Abstract
The management of adults presenting with aplastic anemia (AA) requires careful exclusion of other causes of bone marrow failure. Late-onset inherited forms of AA may present in adulthood with subclinical disease. Recent long-term studies of HLA identical sibling donor BMT show excellent survival for patients under the age of 40 years, but chronic graft-versus-host disease (GVHD) is still a major problem, impacting on quality of life. Recent improvements in outcome after matched unrelated donor BMT may reflect better donor matching and use of reduced intensity conditioning regimens. For patients treated with immunosuppressive therapy (IST), antithymocyte globulin (ATG) and cyclosporin (CSA) remain the standard regimen with excellent overall survival but less impressive failure-free survival due to nonresponse, relapse and later clonal disorders. The benefit of adding granulocyte colony-stimulating factor (G-CSF) to ATG and CSA is unclear and being assessed in a further prospective European study. Patients who are refractory to conventional IST and currently ineligible for BMT represent difficult management problems. For these patients, new approaches to transplantation are being evaluated, such as fludarabine-based conditioning regimens and the potential use of double umbilical cord blood transplants, but there is a need for new immunosuppressive agents. Improved supportive care is likely to be a major factor in improved outcome of all AA patients whether treated with IST or BMT. Robust predictive factors for response to IST are needed to help in decision making at diagnosis and to help justify exploring novel approaches to therapy.
1. Defining Aplastic Anemia
Essential prerequisites to planning therapeutic strategies for adults with aplastic anemia (AA) are firstly, confirmation of the diagnosis and, secondly, defining the disease in terms of (a) whether the disease is acquired or constitutional, (b) etiology, (c) presence and size of co-existing abnormal clones, and (d) disease severity (see Table 1 ).
Confirming the diagnosis
The diagnosis of AA is in most cases straightforward, but other conditions can sometimes present with pancytopenia and a hypocellular bone marrow and mimic AA, particularly hypocellular myelodysplastic syndrome (MDS) (see Table 1 ). Good quality blood and marrow slides and a good length marrow biopsy are essential. Often repeat bone marrow examination is required to confirm the diagnosis when there is doubt on initial bone marrow examination, or if an inadequate sample was taken.1
Excluding an inherited bone marrow failure syndrome
A high index of suspicion for late onset inherited forms of AA is needed. Failure to diagnose an inherited bone marrow failure syndrome may lead to (a) inappropriate treatment with immunosuppressive therapy (IST), (b) delay in planning bone marrow transplantation (BMT), and (c) inappropriate conditioning for BMT which would result in life threatening complications and death. A careful family history should be taken for evidence of similarly affected members and any evidence of blood diseases or abnormalities of blood counts sought from extended family members. Somatic anomalies are absent in about 30% of Fanconi anemia patients and the clinical course is variable, resulting sometimes in delay in diagnosis until later life. Late diagnosis of Fanconi anemia can occur, and because of this, young adults with AA, MDS/acute myeloid leukemia (AML) and epithelial malignancies should be considered to have Fanconi anemia and should be examined for subtle phenotypic markers of the disease.2,3 A diagnosis of dyskeratosis congenita may be apparent when typical clinical features of nail dystrophy, reticulate skin pigmentation and leukoplakia are present. But TERC and TERT gene mutations can be found in about 5% of adult patients with apparent acquired AA who lack the above clinical abnormalities and who typically show a lack or poor response to IST (‘cryptic dyskeratosis congenita’).4,5 Rarely, patients with Shwachman-Diamond syndrome may present in adulthood with AA. Short stature, metaphyseal dysarthrosis, previous neutropenia, pancreatic exocrine insufficiency or a family history of cytopenias may be present.6 Screening for Shwachman-Bodian-Diamond syndrome gene mutations may detect cases missed in childhood.
