Abstract
To optimize treatment outcomes for hematologic malignancies, minimizing the consequences of treatment complications requires as much skill as the choice of the treatment itself. Myelosuppression and immunosuppression are frequent complications and have potentially serious infectious consequences. Invasive fungal infections and infections from respiratory viruses are increasing in frequency and have life-threatening potential. Damage to vital organs, especially the liver, is another important concern. In this chapter, the scope of invasive fungal and respiratory viral infections, recent insights into the pathogenesis of hepatic sinusoidal injury, and recent developments that impact prevention and treatment approaches for these complications are described.
In Section I, Dr. John Wingard describes the advantages and disadvantages of various treatment options for invasive infections by the two chief fungal pathogens, Candida and Aspergillus. Adjunctive therapies and practical considerations that clinicians should weigh in choosing one or another of the various agents are discussed. The studies that have evaluated antifungal prophylaxis and empirical treatment strategies are reviewed. Finally, new approaches such as combination therapy, new diagnostics, and efforts to bolster host immunity are considered.
In Section II, Dr. W. Garrett Nichols describes the epidemiology of community-acquired respiratory viruses (CRV) in patients with hematologic malignancies. Risk factors, clinical syndromes, and possible indirect effects of CRV infections are discussed. Treatment and prevention options are reviewed.
In Section III, Dr. George McDonald describes sinusoidal obstruction syndrome (once known as hepatic veno-occlusive disease). Recent insights into pathogenesis are described. Diagnostic criteria and the advantages and disadvantages of various diagnostic methods are reviewed and prognosis is considered. Prevention and treatment options are discussed.
I. New Treatments for Invasive Fungal Infections in Patients with Hematologic Malignancies
John R. Wingard, MD*
University of Florida, 1600 SW Archer Rd., Rm R4-116, P.O. Box 100277, Gainesville FL 32610-3001
Invasive fungal infections (IFIs) are continuing threats to patients with hematologic malignancies. Factors associated with greater susceptibility for IFIs include prolonged neutropenia, graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT), and the use of antineoplastic therapies that cause intense and prolonged deficiency of T cell immune responses. Candida and Aspergillus are the major fungal pathogens that cause infection. New antifungal agents have been developed and new fungal diagnostics are now licensed. It is hoped that incorporation of these new tools into antifungal strategies will result in improved outcomes.
Candida Infections
For years, the mainstay of treatment for Candida infections has been the use of amphotericin B, a polyene that binds to ergosterol in the fungal cell membrane, given in a dose of 0.6–1.0 mg/kg/day. Alternatively, fluconazole, an azole that inhibits ergosterol biosynthesis, given in a dose of 400–800 mg/day intravenously or orally, is also acceptable. Amphotericin B is active against most Candida species. Exceptions include variable susceptibility by C lusitaniae and C guillermondi strains and rare resistant C albicans strains. Fluconazole is also active against many Candida species, with the notable exceptions of C krusei, C dubliniensis, and some strains of C glabrata. Many strains of C glabrata are susceptible dose-dependent, meaning that the agent is clinically useful for infections due to such strains but higher doses (e.g., 800 mg/day rather than the usual 400 mg/day) are needed; approximately 15% of C glabrata isolates are fully resistant to fluconazole even at higher doses. The lower sensitivity of C glabrata to fluconazole and the widespread use of fluconazole in critical care and oncology units have been associated with a rise in the proportion of C glabrata in numerous hospital fungal surveys (especially of colonizing isolates) in recent years. Notwithstanding, worldwide surveys have been consistent in showing no rise in rates of resistance to fluconazole of Candida bloodstream isolates over time.1
Randomized trials in patients with systemic candidiasis comparing amphotericin B and fluconazole have shown comparable response rates and less toxicity with fluconazole. Amphotericin B in lipid complex has been compared to amphotericin B deoxycholate and found to have comparable response and survival rates, but the lipid formulation was associated with less nephrotoxicity.2 It is important to note that most trials that have evaluated fluconazole were conducted in non-neutropenic patients, and some experts believe that its use in neutropenic patients is not yet firmly established.3 Since susceptibility to fluconazole is well predicted by species, some experts suggest use of fluconazole can be guided by knowledge of the species of infecting pathogen.3 Susceptibility testing can also be used to guide therapy, but it is not widely available.
While both the azoles and polyenes exert their antifungal activity through actions on ergosterol in the fungal cell membrane, a new class of drugs, the echinocandins, acts on the fungal cell wall through inhibition of biosynthesis of β-1,3-glucan, a major constituent of the cell wall. This action is exerted by inhibition of β-1,3-glucan synthase. The echinocandins have potent in vitro activity against all of the Candida species. The first licensed member of this family was caspofungin. Other members of the echinocandin class in clinical trials include anidulofungin and micafungin.
Caspofungin’s half-life is 12–16 hours, permitting once daily dosing. Bioavailability is poor, necessitating administration by intravenous route. In adults, a loading dose of 70 mg on the first day is given, followed by a once daily dose of 50 mg. No dose adjustment is needed for renal impairment; however, the maintenance dose should be reduced to 35 mg/day for patients with moderate hepatic impairment (Child-Pugh score 7–9) and there are no published data in patients with severe hepatic insufficiency. Data on dosing for children younger than 12 years of age are under evaluation. When caspofungin was given to volunteers concomitantly with cyclosporine, a rise in hepatic transaminases was seen, which raises a concern about a potential deleterious interaction; however, two recent series suggest that patients treated with the two drugs concomitantly have not suffered ill effects.4,5
Caspofungin’s clinical activity was noted in case series of systemic Candida infections, in a randomized comparative trial (compared with fluconazole) of esophageal candidiasis in human immunodeficiency virus (HIV)–infected patients, and more recently in a randomized comparison with amphotericin B for systemic candidiasis.6 In this latter study, caspofungin had comparable response and survival rates to amphotericin B. In secondary analyses, in evaluable patients, the response rate in those given caspofungin was significantly higher. Response rates were comparable across different Candida species. This trial was mostly in non-neutropenic patients, but in the small subset of neutropenic patients, response rates were lower but comparable. Caspofungin was well tolerated and considerably less toxic than amphotericin B.
Anidulafungin has been evaluated for treatment of candidiasis in a case series.7 A randomized trial comparing voriconazole, an extended spectrum azole, with amphotericin B has been completed and is being analyzed.
Practical use of current anti-Candida agents
For patients with serious Candida infections, caspofungin or one of the amphotericin B formulations is most appropriate, providing proven efficacy against practically all Candida species. Safety considerations give an edge to caspofungin over amphotericin B, including the lipid formulations.8 Once the infection is stabilized, then fluconazole can be substituted for continued therapy, provided the Candida species is a fluconazole susceptible species. For less serious Candida infections due to fluconazole-susceptible species, fluconazole is appropriate, especially in non-neutropenic patients.
Aspergillus Infections
Amphotericin B administered in a dose of at least 1.0 mg/kg/day has been the treatment of choice for decades. Unfortunately, prolonged courses of amphotericin B in this dose schedule are poorly tolerated in many patients, especially those with antecedent renal impairment or those receiving concomitant nephrotoxins. Lipid formulations are much better tolerated even when given in higher doses and are more advantageous as salvage therapy, but no trial has convincingly shown them to produce significant improvements in response or survival rates as primary therapy.9 Although one trial10 suggested that 1 mg/kg/day of liposomal amphotericin B was as effective as 4 mg/kg/day, it was so underpowered as to make its conclusions moot, and most experts believe higher doses of the lipid formulations are optimal (4–6 mg/kg/day). Nephrotoxicity rates are substantially less with the lipid formulations, and that consideration alone makes them the only reasonable choice for a polyene therapy for most patients with aspergillosis.
