Abstract
Viral infections are important causes of morbidity and mortality for patients with a hematological malignancy. However, the true incidence and consequences of viral infections for these patients who undergo conventional nontransplant therapy are poorly defined. The difference in incidence and outcome of viral infections among patient groups is wide, but dependent upon the intensity and duration of T-cell–mediated immune suppression. Infections caused by cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus (VZV), respiratory syncytial virus (RSV), parainfluenza viruses and influenza viruses have been intensely studied, yet newly recognized aspects of these viral infections including late CMV infection; the emergence of new viral pathogens (human herpesvirus-6, BK virus, adenovirus, and human metapneumovirus); the development of molecular diagnostic techniques, and the potential of new agents for viral prophylaxis (maribavir), or preemptive therapy (valganciclovir) form the basis of this review. Well-designed prospective studies are needed to better clarify the spectrum of these viral infections and develop effective prevention and treatment strategies. Yet the increased use of agents like alemtuzumab that induce profound T-cell depletion demands that we develop a better understanding of viral infections that occur in patients with hematological malignancy who receive nontransplant therapy.
Viral infections are important causes of morbidity and mortality for patients with a hematological malignancy, but the true incidence and consequences of viral infections for these patients who undergo conventional nontransplant therapy are inadequately defined.1,2 Differences in incidence and outcome of viral infections among patient groups are based on the intensity and duration of T-cell–mediated immune suppression, but even allogeneic transplant recipients experience immune dysfunction that varies based on stem cell product, donor-recipient matching, composition of the conditioning regimen, and the occurrence and severity of graft-versus-host disease (GVHD).1
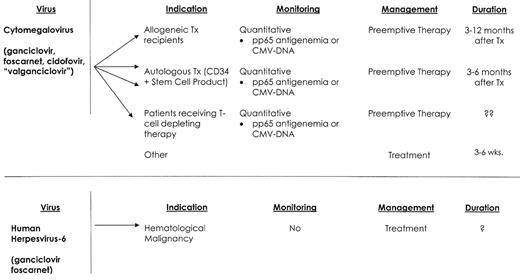
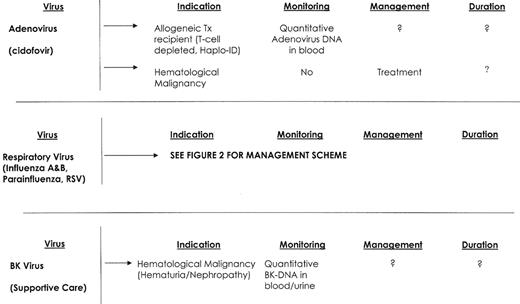
Infections caused by cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus (VZV), respiratory syncytial virus (RSV), parainfluenza viruses and influenza viruses are well recognized.1,3,4 Yet newly recognized aspects of these infections, the emergence of new viral pathogens (human herpesvirus-6, BK virus, adenovirus, and human metapneumovirus),5 and the development of new diagnostic techniques and therapy support the need for this review (Figure 1 ).
Herpesviruses
The group of herpesviruses consists of eight members. Primary and reactivation infections are characteristic of these pathogens. Viral latency can be predicted by serological screening and is useful for disease management. Antiviral therapy is now routinely used for prevention and therapy.1,3,4 Currently available drugs include acyclovir and its prodrug valacyclovir; penciclovir and its prodrug famciclovir; ganciclovir and its prodrug valganciclovir; foscarnet; and cidofovir. Maribavir is a new agent being tested. Viral immunization remains investigational, except for the varicella-virus vaccination.
