Abstract
Peripheral T-cell lymphomas (PTLs) are uncommon, accounting for fewer than 10% of all non-Hodgkin lymphomas. Success in therapy of the PTLs has lagged behind that of aggressive B-cell lymphomas, and most PTLs have a poor prognosis. The molecular pathogenesis of most PTLs is also poorly understood. In the WHO classification, clinical features, in conjunction with morphological and immunophenotypic criteria, are relied on to define most disease entities.
Functionally, T-cell lymphomas are related to the two major arms of the immune system, the innate and adaptive immune systems. NK cells and T cells of the innate immune system recognize antigen in the absence of MHC antigens and are involved in mucosal immunity. The lymphomas derived from these cells often involve cutaneous and mucosal sites. The expression of cytotoxic molecules in these lymphomas may predispose to apoptosis by tumor cells and normal bystander cells. Hepatosplenic T-cell lymphoma is a systemic disease derived from functionally immature innate effector cells, most often of γδ T-cell origin. In contrast, most nodal T-cell lymphomas belong to the adaptive immune system.
Angioimmunoblastic T-cell lymphoma (AILT) is mostly likely derived from follicular helper T-cells (TFH), a finding that explains many of its pathological and clinical features. Studies of these neoplasms may assist in further unraveling the functional diversity of their normal counterparts.
Mature T-cell and NK-cell neoplasms are relatively uncommon, accounting for fewer than 10% of all non-Hodgkin lymphomas (NHL) on a worldwide basis.1 The most common subtypes of mature T-cell lymphomas are peripheral T-cell lymphoma, unspecified (PTLU), and anaplastic large cell lymphoma (ALCL) (Table 1 ).2 T-cell and NK-cell lymphomas show significant variations in incidence in different geographical regions and racial populations. For example, T/NK-cell lymphomas comprise a higher proportion of NHL in Asian populations. These differences result from both a true increased incidence as well as a relative decrease in the frequency of many B-cell lymphomas, such as follicular lymphoma, seen commonly in North America and Europe. Human T-lymphotropic virus-1 (HTLV-1) accounts for an increase of adult T-cell leukemia/lymphoma (ATLL) risk in regions where it is endemic, including southwestern Japan and the Caribbean basin.
Another major factor affecting the incidence of T-cell and NK-cell lymphomas is racial predisposition. Extranodal NK-cell lymphomas, nasal-type and aggressive NK-cell leukemia are much more common in Asians than they are in other races.3 Other groups at increased risk for these Epstein-Barr virus (EBV)–associated diseases are individuals of Native American descent in Central and South America, and Mexico.4 Other rare EBV-positive lymphomas derived from T cells showing a similar racial and geographic distribution include fulminant EBV-positive T-cell lympho-proliferative disorder,5 which has overlapping features with severe chronic active EBV infection,6 and Hydroa vacciniforme–like lymphoma, a form of EBV-positive T-cell or NK-cell lymphoma seen mainly in children.7 Genetic factors linked to defective surveillance of EBV have been postulated to play a role in these epidemiological differences. High viral load at the time of initial viral infection may be an additional risk factor.
γδ PTLs occur with increased frequency in the setting of immune suppression, especially following organ transplantation, a finding that is not well understood.8–10 Overall, the incidence of T-cell and NK-cell malignancies does not appear to be changing, although long-term epidemiological data are not available, as it is only recently that these neoplasms have been reliably distinguished from B-cell lymphomas by using modern immunophenotypic and molecular tools.
Pathophysiology of T-cell Subsets
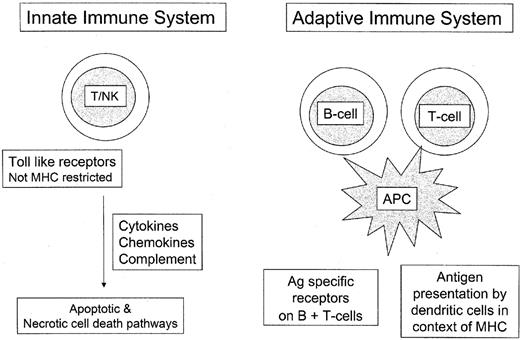
T-cell lymphomas manifest the immunophenotypic features of post-thymic T lymphocytes, being derived from both αβ T cells and γδ T cells.11 This distinction is based on the structure of the T-cell receptor. γδ T cells, along with NK-cells, are components of the innate immune system and do not require antigen sensitization to be active.12,13 The innate immune system is functional based only on genes encoded in the host genome. It is distinguished from the adaptive or antigen-specific immune system; most T cells in peripheral blood and peripheral lymphoid organs belong to the latter (Figure 1 ).
