Abstract
The individual prognosis of patients with chronic lymphocytic leukemia (CLL) is extremely variable. Although clinical stages remain the basis for assessing prognosis in CLL, a number of biological markers, particularly serum markers, cytogenetic abnormalities, IgVH mutations, CD38 and ZAP-70 expression in leukemic cells offer important, independent prognostic information. Before being incorporated into daily practice, however, these markers require standardization and validation in large, prospective trials. Meanwhile, treatment of patients with CLL not included in clinical studies should be decided on the basis of classical NCI/CLL Working Group criteria. An important area of research in CLL prognostication is the identification of markers useful for predicting response to therapy and its duration. Among them, del(17p), reflecting P53 abnormalities, is particularly important. Also relevant is del(11q), which points to ATM defects. There is also some correlation between IgVH mutational status, ZAP-70 and CD38 expression and response to therapy and its duration, although these relationships need further investigation. Finally, there is increasing evidence that response to therapy, particularly in those cases in which minimal residual disease is eradicated, is associated with longer survival.
The overall median survival of patients with chronic lymphocytic leukemia (CLL) is about 10 years. The individual prognosis is, however, extremely variable. Whereas in some patients the disease runs an indolent clinical course and life expectancy is not shortened, in others the disease progresses rapidly, has aggressive behavior, and survival after diagnosis is inferior to 2–3 years.1,2 Considering its variable prognosis and the absence of a curative therapy, management of patients with CLL cannot be planned without taking into consideration their prognosis.
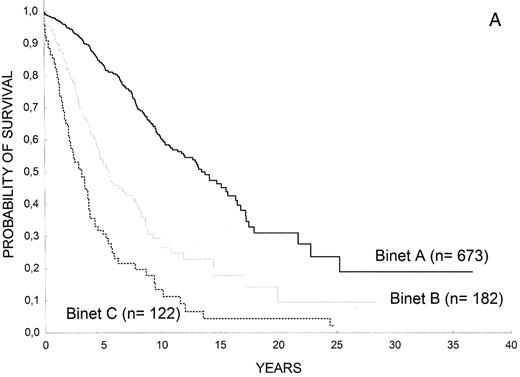
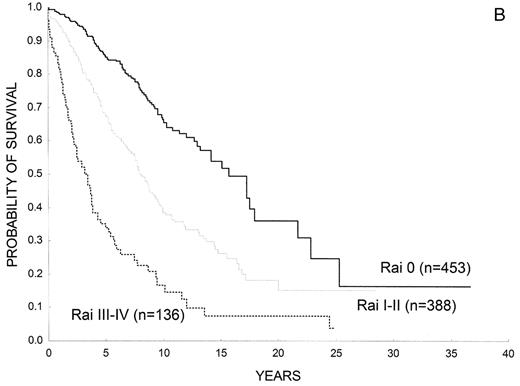
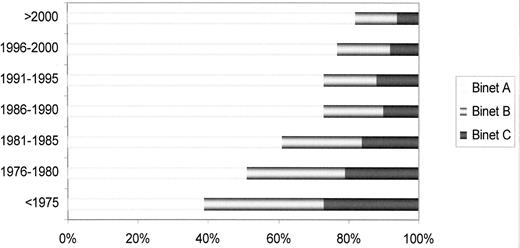
Clinical staging systems are the mainstay for assessing prognosis in patients with CLL.1,2 Patients with low-risk disease (Rai stage 0, Binet stage A) have a median survival that is close to 15 years, those with intermediate-risk disease (Rai stage I or II; Binet stage B) have a median survival of 5–7 years, and most patients with high-risk disease (Rai stage III or IV; Binet stage C) have a life expectancy less than 3–4 years (Figure 1 ). Of note, assigning a clinical stage to a given patient only requires a physical examination and a blood count. Clinical stages have been validated in many studies and, by allowing the comparison of homogeneous groups of patients, they have facilitated important advances in CLL at both biological and clinical levels. Their simplicity and the fact that they have been extensively corroborated in many series constitute the major strength of clinical stages. They, however, have also some limitations; for example, patients who will have progressive disease and those in whom the leukemia will run an indolent course are not identified. On the other hand, the majority of patients are currently diagnosed during routine medical examinations, when still asymptomatic (Figure 2 ). As a result, up to 80% of the patients have low-risk disease at diagnosis, thus limiting the prognostic value of clinical stages as a whole. Finally, the prognosis of a given patient ultimately depends on complex relationships between the characteristics of the patient (age, gender, comorbidity, performance status), the disease (burden, kinetics and biology of the tumor), as well as sensitivity of the disease to treatment. Clinical stages are only one of the parameters in this complex interaction.