Herpes simplex virus
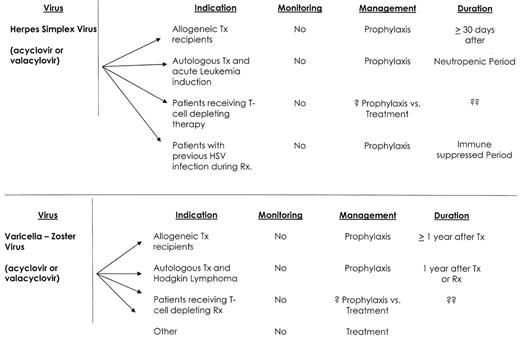
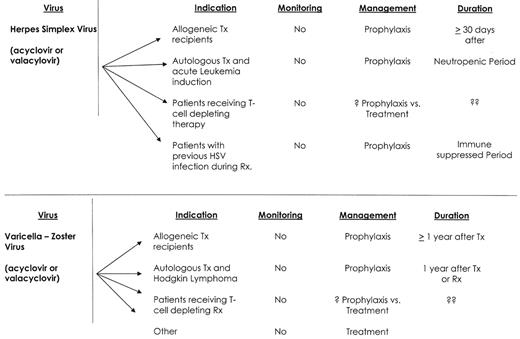
HSV infections in patients with hematological malignancy are almost exclusively reactivation infections.1,3,4 They are common, ranging from 15% among CLL patients treated with fludarabine, to 90% of patients with acute leukemia or stem cell transplant recipients.1,3,4,6 HSV infection and disease occur early after therapy, and frequently recur with future treatment. Mucocutaneous HSV disease will frequently present with an atypical appearance and can mimic other pathogens (i.e., Candida) or treatment-induced mucositis. HSV infections among immunocompromised patients are characteristically more invasive, heal more slowly, are associated with prolonged viral shedding, and may disseminate.
Treatment and prophylaxis of HSV infections can be administered either orally or intravenously, and acyclovir resistance has remained relatively infrequent.1,3,4,7 Treatment for acyclovir resistance is foscarnet, but resistance to foscarnet also occurs. Cidofovir is the only available treatment for double-resistant HSV isolates, but HSV reactivation may occur despite cidofovir treatment. HSV prophylaxis with acyclovir is highly efficacious, but the duration of HSV prophylaxis must be individualized. I recommend that antiviral prophylaxis be continued during the period of neutropenia, and longer for patients with severe immunosuppression, active GVHD or a history of frequent HSV reactivations.
Varicella-Zoster Virus
The clinical manifestations of VZV infections include chickenpox and herpes zoster (shingles). Chickenpox results from a primary VZV infection, while herpes zoster is due to viral reactivation of latent VZV. The incidence of varicella-zoster infection ranges from 2% among patients with CML receiving imatinib mesylate; to 10–15% in patients with CLL receiving fludarabine or alemtuzumab; to 25% of patients with Hodgkin lymphoma or autologous stem cell transplant recipients; and to 45–60% among allogeneic stem cell transplant recipients.1,3,4,6,8,9 Infection risk is greatest within the first 12 months following treatment or transplant, but late-onset disease occurs because of persistent immunosuppression or age-associated immune senescence.8 The majority of VZV infections in adult patients with a hematological malignancy are reactivation infections and 80% present with localized disease.8 Patients who are VZV naïve are at risk for primary infection with either wild type or vaccine strains and should be counseled about the risk of developing such an infection. Primary VZV infection can be very severe, and measures to prevent exposure and intervene early are recommended.10
Unusual VZV syndromes of importance include trigeminal zoster with keratitis and retinal necrosis; encephalitis; Ramsey-Hunt syndrome; secondary bacterial and yeast infections; and post-zoster pain.8 Hepatic or gastrointestinal VZV disease is an important entity, and may present with few or no skin lesions. This presentation may result in delayed diagnosis, and has been associated with significant mortality.11
Treatment of VZV disease should include the early institution of antiviral therapy (valacyclovir, acyclovir or famciclovir).1,3,4,8 There is no evidence that intravenous immunoglobulin or corticosteroids add benefit in the treatment of VZV disease in immunocompromised patients. Prevention of VZV reactivation among patients with hematological malignancy remains controversial. Acyclovir and valacyclovir are highly effective.1,3,4 Yet, despite its efficacy in preventing VZV disease, antiviral prophylaxis is not routinely recommended by many of the clinical care guidelines.1,4 The reluctance to recommend routine prophylaxis appears based on observations that VZV disease may still occur after the discontinuation of VZV prophylaxis. A recently published study using oral acyclovir showed that late VZV infection is likely to be caused by persistent immunosuppression, and that acyclovir prophylaxis does not to predispose to late VZV disease.12 I routinely prescribe antiviral prophylaxis (valacyclovir 500 mg BID) for the first year after stem cell transplantation, and also for patients with Hodgkin lymphoma therapy or those who receive intensive therapy or alemtuzumab.