γδ T cells comprise fewer than 5% of all normal T cells and show a restricted distribution, being found mainly in the splenic red pulp, intestinal epithelium, and other epithelial sites. It is notable that these sites are more commonly affected by γδ T cell lymphomas, which otherwise are relatively rare.10,14 γδ T cells are not MHC restricted in their function, and represent a first line of defense against bacterial peptides, such as heat shock proteins.11 They are often involved in responses to mycobacterial infections, and both mucosal and cutaneous immunity.
More recently, the pattern of cytolytic molecules has been investigated and correlated with both cellular origin and function. For example, to date 5 granzymes have been demonstrated in human cells.15 These enzymes are similar in structure but differ in their substrate specificity and chromosomal locations. Granzyme M, a novel member of this family, has unusual enzyme specificity, preferring cleavage after methionine, leucine, or norleucine. It has been suggested that this enzyme may play a role in the effector phase of innate immune responses.16 Its expression is restricted to NK cells, CD3+ CD56+ T cells, and γδ T cells, but it is absent in other cytotoxic T-cell subsets. Therefore, it may serve as a marker of the innate immune system, as distinguished from cytotoxic T cells belonging to the adaptive immune system.12
Cells of the innate immune system represent a first line of defense, a more primitive type of immune response, and play a role in both mucosal and cutaneous immunity. It is interesting that many T-cell and NK-cell lymphomas observed commonly in the pediatric and young adult age group are derived from cells of the innate immune system.17 These include aggressive NK-cell leukemia, fulminant EBV-positive T-cell lymphoproliferative disease, hepatosplenic T-cell lymphoma, and γδ T-cell lymphomas affecting muco-cutaneous sites.12 ALCL is the most common pediatric T-cell lymphoma, and while it is of cyto-toxic origin, is negative for granzyme M, and thus, most likely part of the adaptive immune system.
The T cells of the adaptive immune system are heterogeneous and functionally complex, and include naïve, effector (regulatory and cytotoxic), and memory T cells. CD4+ T cells are primarily regulatory, acting via cytokine production, while CD8+ (and double negative) T cells are primarily cytotoxic. Recently much has been learned about a unique T-cell subset found in the normal germinal center. These cells, termed follicular T-helper cells (TFH), provide help to B cells in the context of the germinal center reaction. They have a unique phenotype, expressing the germinal center–associated markers BCL6 and CD10, normally found on B cells. TFH express CD4 and CD57, and produce the chemokines CXCR5 and CXCR13. CXCL13 causes induction and proliferation of follicular dendritic cells, and is involved in B-cell recruitment to the lymph node, by facilitating the adhesion of T cells to high endothelial venules and allowing them to transit the vessel wall.
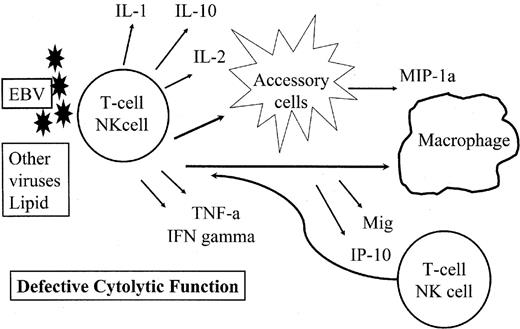
There have been relatively few studies correlating the subclassification of T-cell lymphomas with specific profiles of cytokine or chemokine expression.18 Nevertheless, data are now emerging that can relate the pathological or clinical manifestations of T-cell lymphomas to cytokine or chemokine expression by the neoplastic cells or accompanying accessory cells within the lymph node. For example, the hypercalcemia associated with ATLL has been linked to secretion of factors with osteoclast-activating activity.19 The hemophagocytic syndrome seen in many T-cell and NK-cell malignancies has been associated with secretion of both cytokines and chemokines, in the setting of defective cytolytic function (Figure 2 ).20 And most recently the chemokine CXCL13 has been identified in angioimmunoblastic T-cell lymphomas, and its identification helps to elucidate many of the features of that T-cell lymphoma.21,22
Classification of T-cell and NK-cell Lymphomas