Considering that disease stages and other clinical prognostic factors (e.g., degree of bone marrow infiltration, blood lymphocyte levels, lymphocyte doubling time, lymphocyte morphology) (reviewed in references 3,4) merely reflect the biological diversity of the disease, in the last few years the focus of research in prognostic factors in CLL has changed from clinical to biological factors, the so-called biomarkers or classifiers.
Biological prognostic factors for CLL are shown in Table 1 and the most important of them are discussed below. In addition, the prognostic importance of response to therapy, particularly the achievement of a negative minimal residual disease (MRD) status, and the increasing role of response predictors in prognostication are also outlined.
Biologic Prognostic Factors
Serum markers
Several serologic parameters such as thymidinkinase (TK), β 2-microglobulin (β 2M) and soluble CD23 (sCD23) provide valuable information about disease progression and survival (reviewed in references 3,4). Serum TK levels correlate with tumor mass and the proliferative activity of CLL cells and predict disease progression, even among patients with early-stage disease. High levels of the sCD23 have also been linked to adverse prognostic features such as diffuse bone marrow infiltration, rapid doubling time, and disease progression in early-stage CLL. β 2M shows a positive correlation with clinical stages and has been found to be a strong prognostic marker.5,6
A caveat to be taken into account regarding serological markers is that the predictor cut-off levels may vary depending on the laboratory where the test is performed, this limiting the widespread use of these parameters.
Cytogenetics
In up to 90% of the patients it is possible to detect cytogenetic abnormalities by using interphase fluorescence in situ hybridization (FISH). The incidence of different genomic aberrations depends on the characteristics of the patient, poor prognosis alterations predominating in high-risk patients, refractory to conventional therapy. In a recent review, the incidence of the most relevant genetic abnormalities has been reported to range from 14% to 40% for 13q– as isolated abnormality, 10% to 32% for 11q–, 11% to 18% for +12q, 3% to 27% for 17p–, and 2% to 9% for 6q–, depending on the stage of the disease and whether or not the disease was resistant to conventional therapy.4 Patients with del (13q) as single anomaly have excellent prognosis (median survival, 133 months) whereas those with del(11q), involving ATM, and particularly del(17p), involving P53, do not respond to conventional therapy and tend to have a rapidly evolving disease (median survival, 79 and 32 months, respectively).4,7 Catovsky et al have shown that the higher the percentage of cells showing del(17p) the poorer the prognosis, with 20% of cells expressing the abnormality being the critical cut-off.8 Moreover, del(11q) is associated with male gender, younger age and massive lymphadenopathy; trisomy 12 is linked to atypical morphology and immunophenotype of the leukemic cells, and del(6q) is more frequently observed in patients whose lymphocytes display plasmacytoid features and correlates with intermediate prognosis.3
Cytogenetic abnormalities identify groups of patients with different time to progression and survival. Broadly, two risk groups are recognized: 1) low-risk: patients with normal karyotype or isolated del(13q); and 2) high-risk: patients with del(17p) or del(11q); trisomy 12 conveys high-risk of disease progression but in contrast to patients with del(17p) or del(11q), patients with trisomy 12 usually respond to fludarabine-based therapy and their survival is better (H. Döhner and S. Stingelbauer, personal communication). The prognostic significance of cytogenetic abnormalities is independent from that of clinical stages and IgVH mutational status.4–8
Further work is needed to standardize cytogenetic analysis, including cell processing, DNA probes, and cutoff values for the diagnosis of the different abnormalities. Also, novel technologies such as stimulation of CLL cells by CD40 ligand or other agents that would allow the detection of chromosomal translocations associated with a poor outcome in up to 34% of the patients9 require additional confirmatory studies.