The extended spectrum azoles, including itraconazole, voriconazole, posaconazole, and ravuconazole, have excellent activity against Aspergillus in vitro and case series demonstrate clinical utility for all of them. Only one, voriconazole, has been tested in a randomized trial (compared with amphotericin B) for first line therapy for aspergillosis.11 Patients included in the study were immunocompromised patients who were documented to have proven or probable acute invasive aspergillosis. Both response and survival rates were significantly better for voriconazole in comparison with amphotericin B. Voriconazole was better tolerated than amphotericin B with less nephrotoxicity and fewer switches to alternative therapies due to toxicity.
Voriconazole’s half-life is 6 hours and is administered every 12 hours. It is available in both intravenous and oral formulations. The oral formulation is well absorbed with a 96% bioavailability, generally better absorbed and more palatable than itraconazole. The intravenous formulations of both voriconazole and itraconazole have cyclodextrin excipients. Although the azole is not excreted renally, the excipient is; thus, use of the intravenous formulation of both of these azoles should be avoided in patients with severe renal impairment to avoid potential accumulation of the cyclodextrin. This is not a concern with the oral formulation. The kinetics are nonlinear for individuals over the age of 12: doubling of the dose leads to a 4-fold increase in the blood area under the curve (AUC). In contrast, in children under the age of 12, the kinetics are linear: doubling of dose results in doubling of exposure. In children younger than 12, the clearance is higher, and higher doses are necessary. For example, exposure is comparable for a child given a dose of 4 mg/kg and an adult given a dose of 3 mg/kg. For Aspergillus infections in adults, 2 loading doses of 6 mg/kg are given intravenously on the first day and, subsequently, a dose of 4 mg/kg is given twice daily. When the patient stabilizes, the drug can be switched from intravenous to the oral formulation and dosed at 200 mg twice daily.
In clinical studies, voriconazole has been associated with infrequent hepatotoxicity and nephrotoxicity. However, two unique toxicities are notable: visual disturbances occur in approximately 30%; these tend to be self-limited and not associated with severe or enduring sequelae and rarely require discontinuation of the drug. Dermatologic reactions occur in 6%, often in sun-exposed areas. These are mostly mild to moderate and generally do not necessitate alteration of the treatment course.
Both itraconazole and voriconazole are metabolized by cytochrome P450 enzymes. The interaction with cytochrome P450 isoenzymes results in alteration of the metabolism of other drugs metabolized by this enzyme. Multiple important drug interactions can occur. Two interactions of particular relevance to hematologists include (1) an increase of cyclophosphamide toxic metabolites (thus, clinicians should avoid concomitant use of these azoles when high doses of cyclophosphamide are used) and (2) a predictable increase in blood concentrations of calcineurin inhibitors (thus, clinicians should reduce the dose of cyclosporine by about 50% and reduce the dose of tacrolimus by about 65% when these azoles are instituted and adjust the calcineurin inhibitor doses based on blood level monitoring).
Caspofungin has been evaluated in patients intolerant of first-line therapy or where the infection progressed on first-line therapy. As “salvage” therapy, responses were seen in approximately 40%.12 This is comparable to response rates seen with lipid formulations of amphotericin B and voriconazole used in the salvage setting. To date there are no published data as to caspofungin’s efficacy as first-line therapy for aspergillosis.
Practical use of current anti-Aspergillus agents
Voriconazole is currently the drug of choice for first line therapy. For allergic, intolerant, or non-responsive patients, one of the lipid formulations of amphotericin B is an excellent alternative. Caspofungin is yet another option for salvage therapy.
Combination Therapy
Few controlled trials of combination therapy have been performed, despite this approach being evaluated in numerous in vitro and animal model studies. Combination antifungal therapy (amphotericin B plus flucytosine) is well established for cryptococcal meningitis. In a recent trial, the combination of fluconazole and amphotericin B was compared to fluconazole in high doses (800 mg/day) as therapy for candidemia13: overall, the response rate was higher and time to bloodstream clearance was shorter in the group receiving the combination. This advantage was offset by greater nephrotoxicity in the combination arm.
Despite considerable interest in this concept, there are no controlled trials for aspergillosis. Although several case series suggest benefit,14,15 the majority of Aspergillus cases in which combination therapy was evaluated were only “possible” infections. There are pitfalls with the use of combination therapy including potential antagonism, greater toxicity, and cost.16,17 Thus, controlled trials are clearly needed.
Adjunctive Measures
For catheter-related candidemia, removal of a central venous catheter, if possible, should be strongly considered.3 More rapid clearance of fungemia with catheter removal has been seen in several studies. For Aspergillus infections, consideration should be given for surgical excision of infarcted tissue, especially if the patient faces additional antineoplastic therapy. The role of cytokines such as myeloid growth factors and interferon gamma are supported by preclinical data, but there is a paucity of clinical trial data. Similarly, the use of granulocyte transfusions for neutropenic patients not responding to antimicrobial therapy is intuitively sensible, but this strategy is not without complications, is difficult to implement, and lacks convincing clinical data.
Duration of Therapy
The duration of therapy has not been defined in clinical trials but generally lasts for several weeks to months. In general, treatment of Candida infections should continue for at least 2 weeks beyond the time when cultures become negative, signs and symptoms of infection have improved, and preferably host defenses have improved.3
For Aspergillus infections, treatment should continue until resolution of symptoms and signs, clearance of cultures at previously culture-positive sites, improvement and stabilization of radiological findings, and improvement of underlying host defenses and control of the hematologic malignancy. In the largest trial to date, the planned course of therapy was 12 weeks.11
Prophylaxis
Fluconazole, itraconazole, and low doses of amphotericin B have been shown in randomized trials to be effective as prophylaxis. More recently, micafungin has also been shown to be effective as prophylaxis.18 In general, from meta-analyses of randomized trial data, the benefit appears to be meaningful when the risk of IFI is at least 15% in the patient group treated.19 Most of the antifungal benefit seen in clinical trials has been in the prevention of Candida infection. Trials of itraconazole during neutropenia have been mostly conducted in patient groups at low risk for aspergillosis, and thus no clear benefit against aspergillosis has been shown. A recent meta-analysis20 showed that itraconazole given in oral solution at adequate doses (at least 400 mg/day) was associated with fewer Aspergillus infections. Two randomized trials of prolonged prophylaxis comparing itraconazole to fluconazole after allogeneic HSCT provide an unclear message: although an anti-Aspergillus benefit for itraconazole was suggested (but not definitely shown), issues of excess toxicity were also raised.21,22
High rates of recurrence of IFI occur if the once-infected patient is subjected to subsequent antineoplastic treatment cycles or undergoes hematopoietic stem cell transplantation, and thus “secondary” prophylaxis or chronic maintenance is necessary until the underlying disease is controlled and the full treatment course is completed. Several published case series indicate that hematopoietic stem cell transplantation can be successfully performed in patients given secondary prophylaxis.23 After completion of therapy the patient should be observed to monitor for possible exacerbation.