PTLs show great morphological diversity, and a spectrum of histological appearances can be seen within individual disease entities. The cellular composition can range from small cells with minimal atypia to large cells with anaplastic features. Such a spectrum is seen in ALCL, ATLL, and extranodal NK/T-cell lymphoma, as selected examples. However, cytological atypia does not necessarily correlate with clinical behavior. For these reasons, it has been difficult to apply cytological principles to the classification of PTLs. In a similar vein, immunophenotypic markers have been less useful in the classification of T-cell lymphomas than B-cell lymphomas, as often one marker is shared by multiple disease entities. As an example, CD30, a hallmark of ALCL, is found in diverse lymphoid malignancies of T-and B-cell types. Finally, the molecular pathogenesis for most T-cell lymphomas is as yet undiscovered. For the above reasons, clinical features have played a major role in defining many of the specific entities included in the WHO classification.1
Peripheral T-cell lymphoma, unspecified (PTLU) is the most common category of PTL and by definition is heterogeneous. PTLU is the “diffuse large B-cell” equivalent of PTL. This subtype includes all cases not readily classified as one of the specific entities in the WHO classification. Two morphological variants are delineated as T-zone lymphoma and lymphoepithelioid cell lymphoma, but the evidence that these are distinct entities is lacking, and for that reason, the use of morphological variants in diagnosis is optional. As a group, PTLUs are aggressive neoplasms, often present with advanced stage, and are seen mainly in older adults. They are most often nodal, but can present with extranodal disease. They may contain a prominent background of inflammatory cells, be composed of a diverse population of pleomorphic tumor cells, or be monomorphic, resembling diffuse large B-cell lymphomas. For these reasons the diagnosis of PTLU should always be based on confirmatory tests using immunophenotypic or genotypic methods.
Angioimmunoblastic T-cell lymphoma (AILT) has emerged as a distinctive subtype of PTL with unique pathobiological features. This disease is seen mainly in elderly adults, with an equal male:female ratio. Originally thought to be a form of abnormal immune response, most patients present with generalized lymphadenopathy, hepatosplenomegaly, skin rash, and marked constitutional symptoms. Polyclonal hypergammaglobulinemia is an almost constant finding and the lymph nodes usually contain polyclonal plasma cells, as well as frequent large B immunoblasts, despite the absence of well-formed follicles with germinal centers. The neoplastic T cells have clear cytoplasm and are distributed in a marked inflammatory background. Other features include prominent arborizing high endothelial venules (HEV) and expansion of dendritic meshworks outside the follicle, usually arising from the prominent HEV. The neoplastic T cells are CD4+ T cells that express CD10 and sometimes BCL6, features that suggested the neoplastic cells might be derived from germinal center–based T cells (TFH) 23.
Most recently, two groups have identified increased expression of CXCL13 in AILT, a finding that helps to link together many of these clinical and pathological features.21,22 CXCL13 is associated with expansion of follicular dendritic cells and facilitates the entry of B cells into the lymph node through their attachment to the HEV, thus helping to clarify the B-cell expansion characteristic of this disease.
Another almost constant finding in AILT is the presence of EBV-positive B cells. It has been postulated that this finding is secondary to decreased immune surveillance and reactivation of EBV in the setting of a compromised immune system.24 However, EBV-positive B cells are found even very early in the course of the disease. In some cases this phenomenon progresses to an EBV-positive B-cell lymphoproliferative disorder resembling post-transplant polymorphic B-cell lymphoma. In other instances the EBV-positive B cells may resemble Reed-Sternberg cells, leading to an erroneous diagnosis of classical Hodgkin lymphoma.25
Anaplastic large cell lymphoma (ALCL) is the most common single subtype of PTL. It is most often nodal, but can present in a variety of extranodal sites. It is most common in children and young adults, but can present at any age. The WHO provisionally included cases of ALCL positive and negative for the ALCL-associated tyrosinase kinase (ALK) under the heading of ALCL, but ALK-negative cases differ in a number of respects, being seen in an older age group, having a worse prognosis, and generally showing greater nuclear pleomorphism. These findings suggest that ALK-negative ALCL will ultimately represent a different disease entity with an independent pathogenesis.26 The remainder of the text will focus on ALK-positive ALCL. Besides strong expression of CD30, ALCL are positive for cytotoxic markers and interestingly often lack CD3, with inconsistent expression of other T-cell–associated antigens.
