Abstract
The acute or inducible hepatic porphyrias comprise four inherited disorders of heme biosynthesis. They usually remain asymptomatic for most of the lifespan of individuals who inherit the specific enzyme deficiencies but may cause life-threatening attacks of neurovisceral symptoms. Failure to consider the diagnosis frequently delays effective treatment, and inappropriate diagnostic tests and/or mistaken interpretation of results may lead to misdiagnosis and inappropriate treatment. The four disorders are ALA dehydratase deficiency porphyria, acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria. Other conditions that clinically and biochemically may mimic acute porphyria include lead poisoning and hereditary tyrosinemia type I. The diagnosis of one of these acute porphyric syndromes should be considered in many patients with otherwise unexplained abdominal pain, severe constipation, systemic arterial hypertension, or other characteristic symptoms. Critical to the rapid diagnosis of the three most common of these disorders is demonstration of markedly increased urinary porphobilinogen (PBG) in a single-void urine specimen. The treatment of choice for all but mild attacks of the acute porphyrias is intravenous hemin therapy, which should be started as soon as possible. Intravenous glucose alone is recommended only for mild attacks (no weakness or hyponatremia) or until hemin is available.
The Pathway of Heme Biosynthesis
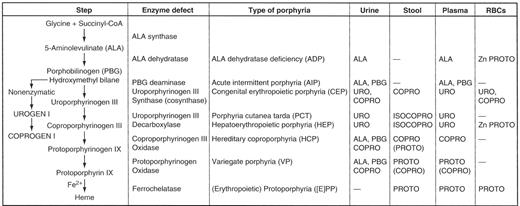
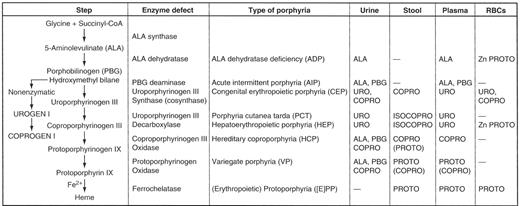
Each of the porphyrias is due to the deficiency of a specific enzyme involved in heme synthesis. The normal pathway of heme synthesis is shown in Figure 1 . The first and normally rate-controlling step is condensation of glycine and succinyl CoA to form 5-aminolevulinate (ALA), catalyzed by the mitochondrial enzyme ALA synthase. There are two forms of ALA synthase, the ubiquitous housekeeping form 1 and the erythroid-specific form 2. These two forms are products of separate genes and are under quite different regulation. ALA synthase-1 can be upregulated markedly by transcriptional and post-transcriptional mechanisms, and an “uncontrolled” upregulation of this enzyme in the liver is believed to be the biochemical sine qua non of acute porphyric attacks. Upregulation of ALA synthase-1 occurs due to three main known causes:
Lipophilic drugs and chemicals, that interact with nuclear receptors and that, in turn, increase activation of drug-response elements (RES) in the upstream enhancer of ALA synthase-1.1
Deficiency of glucose or other gluconeogenic compounds that suppress gene expression of ALA synthase-1 (the so-called “glucose effect”2).
Deficiency of heme, the end-product of heme pathway shown in Figure 1 , which not only represses transcription of the ALA synthase-1 gene,2 but also blocks translocation of the enzyme into mitochondria and decreases the stability of its mRNA.3,4
Downregulation of ALA synthase-1 by avoidance or removal of inducing drugs and chemicals by nutritional means (high carbohydrate intakes) and by administration of exogenous heme remains the cornerstone of management of the acute porphyrias.
As shown in Figure 1 , there is no porphyria associated with a defect in ALA synthase-1, but mutations of the X-linked ALA synthase-2 (the erythroid form) are causative for X-linked sideroblastic anemia. Indeed, one would expect the opposite, namely inadequate porphyrin synthesis, if this enzyme were markedly deficient in activity. It is highly unlikely that such defects would be compatible with life. The other enzymes, when deficient, give rise to the types of porphyrias shown in Tables 1Table 1A,Table 1B and 2 . The last three enzymes of the pathway, namely, coproporphyrinogen III oxidase, protoporphyrinogen oxidase, and ferrochelatase, are also located within mitochondria, whereas the 2nd–5th enzymes are found in the cytoplasm or soluble fraction of the cell.