Empirical Therapy for Neutropenic Patients with Persistent Fever
Early trials demonstrated that rates of IFIs were 15%–30% in neutropenic patients with fever persisting 4–7 days despite antibiotics; fungal morbidity could be reduced by empirical amphotericin B. Subsequent trials with lipid formulations of amphotericin B, itraconazole, voriconazole and caspofungin have been performed. In each study, the test agent was compared with either amphotericin B or liposomal amphotericin B. Since all patients had an active agent, the rates of IFIs in both groups were anticipated to be small and thus a surrogate endpoint of “success” was used as the primary endpoint. Success was judged by defervescence, resolution of an IFI if found at baseline, absence of breakthrough IFI, survival to neutrophil recovery, and no toxicity that necessitated withdrawal of study drug. None of these agents were found to be superior to amphotericin B in the primary endpoint and, in one case, voriconazole failed to meet its protocol-specified non-inferiority bounds. However, a strong trend in favor of itraconazole was noted for the primary endpoint of success (P = 0.055), and differences in secondary endpoints, in terms of rates of breakthrough IFIs (in favor of voriconazole over liposomal amphotericin B) and response of baseline IFIs (in favor of caspofungin over liposomal amphotericin B), were noted in these various trials (reviewed in 24). Considerable differences in toxicity were demonstrated with the various agents. Indeed, tolerability should be considered in the choice of a specific agent for a given patient.
What the Future Holds
Several studies indicate early treatment is key in determining the outcome of IFI treatment. Conventional diagnostic methods are either too slow or fraught with considerable imprecision, and use of invasive procedures is not practical for many patients with aspergillosis. Two rapid diagnostic tests using serum have been licensed. The serum galactomannan assay, testing for a constituent of Aspergillus cell wall released into blood early during the course of invasive Aspergillus infection, has been found to have sensitivity and specificity both exceeding 80% (US Food and Drug Administration [FDA] package insert). This assay used twice weekly detected two-thirds of Aspergillus infections in advance of conventional diagnostic testing.25 This assay clearly has promise. However, concerns as to its performance in children, non-neutropenic patients, patients receiving anti-mould antifungal prophylaxis, and in patients with antibody have been raised.26 Recent reports indicate that false-positive test results can often occur in patients receiving piperacillin-tazobactam.27 More recently, another serum assay, the glucan assay, detecting a cell wall constituent in a wide range of fungal pathogens (rather than limited to only Aspergillus), has received FDA approval. It was found to have high levels of sensitivity and specificity.28 The development of PCR assays also appears promising, and one trial showed that sampling of serum twice weekly accurately identified patients with IFI,29 often earlier than known using conventional diagnostic criteria.
It is hoped that such assays can assist the clinician to better distinguish febrile patients with fungal infections from those who are febrile but not infected. Further experience is needed for all of these assays to determine if and how these assays can assist us in making diagnoses more accurately, earlier, and allow the targeting of antifungal therapy to replace empirical trials in patients suspected of infection.
Ultimately, successful resolution of any IFI is dependent on restoration of the compromised host defenses that led to susceptibility for infection in the first place. Indeed, it can be argued that without immune recovery no IFI can be adequately or durably controlled. There has been substantial progress in our understanding of how host immune responses interact with fungal pathogens.30 These insights are leading to new therapeutic strategies. Cytokines such as IL-12 show promise as adjunctive therapy in preclinical studies. Efforts to enhance Th1 immune responses (or decrease polarization to Th2 responses) also offer promise from preclinical studies. Infusions of common myeloid progenitors provide protection against lethal infection in animal models.31 New fungal molecular structures are being identified that may prove to be novel targets for antifungal drugs or can elucidate crucial cellular receptors, such as the Toll-like receptors, that can be exploited. Vaccine strategies using dendritic cells pulsed with fungal antigens are also under development.32,33
II. Old and New Respiratory Viruses: Potent Pathogens for Patients with Hematologic Malignancies
W. Garrett Nichols, MD, MS*
Fred Hutchinson Cancer Research Center, Program in Infectious Diseases, Seattle, WA Current address: GlaxoSmithKline Research and Development, 5 Moore Drive, Research Triangle Park NC 27709
The community-acquired respiratory viruses (CRVs) are well known for their ability to cause misery during the winter cold and flu season. CRVs such as the influenza viruses, parainfluenza viruses, and respiratory syncytial virus (RSV), however, may be fatal for patients with hematologic malignancies, particularly those who are recipients of allogeneic hematopoietic cell transplantation (HCT). Though most clinicians believe that mortality after incident CRV infection is chiefly mediated via viral pneumonitis and acute pulmonary failure, accumulating data suggest that even upper respiratory tract infections (URIs) with some of these viruses may be associated with increased long-term mortality after HCT. Moreover, the application of PCR-based diagnostics now allows a wider variety of potential pathogens to be identified, including those that are difficult to cultivate (e.g., human rhinoviruses and coronaviruses) and/or newly discovered (e.g., human metapneumovirus [hMPV]). Thus, it is likely that the full spectrum of disease associated with these potent pathogens remains to be elucidated.
Epidemiology of CRV Infections
The CRVs are ubiquitous; otherwise healthy individuals typically experience 2–6 URIs with these viruses per year, with higher attack rates associated with younger age. Though the CRVs are capable of causing pneumonia even in the immunocompetent host (particularly those who are at extremes of age), they are better known for their role in the common cold and other related clinical syndromes, including pharyngitis, tracheobronchitis, and bronchiolitis. Among patients with hematologic malignancies, however, CRV infection may result in interstitial pneumonia and is now more likely to be found than cytomegalovirus (CMV) in patients with pneumonia early after HCT.
Studies conducted at the Fred Hutchinson Cancer Research Center (FHCRC), the MD Anderson Cancer Center, and elsewhere have shown that the epidemiology of CRV infections closely parallels the occurrence of these infections in the community, such that RSV and influenza infections most commonly occur during the well-defined winter and early spring “flu season”; infections due to parainfluenza viruses (PIVs), however, appear to occur year-round.1– 4 The CRVs are also notable for their propensity to spread nosocomially to other patients and healthcare workers. Thus, the occurrence of CRV infection is distinct from the pattern seen with endogenous human herpesviruses, where reactivation knows no season and appears to be primarily correlated with either GVHD and/or the net state of immunosuppression.
Reported rates of respiratory virus infections among HCT recipients have varied widely over time depending on the patient population, transplantation regimen, and type of surveillance instituted.1,2,4,5 Prospective, culture-based surveillance carried out at MD Anderson Cancer Center in the 1990s showed that approximately one-third of patients with upper respiratory tract symptoms (nasal congestion, cough, or sore throat) were infected with community-acquired respiratory viruses such as RSV, PIV, adenovirus, or influenza.4 Longitudinal studies carried out at FHCRC over the past decade have reported rates of CRV infection between 5% and 10% during the early period after transplantation,2 with more recent studies describing overall virus-specific rates of 5%, 7%, and 1%–2% for RSV, parainfluenza and influenza virus, respectively, during the first 100 days after transplantation.1,6,7
These studies likely still underestimate the true incidence of CRV infections in this population. This is because testing has been traditionally applied only to the most symptomatic inpatients rather than to all HCT recipients, and culture-based testing strategies lack sensitivity for the diagnosis of respiratory viral infections. Highly sensitive real-time quantitative reverse transcriptase PCR (RT-PCR) assays have now been developed that permit the detection of even more cases of RSV, PIV, and influenza virus infections than previously possible. van Elden et al investigated the use of RT-PCR to identify CRVs in stored bronchoalveolar lavage (BAL) and nasal wash samples that had been obtained from patients with hematological malignancies and pneumonia, and found that diagnostic yield increased from 19% to 35% in this setting8; in a separate study, PCR-based detection tripled the detection rate for RSV among immunocompromised adults.9 PCR also offers the ability to detect viruses that have heretofore been difficult to isolate in culture (including hMPV, the rhinoviruses, and the coronaviruses), and potentially offers the ability to target therapy according to quantitative virologic cutpoints. Together, these studies indicate a need for re-evaluation of the importance of the CRVs in patients with hematologic malignancies using modern diagnostic assays.