Looking for associated abnormal clones
All patients should be screened for abnormal cytogenetic clones, which can be detected in at least 10% of patients at time of diagnosis, in the absence of morphological features of MDS or AML. There is controversy as to whether this represents AA or hypocellular MDS. A recent study from the UK found an abnormal cytogenetic clone in 12% in newly diagnosed adult AA patients.7 A trisomy (+6, +8 or +15) was the most common abnormality and the clone was usually small in size. For patients with a trisomy, the response to IST, the durability of response, and risk of later clonal disease were similar to those of AA patients lacking a cytogenetic clone, whereas non-numerical karyotypic abnormalities responded poorly to IST. The finding of monosomy 7, in particular, should alert to the likelihood of MDS. Larger multicenter studies are needed to further clarify the prognostic significance of individual cytogenetic clones in the setting of AA. From a practical point of view, treatment with chemotherapy should be avoided; otherwise, irreversible marrow damage is likely. Flow cytometric screening for paroxysmal nocturnal hemoglobinuria (PNH) clones should be performed at diagnosis. The Ham test and sucrose lysis test have been superceded by flow cytometry, which can examine with sensitivity the size of the clone by expression of GPI-anchored proteins on red cells, neutrophils and monocytes.8 Small PNH clones can be detected in many AA patients (the percentage of patients affected depending on the sensitivity of the flow cytometric analysis used) and may help in predicting response to IST.9
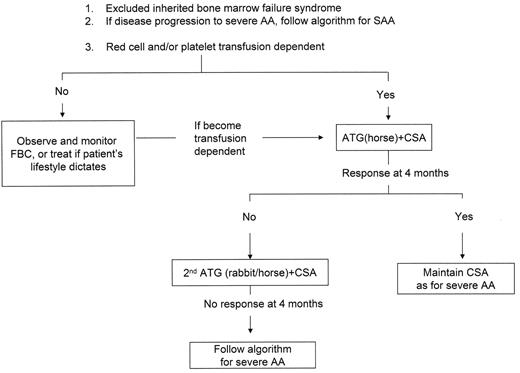
Defining the disease severity
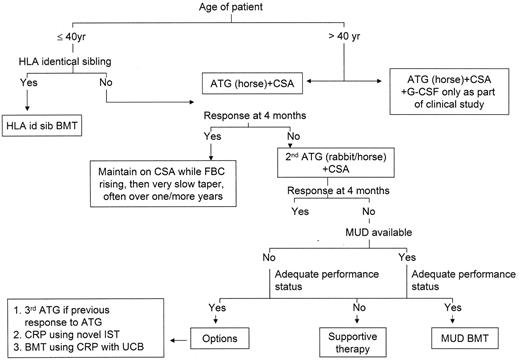
2. Algorithms for Treatment of Acquired Aplastic Anemia
3. HLA Identical Sibling BMT
Very few prospective studies have been performed for BMT in AA and most data come from retrospective European (EBMT) or International (CIBMTR) registries, or single or combined center studies. For severe AA, early ascertainment of whether sibling BMT is an option is important. If eligible for sibling BMT, patients should not be pretreated with IST, unless there are medical or personal reasons not to proceed to transplant. The current most widely used conditioning regimen for HLA-identical sibling BMT is cyclophosphamide (CY) 200 mg/kg and ATG with CSA and methotrexate as GVHD prophylaxis, although the benefit of adding ATG has yet to be proven in a randomized study.10 This is a nonmyeloablative regimen, as patients may have autologous reconstitution after failed transplant, including unrelated donor transplant, and a similar CY dose given without stem cell support can induce complete response in some AA patients.11 Long-term overall survival is 80–90%,12–14 although important differences in survival exist according to patient age. However, critical barriers (in particular, chronic GVHD) to successful outcome remain.
Graft rejection is still a problem for 5–15% of patients. Heavily transfused patients are particularly at risk, emphasizing the importance of early transplant before patients become sensitized to HLA and non-HLA antigens from multiple transfusions. Granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC) have been used in many centers in an attempt to increase the stem cell dose and to accelerate neutrophil and platelet recovery as well as in the hope of reducing graft rejection. However, a preliminary analysis of the retrospective, combined CIBMTR/EBMT study comparing PBSC with bone marrow as the source of stem cells for transplantation in AA, showed no reduction in graft rejection, more chronic GVHD and worse outcome using PBSC.15 Thus, bone marrow remains the recommended source of stem cells for transplantation. The addition of fludarabine to a reduced dose of CY with ATG is being evaluated as a new approach to overcoming graft rejection, particularly in sensitized patients. Good engraftment rates have been reported from NIH in heavily sensitized patients, although a high incidence of acute and chronic GVHD was noted.16 The use of fludarabine-based regimens warrants further evaluation in larger multicenter studies, and may also be more appropriate for older patients. In patients transplanted for AA, fertility is well preserved using high-dose CY and avoiding irradiation. However, more data on fertility are needed for fludarabine-based conditioning regimens.