Classification of the Porphyrias
The porphyrias are usually classified as either hepatic or erythropoietic based on the principal site of expression of the enzymatic defect (Table 1Table 1A,Table 1B ). All of the acute or inducible hepatic porphyrias (5-aminolevulinate dehydratase deficiency porphyria [ADP], acute intermittent porphyria [AIP]; hereditary coproporphyria [HCP], and variegate porphyria [VP]) are hepatic as are the porphyrias associated with deficiency of uroporphyrinogen decarboxylase, namely porphyria cutanea tarda and hepatoerythropoietic porphyria. The erythropoietic forms are congenital erythropoietic porphyria or Gunther’s disease and erythropoietic protoporphyria. Erythroid cells contribute significant amounts of porphyrins in hepatoerythropoietic porphyria, so this form of porphyria may be considered as both a hepatic and an erythropoietic type of porphyria. The major clinical features of the individual porphyrias are summarized in Table 1Table 1A,Table 1B . They consist of attacks of neurovisceral symptoms and cutaneous manifestations. In some forms, liver damage may also occur and an increased risk of hepatocellular carcinoma. This risk is increased in porphyria cutanea tarda, hepatoerythropoietic porphyria, and in all of the acute hepatic porphyrias, as well as in erythropoietic protoporphyria. The remainder of this overview will be limited to discussion of the four acute porphyrias. Their genetic and enzymatic features are summarized in Table 2 .
Pathogenesis of Acute Attacks
As shown in Table 2 , the enzyme deficiencies are partial (usually about 50% of normal in three of the acute porphyrias and usually less than 5% of normal in the fourth [ADP]), and the residual enzymatic activity is usually sufficient to maintain adequate hepatic heme synthesis. Activity of ALA dehydratase normally greatly exceeds that of the other enzymes of the heme biosynthetic pathway in the liver. Therefore, a much more severe deficiency in the activity of this enzyme (less than 5% of normal) is usually needed to cause manifestations of ALA dehydratase deficiency porphyria. Such enzymatic defects are envisioned to predispose affected persons to the deleterious influences of factors that may trigger acute attacks, including drugs, such as barbiturates, hydantoins, rifampin, progestins, endogenous steroid hormones (especially progesterone), prolonged severe fasting or dieting, alcohol, and other intercurrent illnesses or stress. All of these can either increase the demand for hepatic heme or otherwise reduce the regulatory pool for heme and thus induce or upregulate synthesis of ALA synthase (shown in Figure 2 ). Because hepatic ALA synthase-1 is normally rate-controlling, when production of heme pathway intermediates increases, the inherited partial enzymatic deficiency more distal in the pathway becomes rate limiting, and proximal intermediates accumulate. For reasons that are complex and not fully understood, intermediates distal to the inherited enzyme deficiency or their products may also accumulate.
Although the precise pathogenic mechanisms underlying the neurologic damage in the acute porphyrias remain imperfectly understood, it seems likely that symptoms result primarily from accumulation of the porphyrin precursors, rather than deficiency of heme in nerve or muscle tissue.5,6 Symptomatic acute porphyria rarely becomes clinically manifest prior to puberty (highlighting the importance of endogenous steroid hormones, especially progesterone and less so estrogens), and the disease is more often clinically manifested in women, often with cyclical attacks linked to menstrual cycles. Very rare instances of homozygous acute porphyria with severe neurologic manifestations beginning in childhood have been described.5,7,8 Perhaps the most persuasive evidence that overproduction of hepatic ALA and/or porphobilinogen (PBG) are central is the clinical observation of their unique association with diseases that present with neurovisceral attacks. Further evidence was the rapid and dramatic improvement in chronic debilitating neurovisceral attacks that occurred following liver transplantation in a young woman with AIP.6 A patient with a diagnosis of variegate porphyria, who underwent liver transplantation for alcoholic cirrhosis, similarly experienced biochemical improvement after liver transplantation.9 In contrast, liver transplantation was not beneficial clinically or biochemically in a child with severe ALA dehydratase deficiency, suggesting that ALA overproduction did not arise chiefly from the patient’s native liver.10
Common Clinical Features
The most common presenting symptom of acute porphyria is abdominal pain. This is usually colicky in nature and in the lower abdomen. It lasts hours to days. The most common sign in acute porphyric attacks is tachycardia. Table 3 lists other common presenting symptoms and signs with estimates of incidence and other comments. Among neurologic manifestations, evidence of autonomic neuropathy with a sympathomimetic state characterized by tachycardia and systemic arterial hypertension is most common. Severe constipation is also very common. Peripheral neuropathy, which is predominantly a motor neuropathy, initially affecting mainly the proximal rather than distal muscles, is next in order of frequency. Sensory loss over the trunk is also common, although it is often overlooked. Neuropsychiatric manifestations of anxiety, depression, insomnia, disorientation, hallucinations, and paranoia occur somewhat less commonly as do cranial nerve defects, seizures, or coma, and cerebellar, optic nerve, basal ganglion or pyramidal tract involvement. Another common manifestation is hyponatremia, which is usually due to vomiting and replacement of fluid lost with hypotonic solutions such as intravenous glucose without electrolytes, and to excessive secretion of antidiuretic hormone. Although it is often said that this represents inappropriate secretion of antidiuretic hormone, when plasma and blood volumes of patients with acute porphyria were measured, they were found to be decreased.11 In the setting of plasma volume depletion, secretion of antidiuretic hormone can not truly be said to be inappropriate. Sudden death, probably due to cardiac arrhythmia, may occur during acute attacks.12 Death can also be a consequence of progressive paralysis, including paralysis of respiratory muscles and bulbar paralysis with complicating disorders such as pneumonias and aspiration.