Impact of CRV Infections
Direct effects of CRV infection
Several studies have documented high mortality rates associated with incident CRV infection among HCT recipients (Table 1).1,4,6,10 Mortality due to these viruses is host- and virus-dependent, with most studies showing the highest attributable mortality associated with RSV infections (followed by parainfluenza and influenza) and among patients after allogeneic HCT. Differential mortality according to infecting virus is thought to be primarily explained by two factors: the rate at which infection progresses from incident URI to pneumonia, and the mortality rate associated with viral pneumonia once it develops. Thus, viral infections for which effective, easily administered therapy exists (e.g., M2 and/or neuraminidase inhibitors for influenza) may have low rates of attributable mortality due to factors intrinsic to the virus itself (capacity to invade the lower tract) or may reflect the effective use of antiviral therapy (to prevent progression to pneumonia, or to treat it once it occurs). It is clear, however, that all three viruses may cause life-threatening pneumonia in all of these patient groups on their own or may predispose patients to fatal infection with other pulmonary copathogens. Indeed, we have shown that CRV infections (particularly with PIV6) are independent risk factors for invasive aspergillosis after controlling for important confounders.11
Risk factors for pneumonia and virus-associated death have been studied in several cohorts of patients. The cumulative level of immunosuppression appears to correlate well with the risk for progression to pneumonia and virus-associated death: recipients of allogeneic HCT have higher risks than recipients of autologous transplants or conventional chemotherapy, and the presence of GVHD (and the steroids used to treat it) appear to be correlated with risk. The presence of lymphopenia at the time of incident infection also appears to be important; thus, nosocomially acquired infections that occur pre-engraftment are particularly troublesome. It is notable that recipients of alemtuzumab-based reduced intensity conditioning therapy (who commonly experience prolonged periods of severe lymphopenia) appear to experience CRV infection at a high rate and commonly progress to pneumonia, yet virus-related mortality appears to be low.12 Thus, lymphopenia per se does not correlate precisely with risk for CRV-related death. Children appear to do better than adults after CRV infection in some series, which may reflect higher antibody titers prior to the receipt of chemotherapy. Determining patient risk for poor outcomes is important, since treatment for many of these viruses is both expensive and difficult to administer.
Though CRV infections continue to occur at a high rate, some authors have recently concluded that the direct mortality associated with such infections has declined. Lower tract disease developed in 54% of patients with CRV infection in one study, but direct respiratory virus-associated mortality was minimal (2/19 patients, 11%).12 Similar results were documented by Machado and colleagues: though RSV pneumonia occurred in 56% of their patients with RSV infection, only 1 died.13 Only 6/35 patients with URIs developed lower tract disease in the study by Roghmann et al, but none of these patients progressed to respiratory failure.14 RSV was isolated from 37% of cancer and transplant patients studied by Anaissie et al, yet the incidence of serious respiratory complications was not significantly higher among those with (27%) than without (20%) RSV infection.15 Observational cohort studies performed at our center have also documented declining RSV pneumonia-associated mortality over time, from 70%–100% in the early 1990s16 to 20%–30% in more recent series.1,17 Early mortality associated with influenza pneumonia has also been reduced to 15%–30% in our recent series.7
Possible indirect effects of CRV infection
Most studies performed to date, however, have focused only upon early transplant-related mortality (i.e., that occurring before day 100 after transplant), and thus the impact of respiratory virus infection on long-term outcomes has not been measured. Our experience with PIV pneumonia is particularly instructive—though 30-day mortality after isolation of PIV from the lower tract is relatively high (35%), it pales in comparison to the 75% mortality that we have documented at 6 months after diagnosis.6 The concept of pneumonia as a necessary step in the pathway from incident CRV infection to viral-induced death may also obscure the full spectrum of disease that these viruses may produce. Prospective cohort studies conducted at Fred Hutchinson Cancer Research Center over the past decade have shown that, as expected, lower respiratory tract infection with all of the CRVs is associated with a significant increase in the risk for mortality after HCT (Table 2). Interestingly, however, URI with PIV that does not progress to pneumonia is also independently associated with posttransplant mortality, even after controlling for those factors known to predict survival after transplantation (including patient age, CMV serostatus, donor type, underlying disease, disease risk, and year of transplant) (Table 2 ).1,6,7 Though respiratory copathogens may be to blame, severe obstruction of pulmonary airflow may also explain this discrepancy.
Severe airflow obstruction (AFO) has long been recognized as a serious and often fatal complication of HCT. Also known pathologically as bronchiolitis obliterans, this condition presents with respiratory symptoms that may include dry cough (60%–100%), dyspnea (50%–70%), and wheezing (40%).18 The diagnosis is primarily based upon spirometric measurements and clinical exclusion of an active infectious process. Neither BAL nor lung biopsy is currently pursued due to the associated morbidity and the infrequent yield of information that determines either etiology or prognosis. As a result, our understanding of the pathogenesis of AFO after HCT has not advanced significantly since the 1980s, when the condition was deemed a pulmonary manifestation of GVHD. It is notable, however, that the use of immunosuppressive therapy has proven to be ineffective in most cases of late AFO.19
Our recent analysis of more than 1000 patients revealed that over 1 in 4 long-term survivors suffer from severe AFO after allogeneic HCT20; these patients have a crude mortality rate that is double that of those without transplant-related AFO. Importantly, CRV infection appeared to be a risk factor for this complication: those with confirmed CRV infection during the first 100 days after HCT had a 40% higher risk for AFO when compared with those without CRV infection in multivariable models.20 These findings are supported by studies previously conducted at our center, which showed that up to 75% of patients with significant AFO experienced antecedent sinusitis, bronchitis, or “cold or flu” symptoms.21 Studies are underway to determine if CRV-induced AFO is specific to certain pathogens and the degree to which the phenomenon is explained by viral persistence.
Impact of “uncultivated” viruses
hMPV is a newly discovered human respiratory virus that may be associated with as many as 10%–25% of cases of viral upper and lower respiratory tract disease in children. Like RSV, lower respiratory tract disease appears to develop primarily in the very young and the very old, although the rate of hMPV-related pneumonia in previously healthy young children appears to be lower than that associated with either RSV or influenza infection. Clinical features are virtually indistinguishable from those associated with RSV, though wheezing may be more common with the latter. More serious disease has been associated with hMPV infection in immunocompromised patients, with fatalities reported in at least 2 cases—1 child with leukemia22 and 1 adult who underwent bone marrow transplantation.23 We have recently detected hMPV in cryopreserved BAL fluid of 5 HCT recipients who expired with pulmonary failure; diagnosis for 4 of these patients at death was “idiopathic pneumonia syndrome” or “diffuse alveolar hemorrhage” (Englund J, Nichols WG, et al, submitted for publication). hMPV may thus be responsible for at least a proportion of cases of idiopathic pneumonia syndrome after HCT; the full spectrum of disease caused by this virus has yet to be determined.
