Abstract
Data from reporting systems around the world document that non-infectious hazards are the leading cause of serious morbidity or mortality resulting from blood transfusion. Among these non-infectious hazards, mis-transfusion represents the most frequently observed serious hazard and occurs at an estimated rate of 1 in 14,000 transfusions. Mis-transfusion events result from “lapse errors” (slip ups) rather than cognitive mistakes. Lapse errors are more likely to occur during repetitive tasks when individuals are distracted, rushed, or fatigued—conditions to which machines are not susceptible. The final bedside check and the collection of patient samples for pre-transfusion testing are key “error spots” and are candidates for new technology innovation. Existing technology includes non-computerized devices; bedside devices based on bar code technology; and the use of radiofrequency chips. Several commercial systems employing bar-code technology have reached clinical application or are undergoing refinement. Radiofrequency-based systems are on the near horizon. Nearly all systems begin with the application of machine-readable data to the patient’s wristband. The third error spot—the decision to transfuse—will be a challenging area for future application of information technology. Computerized physician order-entry, decision support and ultimately active computer-based decision-making are expected to enhance transfusion decisions. Despite the explosive growth in information technology in modern society, healthcare has lagged behind many other sectors in the use of enhanced information technology. Studies are needed to identify which technologies improve patient outcomes. Healthcare workers, administrators, and regulators need to embrace the use of new technology in order to reduce errors and improve safety for patients.
Case Summary— Ms Johnston
On a Thursday evening, you are paged to consult on the management of a patient (Mary Johnston) who has received incompatible blood. The patient is a 48-year-old woman who underwent surgery that morning for removal of a 2 cm, right pararenal mass. The surgery was uncomplicated and a frozen-section pathology specimen was read as a benign adenoma. She received 2.8 L of crystalloid solution during surgery. Post-operatively, her hematocrit was found to be 24% and she was given one unit of group A packed RBCs over 2 hours. Towards the end of the transfusion, she developed a shaking chill and her temperature rose from 99°F to 101°F. Her urine was red as a result of the renal surgery. A blood culture and complete blood count (CBC) were sent and the CBC specimen was reported as hemolyzed with a Hct = 24%. A transfusion reaction evaluation was sent to the blood bank accompanied by the empty blood bag and a freshly drawn post-transfusion specimen. The blood bank called back to report that a “wrong blood transfusion” had likely occurred. The name and medical record number attached to the empty blood bag tag (Mary Johnson, MRN 2395783) did not match that on the post-transfusion specimen (Mary Johnston, MRN 2395837). There was no pre-op sample on file for Mary Johnston. The post-transfusion specimen was hemolyzed and typed as group O-positive. The Direct Antiglobulin Test was positive. The other patient, Mary Johnson, had a blood bank sample drawn 2 days earlier in the Emergency Room and she was now on the medical service. The sample drawn in the ER was retrieved, retyped, and found to be A-positive and compatible with the unit crossmatched to it. However, a follow-up sample from the medical service on Mary Johnson was found to be O-positive. The hospital risk management department was notified. The clinical staff would like to know your recommendations for management as soon as possible.
Transfusion Safety: More than Just Blood Safety
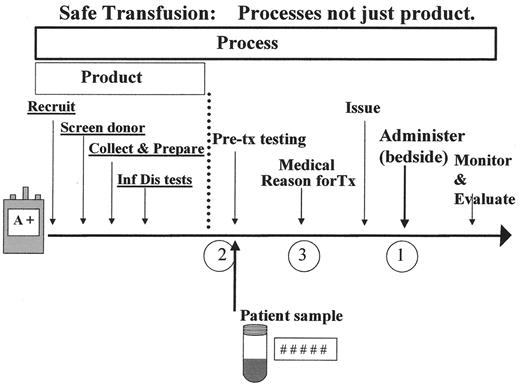
For most patients, clinicians, industry regulators, legislators, and members of the general public, the hazards of blood transfusion are synonymous with concerns about transfusion-transmitted disease. Given fears regarding HIV, prion-diseases, and emerging viral pathogens, this is an understandable perspective. However, the previous 3 decades have witnessed a tremendous focus on blood safety (safety of the fluid) and a corresponding dramatic decline in the risk of transfusion-transmitted diseases. Despite this achievement, overall transfusion safety (safety of the entire therapy) depends upon the coordinated linkage of many processes from donor collection to transfusion1 (see Figure 1 ). At the same time, modern healthcare is undergoing a revolution in information technology.2 This short review will focus on only one small segment of current activity—new technology for enhanced transfusion safety.