Recommendations for Diagnosis
It is important that the diagnosis of acute porphyria be considered early on when patients present to the emergency room or other urgent care settings with compatible symptoms and signs. Too often, acute porphyria is considered only after expensive, time-consuming, unproductive searches for other causes of abdominal complaints have been carried out, sometimes including ill-advised and unnecessary surgery. Careful personal history may reveal recurrent episodes of colicky, usually lower, abdominal pain lasting for hours to days with associated obstipation. ER records of tachycardia and systemic arterial hypertension provide helpful clues. Attacks that occur cyclically in menstruating women, with onset during the bruited phase of the menstrual cycle, are suggestive, although more likely due to endometriosis than acute porphyrias. Severe stress, starvation, “crash” dieting, alcohol excess, or intercurrent infections may trigger acute porphyric attacks. A positive family history of porphyria, if established at a reputable center, is important, although usually not elicited.
The key to establishing or excluding the diagnosis is rapid and simple testing for increased porphobilinogen (PBG) in the urine. This should be done in a single freshly passed urine to which no preservatives are added. Classically, the qualitative test for PBG in the urine has been the Watson-Schwartz test, although the Hoesch test is also useful and may be less prone to misinterpretation.13 The commercially available Trace® PBG kit (Trace American/Trace Diagnostics, Louisville, Colorado) is useful and includes a color chart for semi-quantitative estimation of levels of urinary PBG. These levels are generally markedly increased (20–200 mg or 220–880 μmol/day with a typical reference range of 0–4 mg or 0–18 μmol/day). In all cases of acute porphyric syndromes except for the very rare porphyria due to ALA dehydratase deficiency or in patients with symptoms due to lead poisoning or hereditary tyrosinemia type I, markedly elevated urinary porphobilinogen is expected and readily detectable. If the porphobilinogen level is increased, second line testing including erythrocytic PBG deaminase levels, urinary, fecal, and plasma porphyrin levels will establish the precise disorder of porphyrin metabolism (Table 4 ). The most useful tests are the erythrocytic PBG deaminase level for AIP, the urine and fecal porphyrin levels for HCP, and the plasma porphyrin level and fluorescence pattern for VP. Worthy of emphasis is that about 10% of patients with AIP have normal erythrocytic PBG deaminase levels. There is a single gene encoding PBG deaminase, but erythroid cells utilize different promoters and transcriptional sites than hepatocytes and other cells. As a result, the erythrocyte enzyme lacks the amino terminal residues encoded by exon 1. Therefore, in the 10% or so of patients with AIP who have mutations in exon 1, erythrocytic PBG deaminase activities are entirely normal. Another limitation is that there is a large range of variation in activity of the enzyme, and there is an overlap between normals and carriers of the deficiency.
Diagnosis of ALA dehydratase deficiency, in which PBG is normal or only slightly increased, requires measurement of urinary ALA, which can be done on the same urine sample. Associated findings include elevations in urinary coproporphyrin and erythrocyte Zn protoporphyrin and markedly deficient erythrocyte ALA dehydratase activity. Other causes of this enzyme deficiency must be excluded.