Rhinovirus and coronaviruses circulate widely and are believed to be the most common causes of the common cold in the immunocompetent host. Their association with severe respiratory disease in the patient with hematologic malignancy, however, is controversial. Investigators from the MD Anderson Cancer Center have reported that rhinovirus may cause lower tract disease by itself,24 though two separate analyses from our center2,25 did not provide conclusive evidence for rhinovirus pneumonia in HCT recipients. In the most recent study, 77 BAL samples obtained from HCT recipients with pneumonia were screened with sensitive RT-PCR assays; though 8 were positive for rhinovirus RNA and mortality was high in these patients, all had significant copathogens present.25 The contribution of the human coronaviruses to respiratory disease in this patient population is more obscure. In two recent studies that examined cryopreserved BAL fluid obtained from patients after HCT or with underlying hematologic malignancies, coronaviruses were found only rarely (1 of 43 specimens)8 or not at all (0 of 46 specimens).25 Milder disease and/or long-term pulmonary complications associated with these viruses, however, need to be ruled out with prospective studies.
Treatment of CRV Infections
As discussed above, the direct mortality associated with CRV infections appears to have decreased over the past decade. While newer antivirals may be responsible for some of the improved outcomes, it is more likely that the earlier application of antivirals that have long been available (such as aerosolized ribavirin) accounts for improved outcomes for many infections, including RSV.1 Rapid identification of the infecting virus (i.e., with BAL) is necessary to allow prompt therapy, since mortality is high if pulmonary failure is present when antiviral therapy is applied. BAL is also crucial to identify the significant copathogens that are frequently present in these patients (Table 1), which require additive therapy for optimal outcome.
RSV
Uncontrolled cohort studies performed at the Fred Hutchinson Cancer Research Center suggest that aerosolized ribavirin given alone (2 g over 2 hours 3 times daily or 6 g continuously over 16–18 hours) improves the survival of HCT recipients with RSV pneumonia from 0% (without therapy) to 60%–70%.1 Others have attempted to improve upon these outcomes by adding pooled immunoglobulins, high-titered RSV-IG, or RSV-specific monoclonals (Palivizumab). The latter two approaches may improve survival to 80%–90% (particularly if applied prior to the requirement for mechanical ventilation),17,26,27 though sample sizes were too small to allow for controlling of other factors that may influence survival in these patients. Unfortunately, a multicenter randomized trial comparing palivizumab-based combination therapy with ribavirin alone was recently closed due to poor accrual.
Given the reasonably high rate of progression from RSV URI to pneumonia (~40%), some have advocated the use of aerosolized ribavirin to prevent pneumonia from developing. A multicenter randomized trial designed to determine whether this practice is effective was also recently closed after slow accrual. Due to the expense and inconvenience of this practice, we currently apply preemptive ribavirin to those with extremely high risks (i.e., among those with profound lymphopenia). The orally active fusion inhibitors currently under development will be a welcome addition to our limited armamentarium.
Influenza
Thankfully, active, easily administered agents are available for the treatment of patients with influenza. Currently preferred are the neuraminidase inhibitors (oral oseltamivir and inhaled zanamavir) given activity against both influenza A and B; M2 inhibitors (oral amantadine and rimantadine) are only active versus influenza A and also are associated with emergent antiviral resistance on therapy.28 Small, uncontrolled studies suggest that preemptive therapy with any of these agents may prevent progression to lower tract disease.7,29
Parainfluenza
Unfortunately, effective therapy for PIV pneumonia has yet to be identified. Therapy with aerosolized ribavirin with or without pooled IVIG appeared unsuccessful in the largest series of HCT recipients with pneumonia performed to date.6 Since progression to pneumonia appears highly correlated with dose of corticosteroids given to treat GVHD,6 efforts to decrease the level of immunosuppression at the time of incident CRV infection are warranted. Whether the earlier application of aerosolized ribavirin (i.e., for URI) would improve outcomes is speculative.
Human metapneumovirus
No human or animal studies are available to inform the treatment of patients with hMPV infection. Both polyclonal IVIG and ribavirin appear to be active in vitro, and combination therapy with these agents could thus be envisaged for the patient with hMPV pneumonitis. Monoclonal antibody preparations (such as Palivizumab for RSV) are under development.
Prevention
Stringent infection control practices are critical for decreasing the morbidity and mortality associated with incident CRV infection in the at-risk host. Focus should first be directed toward the prevention of nosocomial infections during the period of highest risk (i.e., during the periods of profound lymphopenia that follow conditioning or induction chemotherapy, when progression to pneumonia appears most likely). Incident infections still may occur later after patients have returned to the community, but these are associated with lower direct mortality. Since large droplets are the most important means of viral transmission, frequent handwashing (or use of alcohol-based hand sanitizers) cannot be overemphasized; targets for this teaching include healthcare workers, close patient contacts, and the patients themselves.
Recommendations for screening and isolation of patients with CRV infections have been summarized by the Centers for Disease Control and Prevention (CDC) and the American Society for Blood and Marrow Transplantation (ASBMT).30 HCT recipients and those at high risk for CRV-related complications should refrain from contact with individuals with symptomatic CRV infections; symptomatic healthcare workers or visitors should thus be restricted from access to wards where these patients are housed. Whether masks for patients or asymptomatic healthcare workers (prior to patient contact) add value to hand hygiene and the restrictions above is debatable.
Inactivated influenza vaccine is an effective means to prevent this important CRV infection and should be offered yearly to nearly all patients with hematologic malignancies (patients in their first year after transplant are the possible exception due to poor antibody responses). Healthcare workers and close patient contacts should also receive the inactivated vaccine to reduce transmission to patients. Live attenuated influenza vaccine cannot currently be recommended for patients or their close contacts (including healthcare workers) due to concerns of vaccine strain transmission and resulting clinical illness in these immunocompromised hosts.
III. Sinusoidal Obstruction Syndrome (Formerly Known as Veno-Occlusive Disease of the Liver): Etiology, Diagnosis, Prevention, and Treatment
George B. McDonald, MD*
Fred Hutchinson Cancer Research Center and the University of Washington School of Medicine, Gastroenterology/Hepatology Section (D2-190), 1100 Fairview Ave N, Seattle WA 98109-1024
Why Sinusoidal Obstruction Syndrome and Not Veno-occlusive Disease?
The clinical syndrome of hepatomegaly, fluid retention, ascites, and jaundice that develops after some myeloablative regimens was initially called venoocclusive disease (VOD) because it resembled a form of toxic liver injury first described in South Africa in 1920. With the recognition that the injury is initiated in hepatic sinusoids1 and that hepatic vein lesions are not required for clinical signs and symptoms,2 this form of toxicity has been renamed Sinusoidal Obstruction Syndrome (SOS).3 High-dose conditioning prior to hematopoietic cell transplantation is the most common cause of SOS. SOS also occurs after ingestion of herbal teas or food containing pyrrolizidine alkaloids; long-term use of azathioprine or 6-thioguanine; and treatment with a number of chemotherapy drugs (gemtuzumab ozogamicin, actinomycin D, dacarbazine, cytosine arabinoside, mithramycin, indicine N-oxide, and chemotherapy combinations).4,5
Pathogenesis of Sinusoidal Liver Injury
Two observations provide clues as to the mechanism of SOS. First, unlike most chronic liver diseases, signs and symptoms of portal hypertension precede evidence of hepatocyte damage. In SOS, disruption of the liver circulation is the cause and not the consequence of liver disease. Second, involvement of hepatic veins is not essential to the development of the clinical picture: 45% of patients with mild or moderate SOS and 25% of patients with severe SOS did not have occluded hepatic venules at autopsy.2
The proximate causes of sinusoidal injury: chemotherapy and irradiation
Cyclophosphamide pharmacokinetics and sinusoidal liver injury.