Cytomegalovirus
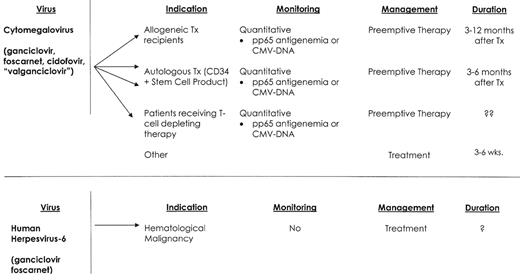
CMV disease manifestations include pneumonia, enteritis, encephalitis, retinitis, hepatitis, cholangitis, cystitis, nephritis, sinusitis and marrow suppression.13–16 T-cell function is paramount in the control of CMV, and T-cell depleting agents (e.g., alemtuzumab) and aggressive chemotherapy (e.g., hyper-CVAD, and acute leukemia induction) appear to increase the risk of CMV infection and disease. In the absence of effective antiviral prophylaxis, the incidence of CMV infection among patients with hematological malignancy ranges from 5–75%.17 Nonmyeloablative transplant-conditioning regimens do not appear to significantly decrease the cumulative incidence of CMV infection.18 Patients undergoing an autologous stem cell transplant have a low risk of CMV infection, but CD34 selection of the autologous stem cell product increases the risk of CMV disease and CMV-associated death.19 The incidence of CMV infection and disease is less clearly defined for patients with hematological malignancies who receive conventional therapy. Investigators at the MD Anderson Cancer Center have reported a series of retrospective studies on the incidence of CMV disease among patients receiving conventional therapy.20–22 These investigators reported an overall increase in CMV gastrointestinal disease during the past two decades, and CMV pneumonia was also increased among patients with lymphoma and acute leukemia. CMV-attributable mortality for these patients ranged from 30% to 57%. An early prospective surveillance study from the University of Maryland Cancer Center reported an incidence of CMV infection in patients with acute leukemia that ranged from 32% to 58%.2 CMV-associated death occurred in 8/130 patients studied. CMV disease in these studies was associated with the use of high-dose cytarabine, fludarabine, or high-dose cyclophosphamide, and increased patient age. More recently CMV infections have been observed among patients who receive alemtuzumab.15,16 Nguyen et al reported CMV viremia in 5/34 (15%) such patients.15 Viremia developed a median of 28 days after starting therapy, and all patients experienced fever, but none of these patients developed CMV disease. A similar incidence of CMV infection was reported for patients with lymphoid malignancies who were treated with alemtuzumab and rituximab.16 CMV antigenemia occurred among 13/48 (27%) patients. Nine patients received anti-CMV therapy, and no patient died as a consequence of this infection.
Diagnosis of CMV infection and disease has been enhanced with the development of techniques for rapid culture, CMV antigen assays and PCR-based molecular tests.23 Treatment consists of ganciclovir, foscarnet and/or cidofovir.1,3,24 Concomitant IVIG appears helpful only when patients suffer from CMV interstitial pneumonia.1 Ganciclovir resistance among CMV isolates is well recognized, and isolates harbor either the more common UL 97 (phospho-transferase) gene mutation or a mutation of the UL 54 (DNA polymerase) gene.25 Treatment with foscarnet or cidofovir is recommended for infections caused by ganciclovir-resistant CMV isolates. CMV-negative or leukocyte-depleted blood products are effective CMV prevention for CMV-seronegative patients.1,17,24 Prophylaxis of infection or early preemptive intervention remains the foundation of effective CMV infection management for seropositive patients.17 Both of these approaches have significantly lowered the risk of early mortality from CMV disease, but CMV disease continues to impact patient survival. Two possible reasons for this lack of overall success is the occurrence of late cytomegalovirus infection and disease, and inadequate CMV prophylaxis for patients with latent CMV infection.
Late CMV infection after stem cell transplant is common (3–17% of allogeneic transplant recipients) and is associated with a 13-fold increase in post-transplant mortality.26 The primary risk factor for late CMV infection is CMV-specific T-cell dysfunction.27 Surrogate markers for this immunosuppression include active GVHD, high-dose steroid therapy, low CD4 cell count, and previous treatment for CMV infection or with donor lymphocyte infusions.26 Late CMV disease can have a varied presentation, with retinitis, sinusitis, encephalitis and marrow failure being more common than in early CMV disease. Treatment of late CMV infection relies on CMV surveillance (3–12 months after transplant or longer in patients with chronic GVHD) and preemptive therapy with ganciclovir. Oral treatment alternatives are needed, but despite studies showing favorable drug bioavailability, there are no published randomized trials using oral valganciclovir as preemptive treatment.28,29
CMV infection prophylaxis remains an attractive option for both early and late CMV infection, but early approaches were associated with increased treatment associated toxicity.30,31 Preliminary results from a randomized, phase II trial of maribavir suggest that infection and disease prophylaxis maybe possible with this agent (personal communication, Stephen A. Villano, MD, Viro Pharma Inc.). Maribavir is a selective UL 97 viral protein-kinase inhibitor and, unlike other currently available anti-CMV agents, does not inhibit CMV DNA polymerase. The early analysis of 111 patients treated after engraftment with maribavir or placebo showed that maribavir decreased the incidence of CMV infection and disease. Maribavir toxicity was mild and did not appear to affect marrow function adversely. However, additional studies are needed to truly clarify the utility of this new anti-CMV agent.