Cytologically a number of histological variants have been identified, including the small cell and lymphohistiocytic subtypes. These two are closely related, occur almost entirely in children, and histologically contain a variable component of histiocytes with homogeneous granular eosinophilic cytoplasm. Misdiagnosis as a chronic inflammatory process, or PTLU, is a pitfall in diagnosis. Some ALCL have prominent spindle cells in a myxoid stroma. Because ALCL can present as a soft tissue or bone mass,27 this variant of ALCL may simulate a soft tissue sarcoma or primary bone tumor.26 Hodgkin-like ALCL28 has proven to be an aggressive variant of classical Hodgkin lymphoma in most instances and shows overlap with mediastinal grey zone lymphomas.29 These cases present mostly in young males, with a mediastinal mass; treatment as Hodgkin lymphoma is often inadequate, and at the National Cancer Institute, mediastinal grey zone lymphomas are treated with dose-adjusted EPOCH plus rituximab, comparable to mediastinal large B-cell lymphoma. More infrequent cases of ALK-positive ALCL have a nodular sclerotic pattern resembling nodular sclerosis Hodgkin lymphoma.30
Hepatosplenic T-cell lymphoma presents with marked hepatosplenomegaly in the absence of lymphadenopathy. The great majority of cases are of γδ T-cell origin,10,31 but an αβ origin has been seen in a small subset of cases.32–34 The clinical presentation is very homogeneous, with most cases presenting in young males, 15–30 years of age. Many patients have a history of chronic antigenic stimulation in the setting of immunosuppression, with a number of reports occurring in patients with solid organ transplantation,10,35,36 or less commonly Crohn’s disease.8,37 Although patients may respond initially to chemotherapy, relapse has been seen in the vast majority of cases, and the median survival is less than 3 years.10 Rare long-term survival has been seen following allogeneic bone marrow transplantation.31
The disease is disseminated at presentation, by virtue of marked sinusoidal infiltration of liver, spleen and bone marrow, despite the absence of lymph node involvement. Abnormal cells are usually present in the sinusoids of the bone marrow but may be difficult to identify without immunohistochemical stains. Peripheral blood involvement is usually not seen until late in the course. The neoplastic cells have a phenotype that resembles that of immature or resting γδ T-cells, often double-negative for CD4 and CD8, and negative for CD5; CD56 is also positive.31,38 The cells express the cytotoxic granule-associated protein, T-cell intracellular antigen–1 (TIA-1), but are generally negative for perforin and granzyme B. Isochromosome 7q is a consistent cytogenetic abnormality, usually in association with trisomy 8.39–41
Enteropathy-type T-cell lymphoma (ETTCL) occurs in adults, the majority of whom have a history of gluten-sensitive enteropathy. Patients usually present with abdominal symptoms such as pain, small bowel perforation, and associated peritonitis. The small bowel usually shows ulceration, frequently with perforation. A mass may or may not be present, and the intestinal involvement is often multifocal. The neoplastic cells infiltrate the overlying epithelium, mimicking normal intraepithelial lymphocytes. In refractory coeliac disease and ulcerative jejeunitis the intraepithelial lymphocytes share clonal identity with the subsequent lymphomas developing in these patients.42,43 The clinical course is aggressive, with poor long-term survival.
The neoplastic cells in ETTCL have a wide morphological spectrum. The cells are generally medium to large in size, but in a subset they are markedly anaplastic and strongly CD30+.44 A marked tissue eosinophilia may partially mask the neoplastic population.45 The cells are αβ cytotoxic T cells mimicking the phenotype of IEL. CD56 positivity is seen in a subset of cases. The neoplastic cells are negative for EBV.
ETTCL must be distinguished from other T-cell lymphomas presenting with intestinal disease, and not all intestinal T-cell lymphomas are ETTCL. The intestinal tract is a common site of localization of extranodal NK/T-cell lymphoma, nasal-type, which can be distinguished by its EBV-positivity. Mucocutaneous γδ T-cell lymphomas may also present with intestinal disease and may appear similar both clinically and morphologically.13,46 They, too, are of cytotoxic T-cell derivation and are associated with extensive apoptosis and necrosis.
