune reconstitution after UCBT is promoted by the increased generative capacity of UCBC HSC, and the decreased GVHD, but is hampered by fewer HSC, decreased adoptively transferred antigen-specific T cells, and functional defects in the T cells. Clinical studies to understand the relative importance of each of these biological properties to outcome are needed. At this time, it is difficult to use relative differences in immune reconstitution between HSC sources as a basis for choosing the mode of HSCT, although BMT might be preferential to UCBT for a recipient with an active viral infection. Some but not all strategies aimed at improving immune reconstitution after HSCT in general may be relevant to addressing the immunological and infectious problems observed after UCBT.
Two pathways for generation of T lymphocytes after hematopoietic stem cell transplantation (HSCT).
Shown at top is the adoptive transfer of post-thymic T cells which then undergo expansion in the host, resulting in a limited repertoire of mature T lymphocytes that can rapidly confer antigen-specific responses, including graft-versus-host disease (GVHD). Below is shown the pathway for differentiation of pre-thymic progenitors by migration into the recipient thymus, resulting in the slow generation of a diverse repertoire that is host-tolerant.
Two pathways for generation of T lymphocytes after hematopoietic stem cell transplantation (HSCT).
Shown at top is the adoptive transfer of post-thymic T cells which then undergo expansion in the host, resulting in a limited repertoire of mature T lymphocytes that can rapidly confer antigen-specific responses, including graft-versus-host disease (GVHD). Below is shown the pathway for differentiation of pre-thymic progenitors by migration into the recipient thymus, resulting in the slow generation of a diverse repertoire that is host-tolerant.
Placental granulocyte colony-stimulating factor (G-CSF) production affects umbilical cord blood cells (UCBC).
G-CSF produced in the placenta may affect both fetal HSC and dendritic cells. Placental G-CSF may induce mobilization of the fetal HSC, resulting in significant numbers of circulating HSC among UCBC, which declines rapidly after delivery. G-CSF may also suppress the immunological potency of fetal dendritic cells by interfering with IL-12 production and co-stimulatory activity. As a result, the immune responsiveness of fetal T lymphocytes is attenuated and skewed away from Th1 responses.
Placental granulocyte colony-stimulating factor (G-CSF) production affects umbilical cord blood cells (UCBC).
G-CSF produced in the placenta may affect both fetal HSC and dendritic cells. Placental G-CSF may induce mobilization of the fetal HSC, resulting in significant numbers of circulating HSC among UCBC, which declines rapidly after delivery. G-CSF may also suppress the immunological potency of fetal dendritic cells by interfering with IL-12 production and co-stimulatory activity. As a result, the immune responsiveness of fetal T lymphocytes is attenuated and skewed away from Th1 responses.