Human Herpesvirus–6
HHV-6 is a ubiquitous herpesvirus that infects most persons early in life.32 Two major viral variants have been identified (A and B), but the B variant is most frequently associated with disease among immunocompromised patients. Longitudinal studies in stem cell transplant recipients found that viral reactivation occurred a median of 20 days after transplantation, and that viral shedding for some patients was prolonged, and correlated poorly with clinical improvement.33 A clinical syndrome consisting of CNS dysfunction, impaired memory, secondary (hypothalamic) hypothyroidism, and delayed platelet engraftment are common disease manifestations.33,34 HHV-6 viremia among allogeneic transplant recipients is associated with an increase in all-cause mortality, and viremia appears to be increased when patients are transplanted for disease other than first remission, when donor and recipient are sex mismatched, and among younger patients.33 We have recently diagnosed and treated 4 patients for HHV-6 viremia who developed CNS dysfunction and delayed platelet recovery. Three of these patients had undergone an autologous transplant for myeloma following melphalan conditioning, and the 4th patient had been treated with hyper-CVAD and imatinib for relapsed ALL. All 4 responded to prolonged antiviral therapy.
Quantitative real-time PCR analysis on blood and cerebrospinal fluid is the method of choice for diagnosis.33 Foscarnet and ganciclovir, alone and or in combination, have been used as treatment for HHV-6 infections. Prospective studies are needed to better understand the importance of HHV-6 infection among patients with hematological malignancy, and to define disease spectrum, and appropriate therapy.
Adenovirus
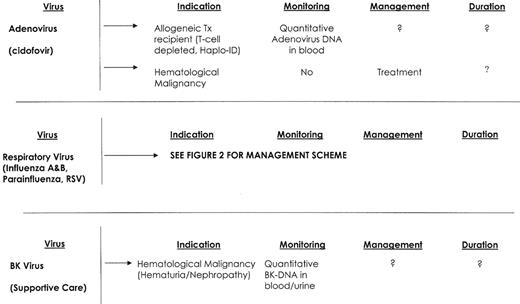
Adenovirus is a DNA virus categorized by 51 human sero-types. Primary infection is acquired from either a respiratory droplet or the oral-fecal route. Most infections among compromised hosts are postulated to be viral reactivation.35 Clinical manifestations vary with serotype, and include viremia, pneumonia, hepatitis, gastrointestinal disease, cystitis, nephritis and conjunctivitis.36 Control of adenovirus appears T-cell mediated, and allogeneic stem cell transplant recipients appear to be at greatest risk of infection and disease.36,37 However, fatal adenovirus infections have been reported in patients with B-cell lymphoma, multiple myeloma and acute myeloid leukemia.38,39 Risk factors for infection and disease include unrelated donor transplantation, GVHD, T-cell depletion, younger patient age, total body irradiation, and viremia.37 The incidence of infection in stem cell recipients has been reported to range from 5% to 29%, with disease occurring in 5–8% of patients. Death secondary to adenovirus disease has been reported to range from 30% to 50%.37,40
Standard detection methods include cell culture, shell vial assays and direct antigen detection. Quantitative PCR assays are now suitable for detection of adenovirus in blood.40 There are no controlled treatment trials for adenovirus infection in immunocompromised patients, but cidofovir has shown promise for the management of clinical disease.41 The availability of techniques for molecular monitoring of adenovirus in blood suggests that preemptive treatment of adenovirus disease is potentially possible.
BK Virus
BK virus is a ubiquitous, DNA polyoma virus that is believed to cause nephropathy and graft loss among renal transplant recipients and also may cause pneumonia.5 There is increasing evidence that BK virus plays an important role in some cases of hemorrhagic cystitis and renal impairment in patients with hematological malignancy, but viral tissue invasion has only recently been demonstrated.42–44 BK viruria is reported to occur in up to 95% of stem cell transplantation recipients, with the onset of viral shedding occurring a median of 41 days after transplant. BK viruria maybe prolonged, can be severe and some patients remain symptomatic for more than 1 month.