Mis-transfusion: More Common than We Realize
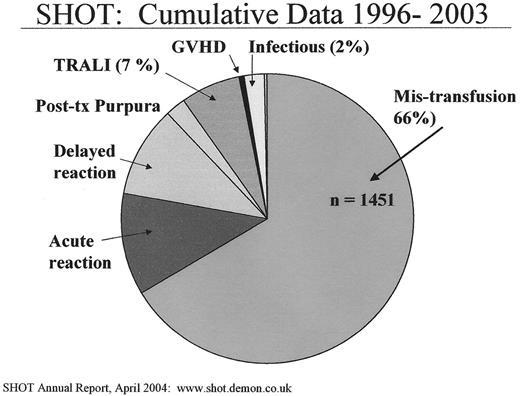
Data from multiple sources worldwide document that mis-transfusion errors are serious and unacceptably frequent. In the United States, mis-transfusion has consistently been a leading cause of death from transfusion reported to the US Food and Drug Administration (FDA) since such reporting began.3 Similar results are found in other nations. For example, the Serious Hazards of Transfusion (SHOT) program collects data on the full range of adverse transfusion events in the UK. As shown in Figure 2 , mis-transfusion accounts for the largest proportion of all adverse events.
Other sources report very similar results. Based on events recognized and reported (an underestimate of the true incidence) to New York State, Linden et al calculated that the risk of ABO mis-transfusion was 1 in 14,000 units.5 Pierre Robillard reports that the hemovigilance program in Quebec identified mis-transfusion as the most common major adverse event, occurring at a rate of 1 in 12,000 transfusions.6 Andreu reports similar findings from the hemovigilance program in France.7 Yet, the true incidence of mis-transfusion is undoubtedly even higher due to failure to recognize many errors. In a very provocative study conducted in three university hospitals in Belgium, mis-transfusion was estimated to occur at the astonishing rate of 1 in 400 units.8
Mis-transfusion events are surprisingly easy to miss. The case of Ms Johnston (see box) illustrates difficulties in their recognition. Signs of the reaction—fever, chill, red urine and visible hemolysis of the plasma—could easily be attributed to other causes. A 101°F post-op temperature is hardly uncommon. The red urine was assumed to be hematuria from renal surgery. A hemolyzed specimen could be attributed to a difficult “line draw.” While pain, vomiting, disseminated intravascular coagulation (DIC), and shock can all occur following transfusion of incompatible blood, sudden dramatic signs or symptoms are, in fact, uncommon. Nor is the outcome of mis-transfusion always fatal. Based on 237 examples of ABO-incompatible RBC transfusions,5 Linden reported that death occurred in 13 (5.5%) of cases, survival with major organ morbidity in 96 (41%) of cases, and survival with no long-term adverse effects in 111 (47%) of patients. However, in many of these cases only a portion of the incompatible unit was administered before the error was recognized and the transfusion stopped. Thus, the above frequencies may underestimate the predicted risk for a patient like Ms Johnston.
Once properly investigated, however, the diagnosis was very easy to make. Proper investigation, in Ms Johnston’s case, hinged on returning the empty blood bag and drawing a fresh, properly labeled sample on the index patient. Too often the empty bag is not returned to the laboratory, thereby discarding critical information that gives an immediate diagnosis. Finally, transfusion mishaps often involve more than one error and can be confusing to sort out at first. Do you recognize in Ms Johnston’s case that a third patient is involved? Read on.