The incidence and severity of acute GVHD has decreased with the introduction of CSA and avoidance of irradiation. However, chronic GVHD remains a problem, occurring in 25–40% of patients and contributing to morbidity and mortality.12,15,17 In a recent study from the Saint Louis Hospital group, comparing their previous regimen of CY and total abdominal irradiation with CY/ATG, chronic GVHD occurred in 40% of patients and was extensive in 80% of those affected.13 Risk factors for chronic GVHD are acute GVHD, irradiation and increasing age. The Seattle study also reported the unexpected finding that a marrow cell dose of > 3.4 × 108 nucleated cells/kg was also a risk factor for chronic GVHD, but data on CD34 cell doses were not available.12 In contrast, the use of anti-CD52 (Campath-1G and more recently Campath-1H, alemtuzumab) monoclonal antibodies results in a low incidence of acute GVHD (14%) and virtually abolishes chronic GVHD (4%). Although the overall graft rejection rate was 24%, using Campath pre-marrow infusion instead of both pre- and post-infusion, reduced rejection to 16% in a group of heavily sensitized patients.18
Mortality increases with increasing age. The survival rate of patients older than 40 years is only 50%; hence, the general recommendation to consider BMT as first-line therapy only for patients < 40 years of age.14 The decision to proceed to BMT also depends on the performance status of the patient and patient choice. Conditioning regimens using fludarabine with low-dose CY (1200 mg/m2) and ATG, similar to the EBMT regimen currently being used for matched unrelated donor (MUD) BMT,19 are more appropriate for older patients (> 40 years). Other predictors of worse outcome are prior treatment with ATG or CSA or androgens, prolonged duration (> 1 year) from diagnosis to BMT, and the use of irradiation.13 Other factors likely to have contributed to improved outcome with time are improved supply and quality of blood products, better antibiotics and antifungal and antiviral agents. Although impossible to prove by statistical significance, the impact on improved survival is most probably major. Irradiation should also be avoided because of the associated long-term complications of infertility and second malignancies.
For those adult patients with apparent acquired AA and TERC or TERT mutations (subclinical DC), it is likely that BMT will provide the only chance of cure of the AA. A reduced-intensity conditioning regimen may help minimize the risk of late complications seen in patients with the clinical syndrome of DC who undergo BMT.20,21
4. Matched Unrelated Donor BMT
The recent CIBMTR retrospective study of patients with severe AA transplanted between 1988 and 1998 (see Table 2 ) highlighted the poor outcome (39% survival at 5 years) and high rates of graft rejection, GVHD and infection after MUD BMT.22 There was also no improvement in outcome with time. In this study, HLA matching was based on low resolution DNA typing for HLA-A, -B, and -DR loci. A Japanese study showed better survival (60%) for HLA-A, -B, -DRB1 MUD transplants, by using high-resolution DNA typing techniques.23
Recent attempts to reduce graft rejection and improve survival include the use of low-dose total body irradiation or a non-irradiation, fludarabine-based regimen (Table 2 ). In a prospective multicenter study, Deeg and colleagues reported a low graft rejection rate of 5% (2% for matched and 11% for mis-matched donors) among 87 patients transplanted from unrelated donors, using a de-escalating dose of total-body irradiation in most patients. However, pulmonary toxicity was observed and GVHD remained a problem.24 In contrast, a study from EBMT using fludarabine, low-dose CY (1200 mg/m2) and ATG reported a higher graft rejection rate (18%), particularly among older patients, but a lower rate of acute and chronic GVHD.19 Other approaches have been to use alemtuzumab instead of ATG in an otherwise similar regimen to EBMT,25 or G-CSF–mobilized CD34+-selected PBSC.26 However, both these latter approaches require further evaluation in larger studies.