After the above first- and second-line biochemical studies have established the presence and type of acute porphyria, DNA studies can identify the disease-causing mutation or mutations in the defective gene. Demonstration of a mutation in each ALA dehydratase allele is important for confirming the diagnosis of porphyria due to ALA dehydratase deficiency. If a mutation has been found in a proband, rapid and accurate testing of asymptomatic-at-risk family members can be carried out. DNA-based studies are rather widely available in European counties at central, government supported laboratories. Mutational analysis for patients and family members with acute porphyria in the United States is currently available through the Department of Human Genetics, Mt. Sinai School of Medicine, New York, NY (contact Dr. Kenneth Astrin for information at kenneth.astrin@mssm.edu).
Recommendations for Management
Key principles of management are summarized in Table 5 . Removal of inciting factors, such as one of the many drugs or chemicals that can trigger or exacerbate acute porphyrias, is essential. Nutritional supplementation, including at least 300 g of glucose given daily, is important because glucose suppresses activity of ALA synthase-1.14 Due to nausea, vomiting, disordered bowel motility, etc., such supplemental glucose usually must be administered intravenously. Because of the danger of severe hyponatremia, sodium should be administered and levels of serum sodium should be monitored frequently. There should also be frequent checks of neurological status, especially looking for evidence of the development of impairment of swallowing, coughing, and respiration. Prompt transfer to an intensive care unit for close monitoring and provision of respiratory support and other intensive nursing care may be life saving.
Tachycardia and/or systemic arterial hypertension can be treated cautiously with beta-adrenergic blocking agents such as propranolol or nadolol. Typical daily doses are 40–240 mg of propranolol or 60–120 mg of nadolol. Caution must be exercised when using beta blockers because hypotension and serious bradycardia have been reported even after low doses.15 Analgesia in the form of parenteral administration of morphine (3–12 mg/dose or meperidine 50–200 mg/dose) is recommended, because these agents have been found to be safe and effective. Such analgesics may depress respiratory drive, such that monitoring of respiratory adequacy (e.g., arterial blood gases) is important.
Addition of a phenothiazine (e.g., chlorpromazine, 10–15 mg/dose) enhances the analgesic and sedative effects of narcotics and helps to treat the agitation and anxiety that often accompany acute porphyric attacks, and are also effective for nausea and vomiting. If seizures supervene, the treatments of choice are benzodiazepines, gabapentin, and vigabatrin. Hyponatremia and hypomagnesemia should be corrected.
Hemin Therapy
Intravenous hemin (the generic term for both heme products marketed for intravenous use), given as quickly as it can be obtained, is the treatment of choice for any patient who is ill enough to require hospital admission for acute porphyria. The standard regimen for hemin therapy is 3–4 mg/kg/day for 3–5 days. In the United States, hemin in the form of Panhematin (Ovation Pharmaceuticals, Deerfield, IL) is the only preparation available. Although the product labeling recommends an initial trial of intravenous glucose, heme should be given without delay. Lyophilized hematin should be reconstituted with human serum albumin to enhance its stability.16 In addition to minimizing the formation of polymers and other degradation products that form rapidly when hematin is reconstituted with sterile water (as currently recommended in product labeling), there are lesser adverse effects on coagulation and irritation to veins (phlebitis, etc.). Heme arginate, which is approved in many other countries, is stable in concentrated solution and somewhat less irritating to veins, although it, too, is commonly given with human serum albumin.
Recommendations for Prevention and Follow-up
All patients with acute porphyria should carry Medic Alert bracelets and wallet cards, as well as a list of safe and unsafe drugs, so that these are readily available to emergency response personnel in the event of accident or incapacity. For women with frequent cyclical attacks, use of gonadotropin-releasing hormone (GNRH) analogs is frequently highly effective. Other women have benefited from the use of low-dose estrogen and progesterone such as in low-dose birth control pills or estrogen patches. Estrogen patches can also be used to help prevent menopausal symptoms in women receiving GNRH analogs. Although pregnancy produces increased levels of progesterone, most women with acute porphyria tolerate pregnancy remarkably well.
Some patients, most of them women with cyclical attacks, seem to require and benefit from regular prophylactic infusions of hemin. These may be given only once a month, shortly before the usual onset of symptoms, or they may be administered weekly or biweekly, especially if the attacks are frequent and mostly unrelated to the cycle. The frequency and doses used are empiric and dependent chiefly upon the symptoms of the patient.
Because of the increased risk of development of hepatocellular carcinoma (HCC) in the hepatic porphyrias, it is becoming more common to do surveillance screening for HCC in affected persons. This is usually done with semiannual serum alphafetoprotein and liver ultrasound (perhaps, alternating with 4-phase, helical, dynamic CT scan). The cost-effectiveness of such surveillance is not established, but the practice has gained in popularity nonetheless.