Why New Technology?
Medical errors are common and can be broadly categorized as either “cognitive” or “lapse” errors. Cognitive errors result when the health care provider lacks sufficient knowledge or misapplies knowledge. Lapse errors or “slip-ups” occur when a repetitive task is performed incorrectly due to distraction, fatigue, or inattention. The labeling of a blood sample or the bedside patient identification check is a repetitive task subject to “slip” or “lapse” type errors.9 Currently, the bedside check is performed without the aid of any supportive technology—even though previous research has documented that the performance of repetitive task functions is aided through the use of technology.10
Technologies to Improve the Final Bedside Check
The single most frequent error resulting in ABO-incompatible transfusion is the administration of properly labeled blood to a recipient other than the one intended.5 This category of error accounted for nearly 40% of the ABO-in-compatible transfusions recorded in the study by Linden et al.5 Other studies support the contention that the bedside check as currently performed is an inadequate patient safeguard. The College of American Pathologists published results of two large observational audits—one conducted in 199411 and one in 2000.12 These audits assessed the frequency with which basic elements of the bedside check were performed including positive patient identification, matching wristband identification to the blood compatibility label, matching patient identifiers with the blood request, and review of compatibility and expiration date information. In each category, there was a substantial decline in the percentage of transfusions that were correctly checked at the bedside between the years 1994 and 2000. Equally disturbing was the actual frequency that checks failed to be performed. For example, in the year 2000, the audit of over 4000 transfusions revealed a failure to match wristband identification with the compatibility label in 25% of transfusions. Nationally, this translates into literally millions of episodes in which patients are not provided basic safeguards against the most common serious hazard of transfusion. Because the pretransfusion check is the “last chance” to stop a flawed, error-prone process from harming the patient, it may be especially beneficial to invest in improving the performance of this final step.
Non-computerizedTechnology
BloodLoc (Novatek Medical, Greenwich, CT) represents a non-computerized technology aimed to reduce the risk of mis-transfusion. The system includes a plastic combination lock that the user must open in order to access the ports on the transfusion bag. The patient’s wristband carries a 3-letter code that is intended to be available ONLY from the wristband. The code is copied from the wristband to the blood sample. In the blood bank, the code is dialed into a plastic lock that seals a plastic bag containing the unit for transfusion. At the time of transfusion, the plastic lock can only be unlocked by dialing-in the 3-letter code found on the patient’s wristband. When used as intended, the 3-letter code links the wristband to the final transfusion product. This system has been used extensively at Dart-mouth Hitchcock Medical Center where all RBCs are released in a BloodLoc container (except uncrossmatched emergency blood). In a decade of use at Dartmouth (covering 240,000 specimens and 100,000 RBC transfusions), the system contributed to preventing 3 mis-transfusion episodes at the bedside (James Aubuchon, personal communication).
Bar Code Check Prior to Transfusion
Within the laboratory, bar coding is a standard technique used routinely by healthcare workers (technologists). Yet once the bag leaves the blood bank, the technology ceases to be used and healthcare workers revert to comparing by eye the confusing array of bag labels found on each bag. Two changes are required to extend the advantages of bar code technology beyond the laboratory. First, hospitals need to begin to recognize the patient wristband as the fundamental infrastructure for positive patient identification and convert patient wristbands from today’s eye-readable only format to a wristband that includes machine-readable technology. Machine-readable technology may include a mixture of both one-dimensional and two-dimensional bar codes as well as radiofrequency identification tags. Second, clinical staff—especially nurses and anesthesiologists who administer blood—will need to incorporate into their daily routine techniques that have become so perfectly natural to medical laboratory technologists, UPS delivery staff, or the check-out people at the supermarket or clothing store. A bar code of the patient’s identity (on the wristband) and a bar code with the identity of the intended transfusion recipient (on the blood bag) is the obvious first choice for machine-readable technology designed to prevent transfusion errors.