A potential advantage of using umbilical cord blood (UCB) as a source of stem cells for unrelated donor BMT in severe AA is that HLA mis-matching is better tolerated and therefore may be considered when a fully matched marrow donor is not available. Published data for acquired AA UCB transplantation are limited. Furthermore, the low cell dose obtained from a UCB donation poses particular problems in AA where a good stem cell dose is important to help maximize engraftment. The recent use of double UCB transplants as a means of increasing the stem cell dose for adult recipients has been successful in achieving a high engraftment rate in high-risk MDS/AML. For individual patients, only 1 of the 2 units engrafted long term. A higher CD3+ cell count, but not nucleated or CD34+ cell count, was found in the engrafted unit.27 In acquired AA, a recent study from China demonstrated engraftment in 7 of 9 adults, with sustained mixed chimerism.28 In 4 of the patients, 2 units of UCB were infused, one of which engrafted each patient. Further studies in acquired adult severe AA are indicated.
5. Immunosuppressive Therapy (IST)
The combination of ATG and CSA remains the currently recommended immunosuppressive regimen for both severe AA and transfusion-dependent non-severe AA (see Figure 1aF1A and bF1B ). The response rate (as defined by transfusion independence) in severe AA is 60–70% at 6 months.29,30 Long-term follow-up from the German prospective study comparing ATG alone with ATG and CSA reported an overall survival of 65–70% at 3–4 years and failure-free survival (as defined by survival in the absence of relapse, no response, PNH, MDS, AML or solid tumors) of 39%. At 11 years, overall survival was 39%.29 Normalization of blood counts occurred in about one third of patients.
Risk of relapse is 30–40% but appears to be less with the more recent practice of prolonging CSA beyond 6 months. CSA dependency is observed in 26–62% of patients, necessitating long-term maintenance of the drug. The actuarial probability of developing hemolytic PNH, MDS/AML or a solid tumor after IST is 10%, 8% and 11% at 11 years, respectively, emphasizing the importance of long-term follow-up of all patients.29 Avascular necrosis may result in serious morbidity, so the minimum dose of prednisolone should be given to prevent serum sickness. The occurrence of osteoporosis, and possibly also avascular necrosis, may also be a manifestation of unsuspected DC. The addition of G-CSF to ATG and CSA when administered daily for 3–4 months results in improved neutrophil recovery but confers no benefit in terms of trilineage hematological recovery of improved survival.31 In children, the use of high-dose G-CSF, or for more prolonged duration with IST, is a risk factor for MDS/AML with monosomy 7, but not to date in adults. A further multicenter prospective EBMT study is currently in progress, but the routine use of G-CSF with ATG and CSA outside of randomized clinical trials is not currently recommended.1
For non-response to (or relapse after) treatment with ATG and CSA, treatment with a second course using the horse preparation again produces a response in 50–60% of patients. A recent retrospective study from NIH showed a response rate of 33% at 6 months using rabbit ATG (Thymo-globulin®, Genzyme) in patients refractory to horse ATG, and 68% response in patients who relapsed after horse ATG.32 In the absence of any prospective study comparing rabbit ATG to horse ATG, the choice of ATG preparation for a second course depends on whether a severe reaction occurred with the previous course, individual center preference and drug availability.
Is there any benefit to giving a third course of ATG? A retrospective study from the UK showed that all of 7 patients who had previously responded to a first or second course of ATG but relapsed, responded again to a third course, whereas only 2 transient responses were observed among 11 patients refractory to the previous 2 courses.33 Multiple courses of ATG are associated with an increasing risk of severe systemic reactions and anaphylaxis so must be given with great caution. Nevertheless, a third ATG course is often useful for relapsing patients, indicating that such patients may require some form of maintenance immunosuppression for presumed ongoing immune attack on hematopoiesis. For refractory patients, alternative therapies should be considered.