Diagnostic tests have been developed, and a highly sensitive quantitative PCR assay for BK virus detection in blood and urine is now available. In a case-control study of hemorrhagic cystitis, BK plasma levels greater than 10,000 copies/mL were highly associated with post-engraftment BK-associated hemorrhagic cystitis among stem cell transplant recipients.43 Treatment of hemorrhagic cystitis is supportive and should be supplemented with hyperhydration and platelet support for patients who are thrombocytopenic. Refractory hemorrhagic cystitis is rare but may be catastrophic. Antiviral therapy for BK virus remains inadequate. Cidofovir has in vitro BK virus activity and some reported clinical activity for renal transplant recipients. Leflunomide, an immunosuppressive agent used to treat rheumatoid arthritis, has been reported to be active against BK virus.45 A recent case report suggests the potential efficacy of bladder-instilled cidofovir when used in allogeneic stem cell transplant recipients with refractory hemorrhagic cystitis.46
Respiratory Viruses
Respiratory viruses, including RSV, parainfluenza virus and Influenza virus A and B, are widespread in the community and easily transmitted to patients with a hematological malignancy.47,48 Infections are spread by air-borne droplets and contact with the hands of infected persons. Infection control measures are critical and should consist of meticulous hand washing, annual influenza vaccination, early infection detection, and both respiratory and contact isolation of infected health care workers and patients. Patients who develop respiratory virus infections prior to the initiation of treatment or transplantation, should if possible, have their therapy delayed.49
RSV and influenza are primarily winter viruses. Parainfluenza virus infections are most prevalent during the summer. The clinical syndromes of these viruses range from the common cold, to sinusitis, pharyngitis, tracheo-bronchitis, bronchiolitis and pneumonia. Respiratory virus infections among patients with hematological malignancy are associated with a more prolonged infection (i.e., RSV shedding may exceed 100 days versus 21 days for immunocompetent children); a higher frequency of noso-comial acquisition (55–83% of exposed immunocompromised patients will become infected), and a higher rate of pneumonia, co-pathogens and death (Table 1 ). The risk of death from a respiratory viral pneumonia has ranged from 9% to 82%, and appears to vary little between the different groups of patients with active hematological malignancy.48,49
Rapid diagnosis is made by viral antigen or nucleic acid detection. An RT-PCR assay that detects RNA from RSV, Influenza A and B, and parainfluenza viruses from nasal wash and nasopharyngeal specimens is highly sensitive and readily available. However, negative tests must be repeated if the patient’s symptoms persist. A scheme for the management of patients with hematological malignancy who develop respiratory virus infection symptoms is included (Figure 2 ). Neuraminidase inhibitors such as oseltamivir appear helpful for both Influenza A and B, but more prolonged treatment may be needed for stem cell transplant recipients and those who develop pneumonia. RSV pneumonia is treated with aerosolized ribavirin, with or without palivizumab.48 Management of patients with non-pneumonic RSV infections is unclear, but I routinely treat such patients who are severely immunocompromised with 7–10 days of TID aerosolized ribavirin. While there continues to be debate regarding the utility of the influenza vaccination in patients with hematological malignancy, my approach is to annually vaccinate all such patients and their household contacts.
Human Metapneumovirus (hMPV)
hMPV is a newly discovered RNA paramyxovirus. Most children by age 5 years are seropositive. This virus infection occurs primarily during winter months and can manifest as both upper and lower respiratory tract disease.50 More serious disease has been reported with hMPV infection among immunosuppressed patients. Results from a recent retrospective study suggested that hMPV infection could be an important cause of idiopathic pneumonia syndrome after stem cell transplantation.51 Five patients among 200 tested had hMPV detected in archived bronchial alveolar lavage specimens. All 5 positive patients had upper respiratory tract prodromes that preceded their pneumonia, and 4 of 5 patients died. Lung tissue obtained at autopsy from the 4 patients who died had histologic changes consistent with idiopathic pneumonia syndrome.
Prospective studies of the role of hMPV as a cause of infection for patients with hematological malignancy are needed. There is no established treatment for hMPV infections although ribavirin appears to have antiviral activity.
Summary
The spectrum of viral infections for patients with hematological malignancy is expanding and diagnosis has increased because of new molecular diagnostic techniques. Well-designed prospective studies are needed to better clarify the spectrum of viral infection, risk factors for disease, and define effective prevention and treatment strategies. Clinical management guidelines for patients receiving conventional T-cell–depleting therapy with agents like alemtuzumab will need to be developed if the maximum benefit from these agents is to be achieved.