PatientSafe TransfuseID™ (AMT Systems, Cheshire, CT) is an example of a bar code-based device. It consists of a handheld portable digital assistant with a built-in bar code reader and a portable printer. The user scans his/her own ID badge, scans the patient’s bar code wristband, and then the blood product. Non-matching data results in an obvious error alarm. A transfusion episode label can be printed for posting into the medical record. Similar devices are available from Bridge Medical, Care Fusion, and Lattice, Inc. Care Fusion received FDA 510k approval for their device in 2004. Whether or not bar code based systems will actually reduce the frequency of mis-transfusion error awaits proper clinical testing13 (see Table 1 ).
Radiofrequency Identification
Radiofrequency identification (RFID) tags carry data similar to bar codes but have the following potential advantages: the tags can hold much more data, can have read-write function (bar codes are read only), and most importantly, are more user friendly because direct line-of-sight is not required to read an RFID tag. Two major functions of RFID tags are asset tracking (location) and item tagging (identification). For asset tracking, the RFID tag emits data that is detected by receivers. Such tags, which emit data over a distance, are called “active tags.” Active tags require battery power to transmit radio-wave energy and are relatively expensive. Examples of active RFID devices are the automobile key clicker, the toll booth drive through tag, and a cell phone. In contrast, for identification of an object (not tracking its location), the RFID tag emits no energy and has no battery. This is called a “passive tag” and is also known as a “smart label.” They are much less costly than active tags and can be made to tag almost any sized object, including blood bags and the patient’s wristband (see Figure 3 ). To function, passive tags must be placed in proximity to a “reader” that emits energy. When the tag is brought into the zone of energy emitted by the reader, the tag’s small antenna activates the data chip to echo back the data to the reader. An example of a passive tag is the theft protection device found on unsold clothing at the store. For most healthcare concerns related to blood transfusion, the major issue is “identity” (and not location tracking). Thus, a wristband with a passive tag can be used much like a barcode to identify the patient. The tag emits no energy and patients wearing this tag cannot be “tracked.” Passive tags can also be applied to blood bags encoding identification information.
In collaboration with Lattice, Inc and Precision Dynamics Corporation, Massachusetts General Hospital (Boston) is exploring the use of RFID for the final bedside check prior to transfusion in the Operating Room (OR). RFID does not require “line of sight” of the patient’s wristband, which is frequently lost when the patient is draped for surgery. OR staff responsible for transfusion may have had limited contact time with the patient and may not “know” the patient except through standard name and medical record number identifiers. The opportunity for error is particularly high as patients are unconscious and thus cannot state their name at the time of the bedside check. In addition, blood transfusions are often given in the OR under circumstances of extreme urgency and distraction—two key elements that may contribute to lapse errors at the time of the pre-transfusion check. Indeed, data submitted to the FDA and reviewed by Sazama et al document that mis-transfusion events are most likely to occur among surgical patients.3 On the other hand, the OR environment is “friendly” to the use of technology to assist in patient safety, and patient-safety devices developed in conjunction with human factors research have been long applied in anesthesia.14,15 Thus, from both a perspective of need and acceptance, the operating room may be an ideal location for the introduction of patient safe-transfusion technology.
Smart Storage or Delivery Systems
Alaris (San Diego, CA) is developing systems in which the “infusion pump” device has built-in technology for reading bar code or RFID information. To run the infusion device, the user scans the patient’s wristband (bar coded, for example) and then scans the bar-coded medication (or blood transfusion product), and software within the device does safety matching prior to activating the pumps that deliver the product. Although designed principally for safe intravenous medication therapy, these products may be easily suitable for routine transfusion therapy. Whether or not such systems will improve patient safety needs to be studied. A recent prospective trial found that the rate of medication error was not improved by introducing smart pumps in an intensive care setting,16 largely due to the failure of health care workers to use the system (non-compliance). This finding underscores the importance and the challenge of making any safety technology technically robust but extremely easy to use.