Cyclophosphamide (CY) is common to conditioning regimens with the highest incidence of fatal SOS. The metabolism of CY is highly variable; patients who generate a greater quantity of toxic metabolites are more likely to develop severe SOS following conditioning with CY and total body irradiation (TBI).6 The endothelial cell toxin generated by CY metabolism is acrolein (a metabolite formed simultaneously along with phosphoramide mustard). The mechanisms behind variability of CY metabolism from patient to patient are not known; current research focuses on the hepatocyte transporter ABCC2 and availability of intracellular reduced glutathione (GSH). When patients conditioned with CY 120 mg/kg and TBI were placed in rank order according to their exposure to the CY metabolite carboxyethyl phosphoramide mustard (CEPM), those in the highest quartile of CEPM exposure were 5.9 times more likely to die of non-relapse causes than those in the lowest quartile.6 Neither relapse nor engraftment bore any relation to variation in CY metabolism, suggesting that CY doses can be reduced in the CY/TBI regimen to prevent toxicity without jeopardizing engraftment or relapse. We have completed a Phase I TBI/CY protocol in which doses of CY are adjusted according to each patient’s metabolism. Using a target AUCCEPM exposure of 325 μM.h, the mean total dose of CY was 86 mg/kg, but with a wide dosing range (54–120 mg/kg). Engraftment was not affected and there were no cases of severe SOS.
Total body irradiation as a cause of sinusoidal injury.
TBI doses used in transplantation (10–16 Gy) are less than the dose that causes radiation-induced liver disease. In combination with CY, however, there is synergism, with irradiation doses ≥ 13.2 Gy causing more severe SOS.6 An irradiation dose of 10 Gy in the CY/TBI regimen causes less liver injury than does 12 Gy.
Role of busulfan in sinusoidal injury.
A relationship between busulfan (BU) exposure following oral dosing and liver toxicity has been reported, but this relationship disappears among cohorts of adults with chronic myelogenous leukemia (CML) in chronic phase and children with acute leukemia. BU contributes to liver injury by inducing oxidative stress and reducing glutathione levels in hepatocytes.7 More importantly, BU profoundly alters CY metabolism, such that exposure to 4-hydroxyCY and CEPM (a reporter molecule for liver toxicity) is increased by > 80% and > 35%, respectively. The regimen CY/BU is less toxic than BU/CY, with a lower frequency of SOS, suggesting that BU predisposes patients to CY toxicity.8 Many centers now dose oral BU according to its metabolism or give BU intravenously to assure less variability in BU exposure, but liver toxicity following BU/CY persists despite these BU dosing improvements.
Other sinusoidal toxins used in hematopoietic cell transplant.
The myeloablative regimen BU, melphalan, and thioTEPA leads to fatal SOS in ~4% of patients. SOS has also been described as dose-limiting for regimens that contain high-dose BCNU, carboplatin, or cytarabine. Gemtuzumab ozogamicin, a conjugate of anti-CD33 and calicheamicin, causes hepatic sinusoidal injury by a different mechanism, as it appears to deliver toxin to CD33+ cells residing in hepatic sinusoids (AML cells, Kupffer cells, possibly stellate cells). The toxicity is characterized by intense deposition of collagen in hepatic sinusoids and a clinical picture similar to that of SOS following myeloablative conditioning regimens.4,5 SOS develops in 10%–90% of patients who receive gemtuzumab ozogamicin that is given either in close proximity to a myeloablative conditioning regimen (particularly if conditioning starts within 3.5 months of gemtuzumab ozogamicin exposure) or given for relapse of AML after transplant.4,5,9
Sinusoidal endothelial cells as the initial site of injury
In vitro, sinusoidal endothelial cells are more susceptible than hepatocytes to drugs that cause SOS. After toxin exposure, sinusoidal endothelial cells round up, and red blood cells begin to penetrate into the space of Disse beneath the rounded up endothelial cells and then dissect off the sinusoidal lining. The sloughed sinusoidal lining cells, i.e., Kupffer cells, sinusoidal endothelial cells, and stellate cells, embolize downstream and obstruct sinusoidal flow.
Hepatocyte necrosis as an ischemic event
By the time hepatocyte necrosis is observed histologically, there is extensive denudation of the sinusoidal lining and alteration of hepatic circulation. In some patients, serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) rise to over 1500 U/L, with peak values at day +23 ± 9. The fact that hepatocyte necrosis peaks ~30 days after myeloablative therapy suggests that ischemia related to altered sinusoidal blood flow is responsible, and not the myeloablative therapy per se.
Biochemical mediators of sinusoidal injury
Role of intracellular reduced glutathione.
Drugs and toxins that cause SOS deplete sinusoidal endothelial cell GSH prior to cell death, and, in animal studies, increased intracellular GSH prevents cell death.10
Role of matrix metalloproteinases.
One explanation for the rounding up of sinusoidal endothelial cells is activity of matrix metalloproteinases (MMPs) in degrading extracellular matrix. Increased MMP activity on the ablumenal side of sinusoidal endothelial cells allows them to detach from the space of Disse.11 Inhibition of MMP activity completely prevents SOS in animals.11 MMP expression and activity are regulated by redox status and can be suppressed by GSH and N-acetylcysteine. Thus, the protective effect of GSH and N-acetylcysteine may in part be due to inhibition of MMP activity.
Role of nitric oxide.
Role of thrombosis.
In the livers of patients with SOS, immunohistology demonstrates deposition of fibrinogen and Factor VIII/von Willebrand Factor (vWF), not within venular lumens, but in the perivenular zone (Figure 1D; see Color Figures, page 506). Animal models do not show any evidence of clotting.1 Some see SOS as a disease of disordered coagulation, in which damage to endothelium leads to initiation of the coagulation cascade. However, heparin and antithrombin III infusions are ineffective in preventing SOS and thrombolytic therapy effects improvement in only a minority of patients. Genetic disorders predisposing to coagulation (Factor V Leiden and prothrombin gene 20210 G-A) have no association with SOS after transplant. Current evidence suggests that disordered coagulation in SOS is an epiphenomenon, not a cause of damage.
Stellate cells and sinusoidal fibrosis
By day +10, procollagen peptide appears in the serum of patients who develop SOS, along with inhibitors of fibrolysis, consistent with intense sinusoidal fibrosis and stellate cell activation seen histologically (Figure 1F, H; see Color Figures, page 506). The stimuli for stellate cell activation and proliferation in the transplant setting have not been identified.
Renal pathophysiology in SOS
Falling fractional excretion of sodium occurs just before the clinical signs of SOS become apparent, reflecting both sinusoidal hypertension, nitric oxide effects, and renal tubular injury. SOS is a risk factor for development of acute renal failure.
Genetic influences on the development of SOS
Current research is examining genetic influences on aberrant CY metabolism and on intracellular availability of GSH as contributors to development of SOS. However, the prevalence of SOS among patients who receive extraordinarily high doses of chemoradiation therapy illustrates that dose-intensity can trump genetics.