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) usually presents with multiple subcutaneous nodules of varying size, primarily affecting the extremities.47–49 It shows a broad age distribution and an equal male:female ratio. The neoplastic cells are generally confined to subcutaneous tissue and frequently infiltrate individual fat cells with a rim-like arrangement at the cell border, which is a helpful feature in the differential diagnosis with benign panniculitis. Dermal and epidermal involvement are generally absent, a feature that helps to distinguish SPTCL from mucocutaneous γδ TCL involving subcutaneous tissue.14 Panniculitis-like features may be seen in both, but SPTCL has a better prognosis. In the latest iteration of the WHO classification, published jointly with the EORTC, SPTCL is limited to a cytotoxic tumor of αβ T-cell derivation.50 The neoplastic cells express an activated αβ CD8+ cytotoxic T-cell phenotype. In addition, the cells are positive for the cytotoxic associated proteins, granzyme B, perforin and TIA-1. These proteins mediate cytotoxicity and apoptosis by T cells and NK cells, and therefore may be responsible for the cellular destruction characteristic of these lesions.
A hemophagocytic syndrome (HPS) is a clinically significant complication of SPTCL.47 Patients present with fever, pancytopenia, and hepatosplenomegaly. The HPS is most readily diagnosed in bone marrow aspirate smears, which demonstrate histiocytes containing phagocytosed erythrocytes and platelets. The HPS usually precipitates a fulminant downhill clinical course. However, if therapy for the underlying lymphoma is instituted and is successful, it may remit. Both in SPTCL and in EBV-positive NK-cell and T-cell malignancies, the HPS appears related to cytokine and chemokine production by the malignant cells, perhaps in a setting of comprised cytolytic function.
Cutaneous T-cell Lymphomas
T-cell malignancies presenting with cutaneous involvement include mycosis fungoides (MF), Sézary syndrome (SS), and the primary cutaneous CD30+ T-cell lymphopro-liferative disorders (CD30-TLPD). Dr. Steven Rosen will cover the clinical and prognostic features of these diseases in a companion article. Most cases of MF and SS are derived from CD4+ T cells showing a loss of CD7 and low levels of activation markers such as CD25 and CD30. However, CD8 expression has been reported in some cases of MF that are pathologically and clinically indistinguishable.50 The hallmark of MF and SS is epidermotropism, but in fact well-formed Pautrier microabscesses are seen in only a minority of cases, and in most skin biopsies the diagnosis of early MF rests of other histological and clinical criteria.51
CD30-TLPD is a spectrum of conditions ranging from lymphomatoid papulosis (LYP) to primary cutaneous ALCL (C-ALCL). A common feature in all is a CD30+ CD4+ T cell, which in C-ALCL can be shown to be clonal. In LYP the atypical cells are in the minority and are associated with a marked inflammatory background. Lesions regress spontaneously, and dissemination never occurs. C-ALCL lies at the opposite end of the spectrum; large atypical CD30+ cells predominate, regression often occurs without therapy, and spread to lymph nodes may be seen. However, widespread disease is relatively rare. C-ALCL is consistently negative for ALK although systemic ALCL may present with cutaneous disease.
Overview of innate and adaptive immune systems.
The innate immune system includes defense mechanisms encoded in the germline of the host. It does not require prior sensitization to antigen, and represents a primitive line of defense, closely linked to mucosal or barrier immunity. The adaptive immune system is based on prior sensitization to antigen and antigen presentation by dendritic cells in the context of MHC. Because it acts based on antigen-specific receptors on T cells and B cells, it has greater specificity.
Overview of innate and adaptive immune systems.
The innate immune system includes defense mechanisms encoded in the germline of the host. It does not require prior sensitization to antigen, and represents a primitive line of defense, closely linked to mucosal or barrier immunity. The adaptive immune system is based on prior sensitization to antigen and antigen presentation by dendritic cells in the context of MHC. Because it acts based on antigen-specific receptors on T cells and B cells, it has greater specificity.
Pathogenetic mechanisms of the hemophagocytic syndrome (HPS).
The HPS is precipitated by an immune stimulus, most often Epstein-Barr virus (EBV), in the context of defective cytolytic function. T cells and NK cells are stimulated to produce a variety of cytokines, including interferon-γ. These stimulate accessory cells, which in turn produce chemokines leading to further stimulation and activation of macrophages and T and NK cells. In the setting of a defective cytolytic response, the cycle of stimulation continues uninterrupted.
Pathogenetic mechanisms of the hemophagocytic syndrome (HPS).
The HPS is precipitated by an immune stimulus, most often Epstein-Barr virus (EBV), in the context of defective cytolytic function. T cells and NK cells are stimulated to produce a variety of cytokines, including interferon-γ. These stimulate accessory cells, which in turn produce chemokines leading to further stimulation and activation of macrophages and T and NK cells. In the setting of a defective cytolytic response, the cycle of stimulation continues uninterrupted.
Chief, Hematopathology Section, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, Bethesda, MD 20892