Viral infections—hematological malignancy.
Abbreviations: TX, transplant; RX, therapy
Viral infections—hematological malignancy.
Abbreviations: TX, transplant; RX, therapy
Respiratory viruses (including respiratory syncytial virus [RSV]) management approach: respiratory virus treatment flow chart.
* Molecular detection or shell vial culture
** Oseltamivir or aerosolized ribavirin + IVIg may be beneficial
***Aerosolized ribavirin (2 g over 3 hrs, TID × 7 d) may be indicated in NPT(+) for RSV
Abbreviations: NPT, nasopharyngeal wash or swab; CXR, chest radiograph; BAL, bronchial alveolar lavage
Respiratory viruses (including respiratory syncytial virus [RSV]) management approach: respiratory virus treatment flow chart.
* Molecular detection or shell vial culture
** Oseltamivir or aerosolized ribavirin + IVIg may be beneficial
***Aerosolized ribavirin (2 g over 3 hrs, TID × 7 d) may be indicated in NPT(+) for RSV
Abbreviations: NPT, nasopharyngeal wash or swab; CXR, chest radiograph; BAL, bronchial alveolar lavage
Mariette C. and Philip W. Orth/Tom Anderson Professor of Oncology, Professor of Medicine, and Chief of the Division of Neoplastic Diseases and Related Disorders, Medical College of Wisconsin, Milwaukee, Wisconsin

![Figure 2. Respiratory viruses (including respiratory syncytial virus [RSV]) management approach: respiratory virus treatment flow chart. / * Molecular detection or shell vial culture. / ** Oseltamivir or aerosolized ribavirin + IVIg may be beneficial. / ***Aerosolized ribavirin (2 g over 3 hrs, TID × 7 d) may be indicated in NPT(+) for RSV. / Abbreviations: NPT, nasopharyngeal wash or swab; CXR, chest radiograph; BAL, bronchial alveolar lavage](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2006/1/10.1182_asheducation-2006.1.368/5/m_wade_fig2.jpeg?Expires=1768080856&Signature=AKxAQHMaHuIMjlIWMEhr6H7o3gO09vM6zbKCZLi9HnRkZnc81EM5m9voG3H2uVKjmnSun8cNAtBkwlnqFlvIvMPUG09FnSvSSynBx~zxgWVz-uZfBMEm2V-ME04681WD1p93zIRIMKzTL2aix4YRrKjFybxj90Vpf0S1mlNlIoeInDAkFljBcSdszf~oxysAjl~ocQlXoWcsSq3YCehjmVXqiBlZXa0sGC4aGZaWXuUNF8UOEJZd3YA186IXuieQJU~uK2A0yZ8fhmnRJLe8OnqjpTgDOMVOEoPyErTPxkVcTuZ~vkqXkIEs-FRuK1aazKsiLF4gCCjJMqey4q9fzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Respiratory viruses (including respiratory syncytial virus [RSV]) management approach: respiratory virus treatment flow chart. / * Molecular detection or shell vial culture. / ** Oseltamivir or aerosolized ribavirin + IVIg may be beneficial. / ***Aerosolized ribavirin (2 g over 3 hrs, TID × 7 d) may be indicated in NPT(+) for RSV. / Abbreviations: NPT, nasopharyngeal wash or swab; CXR, chest radiograph; BAL, bronchial alveolar lavage](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2006/1/10.1182_asheducation-2006.1.368/5/m_wade_fig2.jpeg?Expires=1768080857&Signature=Z6q2BpK1YWgZ4J4olwGhZ7J6W6guXc9cHMy488QHH5kZ8~v0EWcgeG7jdW6D6dhpUwf9VMd-u~dkVST5-qzQWx2bTIlngnl4sOCJAzlhS8MJMypmUpzLiygrXV8DuxF1JovJ8IQ2Fq53YQbVOLTqKkP6Rncr64PVG3J4D1usUc2XAFr0c3mmnNW3uQog-XhEkKBM81T3JFkkwbPzt4Jr3Pgrgup4yOqjdw~oXl11Ie1O5ncXxkpTj5ElvoTFIDfsNrcjoJ25lEWXAwZi~syroLbCUBpsUtIHDSJJ8X0tbFkFedDowIDJ4XMNt6yvi9FoqaDiyPXRUtSMalmOCdDhVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)