Diagnosis of SOS after Myeloablative Therapy
Incidence
The incidence of SOS varies because of differences in the liver toxicity of conditioning regimens. The incidence is highest in regimens that contain CY in combination with TBI or BU or BCNU/etoposide. At our center, the overall incidence of SOS among patients with hematological malignancy conditioned with CY 120 mg/kg/TBI 12–13.2 Gy is 38% (7% severe) and among patients with MDS conditioned with targeted BU 16 mg/kg/CY 120 mg/kg, 12% (2% severe). There is no sinusoidal liver toxicity from nonmyeloablative fludarabine plus low-dose TBI. The substitution of fludarabine for cyclophosphamide, that is, a BU/fludarabine regimen, results in less liver toxicity than BU/CY. The frequency and severity of SOS have fallen dramatically over the last decade. However, some current patients come to transplant at high risk for fatal SOS because of pre-existing inflammatory or fibrotic liver disease.
Clinical presentation
The onset of SOS is heralded by an increase in liver size, tenderness, renal sodium retention, and weight gain, at an average of day +1 after CY-based transplant. Patients then develop hyperbilirubinemia, usually before day +20. A syndrome of “late VOD” may develop after conditioning with some BU-containing regimens, where signs of liver disease are first recognized after day +30.
Diagnosis
There has been confusion in the literature over what is needed to make a diagnosis of SOS. For research studies, the criteria of hepatomegaly, weight gain, and jaundice, in the absence of an alternative explanation, were established to have a high positive predictive value for sinusoidal injury as proven by liver histology. However, the spectrum of clinical signs of sinusoidal injury is far wider, encompassing ALT elevations, isolated jaundice, isolated weight gain, gall bladder wall edema, and pain.
Are laboratory studies useful in diagnosis? Total serum bilirubin is a sensitive test for SOS but not a specific one, as there are many causes of jaundice after transplant. Several plasma proteins are high in patients with SOS (endothelial cell markers, thrombopoietin, and cytokines) and some values are low (protein C, antithrombin III and platelet counts). It is not clear whether any of these tests have diagnostic utility beyond the clinical criteria of weight gain, jaundice, and hepatomegaly. Serum levels of collagen peptides, however, appear to reflect the extent of sinusoidal fibrosis, an important prognostic variable.
Are imaging tests useful in diagnosis? Doppler ultrasound and MRI can show hepatomegaly, ascites, and attenuated hepatic venous flow consistent with SOS, and the absence of biliary dilation or infiltrative lesions that might explain hepatomegaly and jaundice. Other findings that are more frequent in patients with SOS than in controls include gallbladder wall thickening, splenomegaly, a paraumbilical vein, enlarged portal vein diameter, slow or reversed portal vein flow, high congestion index, portal vein thrombosis, and increased resistive index to hepatic artery flow. Unfortunately, findings early in the course of SOS do not appear to add to the information provided by clinical criteria.13
Liver biopsy and hepatic venous pressure gradient: the gold standard for diagnosis. In cases where the diagnosis is unclear, a transvenous approach that allows both biopsy and hepatic venous pressure measurements is the most accurate diagnostic test. Transvenous biopsy methods can be done safely with platelet counts as low as 30,000/mm3. In HCT patients, a hepatic venous pressure gradient (using an occlusive balloon technique14) greater than 10 mm Hg is highly specific for SOS. The liver histology of SOS is characteristic, involving structures in the centrilobular area: disruption of sinusoidal endothelium, extravasation of red cells through the space of Disse, hepatocyte necrosis, sinusoidal fibrosis, and stellate cell activation—findings that often appear much more widespread and severe than the extent of venular injury (Figure 1; see Color Figures, page 506).3 The later stages of fatal SOS are characterized by extensive collagenization of sinusoids and venules. A coded review of histological features `found that the strongest statistical associations with clinically severe SOS were hepatocyte necrosis and sinusoidal fibrosis.2
Differential diagnosis
(1) Sepsis syndrome requiring large volumes of crystalloid, followed by renal insufficiency and sepsis-related cholestasis; (2) cholestatic liver disease, hemolysis, and congestive heart failure; (3) hepatic vein obstruction (Budd-Chiari Syndrome), and (4) hyperacute GVHD and sepsis syndrome. SOS commonly co-exists with cholestatic liver disease.
Outcome and Prognosis
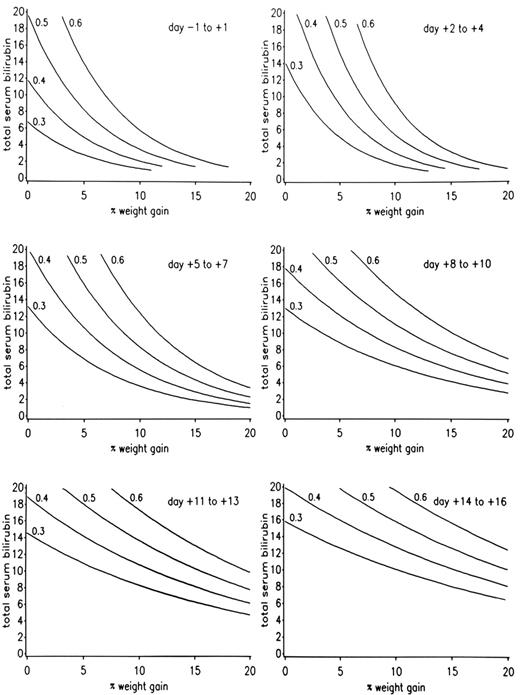
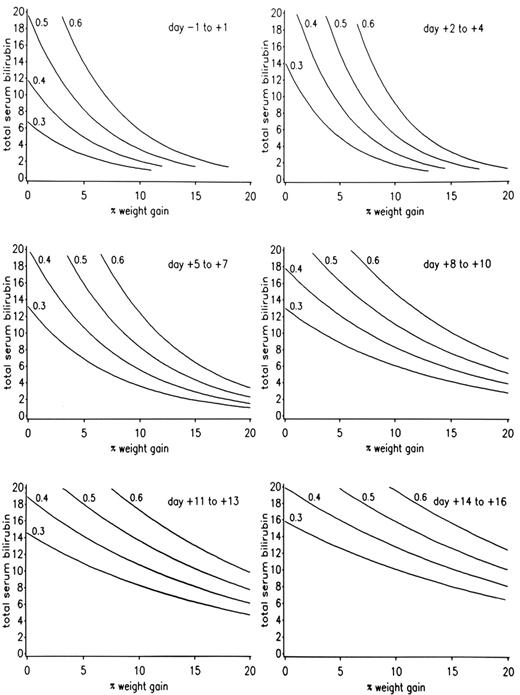
The severity of SOS has been classified as mild (clinically obvious, requires no treatment, and resolves completely), moderate (signs and symptoms require treatment such as diuretics or pain medications, but resolve completely), or severe (requires treatment but does not resolve before death or day 100). A range of clinical and laboratory findings correspond to these operational definitions of disease severity (Table 3 ). Recovery from SOS is seen in over 80% of patients whose SOS follows myeloablative regimens. A model has been developed that predicts the outcome of SOS after CY-based regimens, derived from rates of increase of both bilirubin and weight in the weeks following transplant (Figure 2 ). A poor prognosis also correlates with hepatocyte necrosis, higher portal pressures, and renal and pulmonary failure.