Is there an upper age limit for using ATG? The recent interest in using ATG in low-risk MDS has justifiably raised concerns about its safety in older patients. It is established that older patients with AA (> 60 years) show similar response to ATG compared with younger patients, but are at increased risk from infections and bleeding with poorer survival. A recent phase I/II study evaluated the use of low-dose ATG (5 mg/kg/day, one third the standard dose, of Lymphoglobuline®, horse ATG, Genzyme) to assess its efficacy and tolerability in patients > 60 years of age.34 Previous in vitro data had shown serum drug levels obtained with standard dose Lymphoglobuline were toxic to bone marrow hematopoietic progenitor cells. Of 12 evaluable patients, only 1 partial response was seen, indicating that this dose of ATG was insufficient to achieve a response rate similar to that with standard dose Lymphoglobuline. In practical terms, the decision whether to use ATG in older patients should be based on patient performance status. Careful clinical assessment is required before giving ATG to patients over 60 years, and especially in those over 70 years of age. It is unusual to consider its use in patients over 80 years old.
Possible reasons for nonresponse to IST have recently been discussed, namely a nonimmune pathogenesis of AA, misdiagnosis or exhaustion of stem cell reserve from immune attack. In addition, insufficient immunosuppression may account for nonresponse if there is ongoing immune attack.35 The last hypothesis has been tested by administration of additional immunosuppressive drugs with ATG and CSA, or by using drugs more immunosuppressive than ATG, such as high-dose CY and alemtuzumab. One approach has been to add a drug which works through a different mechanism to ATG and CSA. Mycophenolate mofetil inhibits inosine monophosphate dehydrogenase, which is required for purine synthesis, particularly in activated lymphocytes. In a recent study of 104 patients, the combination of ATG, CSA and mycophenolate mofetil produced a similar response rate (62% at 6 months) and relapse rate (37%) compared with historical data from patients receiving ATG and CSA. Most relapses (63%) occurred while receiving mycophenolate mofetil despite continuing the drug for 18 months.36 High-dose CY given without stem cell support can induce durable responses in AA, but its more widespread use has been tempered by slow recovery of blood counts and a high incidence of invasive fungal infections, when compared with standard IST. In a series of 17 patients who had not responded to one or more courses of ATG-based regimens or CSA alone, 53% achieved transfusion independence and 59% survived at a median follow-up of 29 months. However, the increased immune suppression associated with CY did not prevent later clonal disorders in 2 patients and did not eradicate PNH clones in all affected patients.11 The use of alemtuzumab as a means of producing more profound and more prolonged immune suppression than ATG, is currently being evaluated in two prospective studies at NIH for both new and refractory patients. The management of patients with refractory AA is problematic and reflects a lack of robust tests to predict response to IST and lack of availability of new immunosuppressive drugs. Only a modest effect of recombinant humanized anti-IL-2 receptor antibody (daclizumab) was observed in a series of untreated non-severe AA, although there was a positive correlation between response and CD8+ T-cell positivity for interferon-γ.37
6. Quality-of-Life Issues in Aplastic Anemia
With time, outcomes following BMT and IST for AA continue to improve such that differences in overall survival between the two treatment modalities have diminished. Hence, the importance of failure-free survival and quality-of-life issue analyses. One retrospective study compared quality-of-life outcomes between 52 transplanted patients and 155 patients receiving IST during the period 1976 to 1999. Overall and event-free survival were similar between the two groups. However, quality-adjusted time without symptoms and toxicity analysis showed that, compared with BMT, IST treated patients had longer periods of time with (a) symptoms from drug toxicity, (b) transfusion dependency, (c) partial remission, and (d) secondary clonal disorders. Transplanted patients spent more time in complete remission without drugs and had longer periods free from symptoms (as well as time with extensive chronic GVHD).38
7. Management of Pregnancy in AA
In rare cases, AA may occur in association with pregnancy and spontaneous remission may occur at time of delivery or after termination of pregnancy, although not in all patients. There is no evidence of reduced fertility in acquired AA following treatment with ATG and CSA, but AA may relapse in more than a third of patients in pregnancy, regardless of the remission status.39 The final decision to proceed with a pregnancy is the patient’s after being fully informed of the potential risks. Treatment of de novo or relapsed AA in pregnancy may require intensive transfusional support with increased risk of alloimmunization to red cell, HLA and platelet antigens. Data from renal transplantation studies show no increase in birth defects or spontaneous abortions in infants born to mothers taking CSA compared to the general population,40 so CSA therapy may be considered during pregnancy in AA. ATG has been used rarely in pregnancy.1 Pregnancy following successful BMT does not result in relapse of AA, in contrast to the experience in IST-treated patients.