IgVH mutational status
An important advance in CLL, not only at biological level but also in its prognostication, was the demonstration that about 50% of patients present in their leukemic cells somatic hypermutations in the rearranged variable regions of the immunoglobulin heavy chains (IgVH). In 1999, the Hamblin and Stevenson and Chiorazzi groups independently reported on the prognostic importance of IgVH genes in CLL, showing that IgVH mutational status separates CLL into two different forms of the disease.10,11 Patients with IgVH unmutated genes (unmutated-CLL) have a more malignant condition, including evidence of advanced, progressive disease, atypical peripheral blood cell morphology, adverse cytogenetic features, clonal evolution, and resistance to therapy than those with mutated IgVH genes (mutated-CLL). The definition of mutated or unmutated CLL is based on a arbitrarily defined threshold of 98% homology to the most similar germ-line gene, although this cut-off value has been disputed.12
In most studies the prognostic significance of IgVH mutations is independent from that of clinical stages and cytogenetic abnormalities, particularly in patients with early-stage disease. There is, however, an exception to this rule and that is the expression of the immunoglobulin variable region gene V3-21, which is associated with an inferior outcome independent of mutational status.13 The prognostic importance of IgVH mutations has been corroborated by many groups.
Since determining IgVH mutations requires specific equipment for DNA sequencing and is time consuming as well as expensive, it is not possible to study IgVH mutations on a routine basis, particularly in nonspecialized laboratories. Accordingly, many attempts have been made to identify a marker that could be as useful as IgVH mutational status in the prognostic assessment of patients with CLL.
CD38 expression
CD38 expression on leukemic lymphocytes was the first marker that was found to correlate with IgVH mutations.11 Eventually, however, it was found that the relationship is not absolute and that, according to some studies, CD38 expression may vary over time.3,10 CD38 expression and IgVH mutations are independent prognostic factors.
On the other hand, the most appropriate threshold to define CD38-positivity is controversial, the proposed cutoffs being 5%, 7%, 20% and 30%.3,4,14 It has been suggested that rather than a fixed, arbitrarily predetermined cut-off level, CD38 should be evaluated by its modal expression in flow cytometry or by the antigen density as measured by the antibody-binding capacity. Clearly, CD38 analysis and the most reliable method for using it to determine CLL prognosis requires standardization and additional, prospective studies.
ZAP-70 expression
In a seminal study on the genetic signature of CLL, Rosenwald et al found that a small number of genes allow the separation of mutated and unmutated CLL, the most specific of them being a gene that encodes for a 70-kD zeta-associated protein (ZAP-70). The majority of mutated cases are ZAP-70 negative, whereas unmutated forms are ZAP-70 positive.15
The expression of ZAP-70 in leukemic cells can be assessed by different methods, such as Western blotting, quantitative RT-PCR, immunohistochemistry, and flow cytometry.16–19 The expression of ZAP-70 in peripheral blood lymphocytes depends on the cell lineage, the highest levels of ZAP-70 being observed in NK and T cells while the expression on B cells is low or absent. Consequently, the assessment of ZAP-70 in CLL cells by flow cytometry implies a multiparametric staining to independently identify CLL cells from T and NK cells such as that implemented by our group.16 The European Research Initiative on CLL (ERIC) has launched an international project to standardize the flow cytometry technique to determine ZAP-70, including the use of directly labelled anti-ZAP-70 mono-clonal antibodies.