Heme biosynthetic pathway showing the sites of enzymatic defects in the porphyrias and the major biochemical abnormalities in biochemically active disease. Only the major increases in the urine, stool, plasma, and erythrocytes (RBCs) are shown. The dashes (—) represent no abnormalities. For several of the diseases, many patients are biochemically silent (“latent”) carriers of the enzymatic defects for most of their lives.
Abbreviations: COPRO, coproporphyrin; COPROGEN, coproporphyrinogen; ISOCOPRO, isocoproporphyrin; PROTO, protoporphyrin; URO, uroporphyrin; UROGEN, uroporphyrinogen; Zn, zinc.
Reprinted with permission from
Heme biosynthetic pathway showing the sites of enzymatic defects in the porphyrias and the major biochemical abnormalities in biochemically active disease. Only the major increases in the urine, stool, plasma, and erythrocytes (RBCs) are shown. The dashes (—) represent no abnormalities. For several of the diseases, many patients are biochemically silent (“latent”) carriers of the enzymatic defects for most of their lives.
Abbreviations: COPRO, coproporphyrin; COPROGEN, coproporphyrinogen; ISOCOPRO, isocoproporphyrin; PROTO, protoporphyrin; URO, uroporphyrin; UROGEN, uroporphyrinogen; Zn, zinc.
Reprinted with permission from
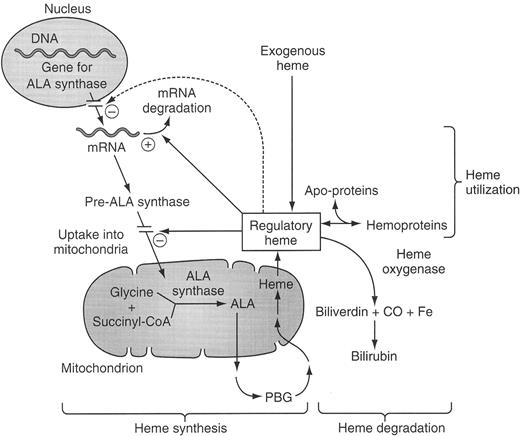
Regulation of the hepatic heme biosynthetic pathway and subcellular localization of the enzymes of the pathway.
Schematic of the synthesis of heme and its regulation. The regulatory heme pool acts to stimulate (+) or down-regulate (−) the indicated steps. Those steps indicated as not within the nucleus or mitochondrion take place in the cytosol. The dashed line indicates a still controversial regulatory effect of heme to decrease transcription of the gene of ALA synthase 1.
Abbreviations: ALA, 5-aminolevulinate; CO, carbon monoxide; CoA, coenzyme A; PBG, porphobilinogen.
Reprinted from
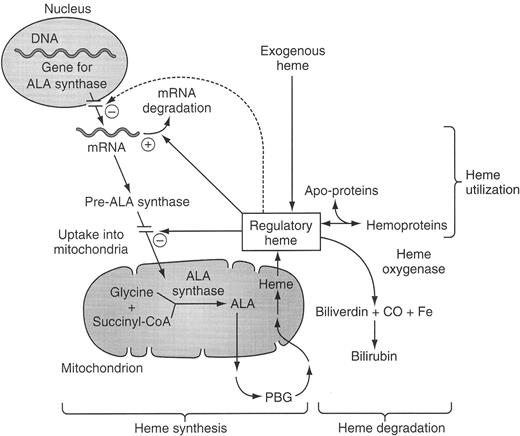
Regulation of the hepatic heme biosynthetic pathway and subcellular localization of the enzymes of the pathway.
Schematic of the synthesis of heme and its regulation. The regulatory heme pool acts to stimulate (+) or down-regulate (−) the indicated steps. Those steps indicated as not within the nucleus or mitochondrion take place in the cytosol. The dashed line indicates a still controversial regulatory effect of heme to decrease transcription of the gene of ALA synthase 1.
Abbreviations: ALA, 5-aminolevulinate; CO, carbon monoxide; CoA, coenzyme A; PBG, porphobilinogen.
Reprinted from
Grant Support: Supported by NIH grants 1RO1-DK38825; MO1-RR01692 and NIH contracts NO1-DK92326, and U01-DK065193.
Acknowledgments: I thank Jean Clark for help with assembling and preparing the manuscript.