Technologies to Improve Blood Sample Collection
Errors made in the collection of the patient sample are serious because they may set into motion the assignment of blood with the incorrect ABO group for the intended recipient. Two categories of error are defined: mis-labeled tubes and mis-collected tubes. Mis-collected tubes are more dangerous errors and have the blood from patient “A” in a tube properly labeled with the identifiers of patient “B,” so called “wrong blood in tube” (WBIT). Linden et al documented that 14% of ABO incompatible transfusions reported to New York State were due to sample collection errors.5 Lumadue et al17 found that 1.4% of 40,770 samples (1 in 71) were mis-labeled and that 0.035% (1 in 2800) were WBIT. Samples with labeling errors were 40-fold more likely to have WBIT. This finding alone is sufficient reason for laboratories to refuse to process mis-labeled samples. We reported results of a large multicenter international study that examined the frequency of mis-labeled or mis-collected blood bank samples submitted to 62 hospitals.18 We found that the median hospital performance resulted in a rate for mis-labeling of 1 in every 165 samples (6.1 per 1000; inter-quartile range 1.2–17 per 1000). Mis-collected samples demonstrating WBIT occurred at a median rate of 1 in every 1986 samples (0.5 per 1000; interquartile range < 0.3–0.9 per 1000).
Sample labels—“From the Patient, at the Bedside”
There is little doubt that a humble can of peas at the supermarket is more accurately and consistently and correctly labeled than patient specimens used for blood transfusion. The two principal flaws to overcome in sample collection labeling are failure to use the actual patient as the source of the data and failure to label at the bedside. The use of pre-printed specimen labels invites sample mis-collection. Drawing the blood sample then carrying it away to a ‘labeling site’ (e.g., a nursing station) invites labeling the sample with data from another patient. Such systems, in widespread use, are designed to fail, and do. Even systems that print labels from a local computer (rather than from the patient as the source) risk having the wrong individual on the computer screen and thus printing the wrong patient’s data. To overcome these weaknesses, most commercial safety-based systems have focused on using the patient’s wristband as the source of the data and a portable label printer to allow specimen labeling “from the patient at the bedside.”
Non-electronicTechnology Designed for Error Reduction
One simple approach to blood bank sample errors is the use of specially designed wristbands unique to blood bank activity. These bands (Bio-Logics, West Jordan, UT) carry an embossed card with a pre-assigned unique number. After sample collection, the card is removed from the wristband and used as a template to make labels from a bedside printer. These labels are attached to the tube and requisitions. While the system promotes bedside labeling, it introduces a second numbering system that must be linked to the medical record number. A more robust extension of non-computerized wrist-band technology is provided by Novatek Medical (Greenwich, CT) who markets BloodLoc (described above). When used as intended, the 3-digit code that drives the system can only be obtained from the patient’s wristband. This code is transcribed to the blood sample, thus forcing a process “from the patient and at the bedside.” Over a 10-year period of use at Dartmouth Hitchcock Medical Center, the BloodLoc device was solely responsible for detecting 35 WBIT samples (James Aubuchon, personal communication).
Bar Code-based Systems for Sample Collection
A bar coded patient wristband is an obvious platform upon which to build machine-readable positive-patient identification for patient samples. These systems share common features including either fixed (bedside computer) or mobile (hand-held portable digital assistant, PDA) integrated bar code reader and a bedside printer for producing sample labels. Half a dozen companies already have leading edge technology (see Table 1 ).