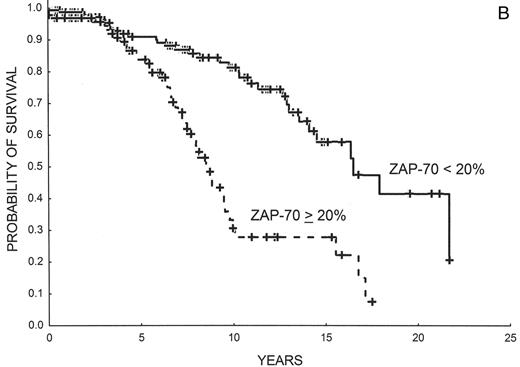
In our study it was found that there was a very good correlation between ZAP-70 expression as detected by flow cytometry and IgVH mutations and, not surprisingly, that ZAP-70 had a prognostic value equivalent to IgVH mutational status.16 The best threshold to separate ZAP-70–negative from ZAP-70–positive cases was found to be 20%. Many other groups have validated these results16,17,20–23 (also reviewed in reference 24) (Table 2 ). The reasons for discrepant cases, which range from 8% to 25%, may depend on patient selection and methodological variables. Interestingly, Krober et al have shown that discordant cases frequently present other biological features carrying poor prognosis such as del(17p), del(11q) or V3-21 expression.25 Rassenti et al have shown that ZAP-70 is more useful than IgVH mutations to predict time to progression21 and the same conclusion has recently been reached in an Italian study.22 Updated, unpublished results from our group showing progression-free and overall survival in 220 patients with CLL on the sole basis of ZAP-70 expression are shown in Figure 3 .
Of note, ZAP-70 expression remains quite stable over the course of the disease.24 We have sequentially studied ZAP-70 in 111 patients; median number of determinations was 2 (range, 2 to 6) and the median time between the first and the last analysis was 29 months (range, 1.3 to 102). The initial ZAP-70 determination was positive (≥ 20%) in 41 (37%) and low (< 20%) in 70 (63%) patients, respectively. Over time, ZAP-70 only changed its categorical value in 9 patients (8%), this event being extremely infrequent in patients with low ZAP-70 expression (3%). No apparent reasons for the changes in ZAP-70 values (e.g., genetic markers, treatment, infections, development of autoimmune phenomena) could be detected.26
ZAP-70 and CD38 may provide complementary prognostic information.27–29 Thus, patients who express both markers would have the poorest prognosis, those in whom none of these marker is present would have good outcome, and discordant cases would fall in a intermediate-risk category. These results should be validated in prospective studies.
Additional surrogates for IgVH mutational status and other prognostic parameters
Other surrogates of IgVH mutations have been suggested. The high expression of activation-induced cytidine deaminase (AID) mRNA is associated with unmutated IgVH gene status and unfavorable cytogenetic aberrations30 and similar observations have been made for lipoprotein lipase A (LPL) and ADAM29, which are not expressed in normal lymphocytes and can thus be analyzed by quantitative RT-PCR.31,32 Recently, a unique set of 13 microRNA genes has been found to correlate with ZAP-70 expression and IgVH mutational status.33 Also, a direct relationship between plasma thrombopoietin (TPO) levels and unmutated IgVH genes has recently been reported.34 All these tests require validation in future studies.