Treatment Options for Patients with SOS
It is reassuring that ≥ 80% of patients with SOS will recover completely, some without any treatment, others with just management of sodium and water balance and diuresis, and others after repeated paracenteses, dialysis, or hemofiltration. A management algorithm starts with stratification of risk.
Identification of patients at risk for a fatal outcome
For patients who received CY-based regimens, prognosis can be predicted by plotting the rate of rise of total serum bilirubin and weight gain (Figure 2 ). Doubling of the baseline serum creatinine, falling oxygen saturation, refractory thrombocytopenia, AST/ALT values over 750 U/L, and encephalopathy are adverse signs. The more failing organs, the more likely a fatal outcome. An adverse prognosis is usually readily apparent before day 7–14 post transplant.
Management of patients likely to recover completely
Patients with mild SOS may need no treatment, while those with moderate SOS often require close attention to renal sodium handling, intravascular volume status, and salt and water balance. Opioid medication may be needed to control liver pain during an increase in liver size. If ascites or pleural effusions are problematic, drainage can be more effective than vigorous diuresis, which tends to contract intravascular volume. Renal toxins (amphotericin, gentamicin, non-steroidal anti-inflammatory drugs [NSAIDs], contrast) should be avoided. Some patients may require hemofiltration or hemodialysis to control volume excess and renal failure. Respiratory failure due to interstitial fluid accumulation and pleural effusions can be reversible in the setting of SOS.
Management of patients likely to die from SOS and multiorgan failure
There is no completely satisfactory treatment for severe SOS—the best reported therapy (defibrotide) is effective in only half of patients. Whenever possible, patients with severe SOS should be enrolled in clinical trials. In severely ill patients with SOS, guidelines that define futility of treatment have been published, allowing patients and their physicians to eschew life-support measures or to consider a liver transplant.
Defibrotide:
Defibrotide, a single-stranded polydeoxyribonucleotide drug derived from animal tissue, has anti-thrombotic, anti-ischemic, and thrombolytic properties. In treatment of patients with SOS, the dose range is 25–40 mg/kg/day for 14 days or longer. The mechanism for complete resolution of severe SOS in ~50% of patients who receive defibrotide for severe SOS is unknown.15 Hypotension during infusion has been reported.
Thrombolytic therapy:
Tissue plasminogen activator and heparin infusions effect improvement in < 30% of patients with severe SOS, but never in patients with either renal or pulmonary failure. Thrombolytic therapy is further limited by the risk of fatal intracerebral and pulmonary bleeding.
Other medical therapies:
Patients with SOS treated with intravenous N-acetylcysteine improved.16 Infusions of human antithrombin III concentrate or activated protein C are ineffective. Improvement has been reported after therapy with prostaglandin E1, prednisone, topical nitrate, and vitamin E plus glutamine therapy. Inhibition of collagen production by stellate cells is a logical but unproven approach.
Transjugular intrahepatic portosystemic shunt:
Transhepatic shunts have been placed in patients with SOS to reduce portal pressure and mobilize ascites, but this has no effect on either serum bilirubin levels or patient outcomes. Transjugular intrahepatic portosystemic shunts (TIPS) may lead to acute respiratory distress syndrome.
Surgical approaches:
Peritoneovenous shunts for intractable ascites are unsuccessful. Patients have received successful liver transplants for severe SOS. Liver transplant should be considered when severe SOS develops in a patient with a condition with a favorable outcome (for example, CML in chronic phase). Artificial liver support may be useful as a bridge to transplant.
Prevention of SOS in Patients Receiving Myeloablative Therapy—The Future
The only certain way to prevent fatal SOS is to avoid giving hepatotoxic conditioning therapy, particularly to patients at high risk of severe SOS.
Identification of patients at high risk for fatal SOS
Some patients come to transplant with pre-existing risk factors for fatal SOS (the estimated risk of a fatal outcome when these conditions are present at the start of conditioning therapy is 20%), for example, chronic viral hepatitis; non-alcoholic steatohepatitis; systemic bacterial or viral infection before conditioning; extramedullary hematopoiesis with matrix deposition in sinusoids; previous radiation therapy to the liver; previous hematopoietic cell transplant; and recent gemtuzumab ozogamicin therapy. For patients who have had recent gemtuzumab ozogamicin exposure, the frequency of SOS following myeloablative hematopoietic cell transplant was 90% when the interval between exposure and transplant was less than 3.5 months, and nil (0 of 4 patients) when the interval was greater than 3.5 months.9 Marginally compensated cirrhosis is a contraindication to myeloablative regimens. The risk of mortality among patients with compensated cirrhosis is also very high, even after nonmyeloablative regimens.
Modification of the CY/TBI regimen
If a CY/TBI regimen must be used for a patient at high risk for fatal SOS, modifications should be considered for both CY and TBI dosing, with the understanding that clinical trials to prove efficacy and safety have not been done. The total dose of CY should be in the 75–100 mg/kg range, and TBI doses should not exceed 12 Gy. Shielding the liver during TBI will lessen liver injury but leads to hematologic relapses. In patients without risk factors, a case can be made for limiting CY doses to no more than 100 mg/kg, pending clinical trials of CY dosing based on its metabolism.
Modification of the BU/CY regimen
If a BU/CY regimen must be used for a patient at high risk for fatal SOS, clinical trials support the following modification: Because BU profoundly affects CY metabolism (more CY toxins are produced), liver toxicity appears to be less frequent if CY is given before targeted BU (this requires intravenous BU, as oral BU is poorly tolerated after CY infusions)8 or if dosing of CY is delayed for 1–2 days after completion of BU.17 It is not clear whether intravenous BU offers any advantage over oral BU with regard to liver toxicity from a BU/CY regimen when both are dosed to the same steady state concentration18; however, intravenous BU is always preferable to non-targeted oral BU because of less variable BU exposure. An optimal BU/CY regimen may target the dose of both drugs to metabolic endpoints. Alternatively, substituting fludarabine for CY may reduce liver toxicity.19
Possibly useful preventive strategies
There may be value in prophylaxis of SOS with repletion of intracellular GSH,10,16 or inhibition of MMP enzymes,11 or infusion of defibrotide.20 Large-scale clinical trials of these modalities have not been reported, and there is concern about tumor protection from GSH supplementation. Regimens that do not contain sinusoidal toxins should be considered for patients at risk.
Preventive strategies that are not effective
Prospective studies have shown no benefit from use of heparin, ursodiol, or antithrombin III for prevention of fatal SOS. Ursodiol prevented cholestatic liver injury in a well-designed trial, but had no effect on SOS.21
A model for predicting the outcome of sinusoidal obstruction syndrome (SOS) among patients conditioned with cyclophosphamide-based regimens.
The contour lines estimate the probability of developing severe SOS as ≥ 30%, ≥ 40%, ≥ 50%, and ≥ 60%, using total serum bilirubin in mg/dL and percentage weight gain above baseline from day −1 through day +16. If a plotted point lies above a given probability line, the probability of severe SOS is or exceeds the percentage of that line.
Reprinted with permission from the
A model for predicting the outcome of sinusoidal obstruction syndrome (SOS) among patients conditioned with cyclophosphamide-based regimens.
The contour lines estimate the probability of developing severe SOS as ≥ 30%, ≥ 40%, ≥ 50%, and ≥ 60%, using total serum bilirubin in mg/dL and percentage weight gain above baseline from day −1 through day +16. If a plotted point lies above a given probability line, the probability of severe SOS is or exceeds the percentage of that line.
Reprinted with permission from the