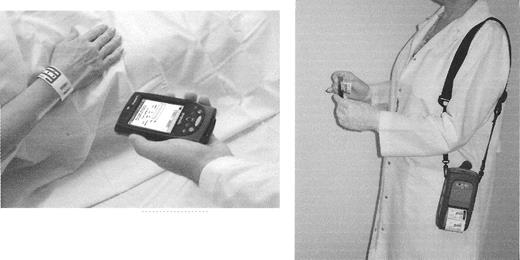
MediCopia by Lattice, Inc (Wheaton, ILL) represents an example of a well-developed device for bar code-based sample collection. MediCopia consists of a handheld PDA, built-in bar code reader, and portable label printer. The PDA receives census information from the hospital information system (HIS) and order information from the laboratory information system (LIS). The system uses this information to provide electronic draw lists of patients including tests, tube types, and special instructions. The information from the HIS/LIS is transferred to the PDA in real-time over the wireless network and/or transferred in batch by inserting the PDA into a cradle that has an interface to the HIS/LIS. At the time of phlebotomy the user scans the patient’s bar-coded wristband and prints a sample label from a portable printer, achieving the goals of “from the patient and at the bedside.” Caregivers are prevented from printing labels in the absence of patient identification (see Figure 4 ). Other phlebotomy systems using bar code technology to achieve positive patient identification include those by Care Fusion, Becton Dickinson, and others (see Table 1 ).
A common challenge for all these devices is the deployment of conveniently sized handheld devices within easy reach of the patient’s wristband. In addition, devices add value when they “know” the requested laboratory tests to be performed. Published accounts by hospitals that have implemented bar code technologies document that successful adoption depends upon “ease of use,” a proper fit with local practice, and a strong commitment on the part of the hospital to adopt a new technology approach to patient safety.19
Radiofrequency Identification
RFID has not yet been routinely applied to blood sample collection. However, Maxell Corp of America (Fair Lawn, NJ) is developing an RFID smart-tag (passive) approach to blood sample labels. The patient’s identity and other information commonly written to paper slips attached to tubes (e.g., names of requested tests, billing information, physician name, location, etc.) are written to a small chip located at the base of the test tube (see Figure 5 ). Using a specially designed 96-test tube rack, the RFID encoded tubes can be “read” and the data transferred to the laboratory computer system. Given the sheer volume of blood samples collected annually in the developed nations, the application of RFID to individual tubes of blood may or may not prove to be cost effective. However, the unit cost of passive RFID tags continues to decrease and some experts believe such tags will be found on most objects sold in stores.
Technologies to Improve Clinical Decision-making
In the opening case of Ms Johnston, readers will note that the RBC transfusion was given for a post-operative anemia (Hct 24%) in a well-perfused 48-year-old female, with no known coronary disease, who was not under conditions of increased oxygen demand. There is little or no evidence that blood transfusion was indicated in this patient, although millions of RBC transfusions are given annually to patients under similar circumstances.
Currently, the real-time application of information technology to medical decision-making is in its early stages of development. Three levels of development are in progress: improved documentation; computer-assisted decision support; and active computerized decision-making. Computerized physician order entry (POE) has been implemented in many medical centers20 and has been shown to reduce serious errors by 50%21 and all errors by 80%.22 Computerized POE allows for more structured, legible, and traceable communication of the physician with pharmacy, blood bank, radiology, and other hospital teams. However, simple order entry systems do not reduce errors in the selection, dosing, or appropriateness of treatments.23
Computer-assisted decision support, often linked to POE, has emerged as the second level of technology in clinical decision-making. Decision support supplies passive but readily available information intended to assist the clinician in making a proper decision. Examples include drug-allergy alerts, dose-range recommendations, antibiotic selection options, user-adjustable order templates (pathways) and many others.2 In transfusion medicine, such alerts can provide valuable guideline information at the time of blood requests and provide feedback to clinicians on indications for transfusion. Recently, Kucher et al published a randomized controlled trial testing the value of electronic alerts sent to physicians caring for patents at risk for venous thromboembolism.24 In the test group, the computer alerted physicians to the patient’s risk and advised physicians to consider prophylaxis treatment. No alert or advice was provided to the control group. The study found that more patients in the physician test group were treated with pneumatic compression boots and/or heparin and the study also found that patients treated by physicians in the intervention group had a lower frequency of clinical venous thromboembolic events.