Response to Therapy
Response to therapy is an important prognostic parameter by itself, whereby the higher the degree of response the longer the survival. MRD can be assessed by either allele-specific polymerase chain reaction (PCR) or four-color cytofluorometry. Although PCR has a slightly higher sensitivity than four-color cytofluorometry, both methods are considered to be similarly useful from the clinical point of view, and four-color cytofluorometry is more widely applicable.35
Patients who achieve MRD-negative status have a better prognosis than those with an inferior response to therapy, including those in complete response by NCI-CLL/WG classical criteria but with persistence of MRD after alemtuzumab therapy.36 Also, the detection of MRD predicts clinical relapse in patients treated with chemotherapy or chemo-immunotherapy.3,5,6 Although it is possible that those patients who achieve MRD-negative CR have an intrinsically less aggressive disease, which could explain their better prognosis, obtaining an MRD-negative status is a desirable goal in patients with CLL.37
Predictors of Response
Achieving response to therapy is one of the most important prognostic factors for survival. It is not surprising, therefore, that predictors of response and prognostic factors are somewhat redundant. However, predictors of response are gaining interest because of the plethora of new treatments for CLL and the variable response to them. Predicting subsets of patients who will respond to a given therapy should be useful to avoid unnecessary toxicity and, theoretically at least, avoid the emergence of clones resistant to treatment.3–6,8,38,39 Abnormalities of 17p reflecting alterations of P53 convey resistance to fludarabine; del(11q) pointing out at ATM defects correlate with a lower response rate to fludarabine and early relapse after autologous stem cell transplantation. The power of other markers such as IgVH mutations, ZAP-70 or CD38 expression to predict response to therapy has not been fully investigated in large series of homogeneously treated patients, although there is some indication that the overall response rate and particularly the response duration could be worse in those patients expressing these markers.24 High serum β 2M levels have been found to correlate with inferior response to chemo-immunotherapy.5,6
Conclusions
Clinical stages have been the basis for assessing prognosis in patients with CLL. However, a number of biological parameters, particularly serum markers, cytogenetics, IgVH mutations, CD38 and ZAP-70 expression in leukemic cells, are important independent prognostic markers. ZAP-70 and IgVH mutations basically provide similar prognostic information and therefore they can substitute each other. Studying ZAP-70 by flow cytometry can be easier than determining IgVH mutations, particularly in general laboratories.
It is not difficult to envisage a prognostication system for patients with CLL in which clinical stages are complemented, if not replaced, by biological markers. Nonetheless, before this can happen a number of conditions must be met: 1) the methods to determine biologic prognostic parameters should be fully standardized; 2) their prognostic value should be validated in large prospective clinical trials; and 3) only the most important markers—namely, those with independent prognostic value, identifying a substantial proportion of patients and therapeutically relevant—should be retained. Meanwhile, treatment decisions based on biological parameters are only justified within clinical trials. Patients not included in these trials should continue to be treated based on classical criteria.40
(A) Overall survival of patients with CLL according to Rai stages (Barcelona series). (B) Overall survival of patients with CLL according to Binet stages (Barcelona series).
(A) Overall survival of patients with CLL according to Rai stages (Barcelona series). (B) Overall survival of patients with CLL according to Binet stages (Barcelona series).
Chronic lymphocytic leukemia: stage at diagnosis over the years (Barcelona series).
Chronic lymphocytic leukemia: stage at diagnosis over the years (Barcelona series).
(A) Progression-free survival in 220 patients with CLL according to ZAP-70 expression as detected by flow cytometry. ZAP-70 negative (< 20% of leukemic cells): 140 patients; ZAP-70 positive (> 20% of leukemic cells): 80 patients (Barcelona series; updated). (B) Overall survival in 220 patients with CLL according to ZAP-70 expression as detected by flow cytometry. ZAP-70 negative (< 20% of leukemic cells): 140 patients; ZAP-70 positive (> 20% of leukemic cells): 80 patients (Barcelona series; updated).
(A) Progression-free survival in 220 patients with CLL according to ZAP-70 expression as detected by flow cytometry. ZAP-70 negative (< 20% of leukemic cells): 140 patients; ZAP-70 positive (> 20% of leukemic cells): 80 patients (Barcelona series; updated). (B) Overall survival in 220 patients with CLL according to ZAP-70 expression as detected by flow cytometry. ZAP-70 negative (< 20% of leukemic cells): 140 patients; ZAP-70 positive (> 20% of leukemic cells): 80 patients (Barcelona series; updated).
Institute of Hematology and Oncology, Department of Hematology, Hospital Clínic, IDIBAPS, University of Barcelona
Acknowledgments: Thanks are due to F. Bosch, M. Crespo, E. Gine, C. Moreno, A. Muntañola and N. Villamor (Barcelona) for their continued contributions to CLL studies at our institution, and to H. Döhner and S. Stingelbauer (Ulm) and D. Catovsky (London) for helpful comments and discussion.