Finally, the third level of technology involvement in medical decision-making will be the use of active computer-based decisions. Doctors of the future may monitor the computer’s suggested orders and exert “veto power” when the computer’s suggested decision is not the best for the patient. Such artificial intelligence is clearly for the future but will arrive in small ways. For example, a recent randomized controlled trial examined influenza and pneumococcal vaccination. The study compared outcomes resulting from “physician alerts” (computer suggests treatment) versus “computer generated orders” (computer activates order that can be vetoed). The investigators found that a higher proportion of eligible patients received their vaccinations in the group assigned to computer-generated orders compared with electronic alerts.25
Apart from these early successes, computer-assisted medical decision-making is still in its infancy and has a long development path ahead. A study found that computerized physician order entry, if poorly designed, can facilitate new types of medication errors even as they reduce transcription errors.26 In another study computer-generated treatment suggestions for chronic heart failure were deemed inappropriate by treating physicians for their patients’ needs.27 Thus, well-intentioned new technology may solve one problem while introducing new sources of error. Currently there is a paucity of good clinical trial data28 and more studies are needed to evaluate and demonstrate the value of new technology solutions to the problem of medical errors. Despite these reports, the 21st century will see unprecedented advances in information technology.
Case of Ms Johnston Concluded
Ms Johnston’s case demonstrates all 3 major weaknesses in the process of safe transfusion therapy. The sample in the Emergency Room was collected from an unknown third patient (who is group A) and was labeled with the name and medical record number of Ms Johnson on the medical service. This was a “wrong blood in tube” labeling error. A follow up sample (correctly drawn) on Ms Johnson showed that she is, in fact, group O. As a result of the initial wrong-blood-intube error, group A blood was cross-matched on Johnson. Fortunately for her, no blood was requested for transfusion (a “near-miss” event for Johnson). Meanwhile, the surgical patient, Ms Johnston, received Ms Johnson’s blood in error. This was a mis-transfusion error that could have been caught at the bedside with a better clerical check. Finally, Ms Johnston’s transfusion was not indicated in the first place. This was the third error—a medical decision error.
Apart from the initial symptoms of chill and fever, Ms Johnston had no other immediate clinical evidence of having received the wrong transfusion. Her urine output, however, rapidly declined and she did not respond to furosemide. By morning she was anuric. The error in transfusion was explained to the patient. She required no further transfusions. On day 10 she was begun on dialysis for volume and potassium control and remains on chronic hemodialysis.
Treatment of major ABO-incompatible transfusions remains largely supportive and little improved in recent decades. Our best national response to improve transfusion safety is to introduce clinically tested new technology for error prevention.
Other Internet Resources for More Information on Patient Safety Initiatives
Joint Commission on Accreditation of Healthcare Organizations: http://www.jcaho.org/accredited+organizations/patient+safety/
National Patient Safety Organization: http://www.npsf.org/
Agency for Healthcare Research and Quality: http://www.ahcpr.gov/qual/errorsix.htm
The Leapfrog Group: http://www.leapfroggroup.org/safety.htm
Veterans Administration National Center for Patient Safety: http://www.patientsafety.gov/
Patient Safety Institute: http://www.ptsafety.org/
Safe transfusion from donor to recipient. The circled numbers refer to three major points of weakness in the process of transfusion.
Safe transfusion from donor to recipient. The circled numbers refer to three major points of weakness in the process of transfusion.
Serious Hazards of Transfusion (SHOT) national data from the United Kingdom.4
A patient wristband with eye-readable, one-dimensional bar code, two-dimensional bar code, and passive RFID tag seen from back.
A patient wristband with eye-readable, one-dimensional bar code, two-dimensional bar code, and passive RFID tag seen from back.
A bedside bar-code based system designed to improve the accuracy of patient sample collection. (Photo courtesy of Lattice, Inc).
A bedside bar-code based system designed to improve the accuracy of patient sample collection. (Photo courtesy of Lattice, Inc).
A small RFID chip on the bottom of a test tube. (Photo courtesy of Maxell Corp.)
A small RFID chip on the bottom of a test tube. (Photo courtesy of Maxell Corp.)