Abstract
Epstein-Barr virus (EBV) is detected in some Hodgkin lymphoma (HL) tumor cells. Primary infection is associated with infectious mononucleosis and EBV+ HL. Vaccines and antiviral drugs show promise in modulating the clinical course of infectious mononucleosis. Their impact on HL is entirely unknown. T-cell function may be important in the pathogenesis of HL. In HIV patients, higher CD4 counts are associated with an increased incidence of EBV+ HL. One of the roles of the virus in the pathogenesis of HL may be to mimic signals associated with surface immunoglobulin molecules. New approaches to imaging EBV-associated tumors may be on the horizon. Adoptive immunotherapy and virus-specific pharmacologic therapies offer promise for future treatment.
EBV and Hodgkin Lymphoma
Recognition of an association of infectious mononucleosis with Hodgkin lymphoma (HL) predates the discovery of Epstein-Barr virus (EBV) and the present understanding that the disease entity is a malignancy of B lineage cells. In the last several years, our understanding of that association has advanced considerably. There are new insights into infectious mononucleosis and perturbations in cellular immunity, new insights relating to the role that virus may play in the molecular pathogenesis of HL, an emerging appreciation of the increased incidence of HL in HIV and its relationship to immune suppression, and new approaches to clinical monitoring and therapy. The terminology used to describe the association between the ubiquitous virus, the symptomatic and asymptomatic primary infection, and virus-associated tumor is sometimes ambiguous. Please refer to Table 1 for clarification of terms as they are used here.
EBV in Tumor Cells
The detection of EBV nucleic acids and proteins in tumor cells defines EBV+ HL. In situ hybridization for high abundance viral RNAs referred to as EBERs, and immunohistochemistry for the Latency Membrane Protein 1 are widely used in diagnostic laboratories.1 These detection techniques have been compared, and their reproducibility has been assessed with some rigor.2 At a first approximation, both are reliable indicators of the presence of virus. Sensitivity is modestly increased when both assays are used in combination. In North America and Western Europe, 30% to 50% of HL patients’ tumors are EBV+. Curiously, detection of EBV in tumors in these regions is least common in young adult disease. In some parts of Latin America, Africa and Asia the percentage is much higher, with the percentage in children approaching 100%.3 Infectious cofactors other than EBV have been suggested, but no consensus in support of any other particular association has emerged.4 Many investigations have focused especially on EBV− cases, but the association of malaria and EBV+ Burkitt lymphoma (BL) highlights the possibility that more than one infectious cofactor may play a role in a particular tumor.
Infectious Mononucleosis
Recognition of the viral etiology of infectious mononucleosis followed the serendipitous observation that a technician working in a laboratory studying the serology of African children with BL became EBV seropositive as she recovered from the illness. Studies of college students followed that helped better define the syndrome. In a recent cohort study from the United Kingdom, more than 500 seronegative university students were followed for 3 years. Seroconversion occurred in 46%. Among seroconverters, infectious mononucleosis developed in 25%.5 But why some primary infection is associated with symptoms in some but not others remains a matter of speculation. Sexual activity, host age, host immune response polymorphisms, infection with particular strains of virus, and the size of the primary innoculum are all possible determinants. Recently, it has been recognized that infection with multiple viral strains is the rule rather than the exception.6 Viral copy number in whole blood correlates with severity and duration of symptoms.7 Whole-blood measurements do not distinguish between viremia (virions) or latently infected lymphocytes.8 Fine mapping of the evolution of the specific cellular immune response to viral antigens has progressed,9,10 as has an appreciation of the importance of innate immunity.11 Investigators have reported that EBV infectious mononucleosis is associated with a lifelong “immunologic scar.”12 Individuals with a history of primary symptomatic disease differ from other healthy EBV seronegative and EBV seropositive individuals in that they lack CD8 T cells and natural killer (NK) cells expressing IL-15 receptor (recognized by flow cytometry for IL-15Rα). Assays of IL-15 responsiveness indicate that the absence of these cells have functional correlates in vitro. Whether they have clinical correlates is unknown. Remarkably, the change in lymphocyte cell populations is sustained over years and perhaps decades. No similar sustained change in the phenotype of lymphocytes accompanies the infectious mononucleosis-like syndrome associated with primary cytomegalovirus infection (or acute viral illnesses such as influenza). But what is the significance of the scar? Are there long-standing consequences with regard to health? As discussed below, EBV+ HL may be one of the consequences.
Infectious Mononucleosis and HL
A history of infectious mononucleosis was first linked to HL in reports in the 1950s. In the 1980s, viral antigens, DNA and RNA were found in Reed-Sternberg cells in some patients with HL. The percentage of cases associated with virus in tumor cells varied as a function of age, histology, geography, ethnicity, locus of tumor, and underlying immune deficiency. However, the character of the relationship between symptomatic primary infection (infectious mononucleosis) and HL remained murky. The association of infectious mononucleosis and HL is strongest in young adults, but virus in tumor cells is least frequently detected in tumors in young adults. This paradox led to concern that there were uncontrolled confounding factors in cohort studies that had been missed, or that the childhood environment characteristics associated with risk of HL in young adults might also be associated with an increased risk of infectious mononucleosis. Alternatively, EBV might be an etiologically innocent passenger, or the virus might be lost from the neoplastic cells during progression (a phenomenon that is readily demonstrated in EBV BL cell lines in vitro but that is largely hypothetical in vivo). Several studies addressed the relationship between symptomatic mononucleosis and its association with EBV+ and EBV− HL.13–16 In a Scandinavian study of 586 patients and 3187 healthy controls, HL risk was associated with self-reported infectious mononucleosis.16 The increased risk varied by the duration of time since symptoms and was confined to EBV+ tumors. The statistical analysis suggested that HL tended to occur at a median of 2.9 years after infectious mononucleosis. It is still not clear whether primary infection per se is the risk factor for EBV+ HL or whether primary symptomatic infection is the risk factor. This distinction might be important if vaccines, antivirals or anti-inflammatory agents were found to modulate the manifestations of primary infection but not prevent primary infection as discussed below.
Infectious Mononucleosis: Prospects for Treatment and Prevention
Steroids have long been used in some cases of infectious mononucleosis, but a recent literature review was inconclusive.13 Phase 1/2 clinical trials of a recombinant glycoprotein subunit vaccine with and without adjuvant demonstrated safety and immunogenicity in healthy adults.17 All vaccine formulations induced antigen-specific antibody responses (including neutralizing antibodies) and cell-mediated responses. Only one possible case of infectious mononucleosis was reported, among a total of 117 seronegative patients. Several patients developed antibodies to nonvaccine EBV antigens during the 7-month follow-up of the trial, suggesting primary infection. These results suggested that the vaccine would likely not induce sterile immunity, but might reduce the incidence of symptomatic infection.
In a small randomized pilot trial, valacyclovir treatment reduced viral copy number in the oropharynx and reduced symptoms.18 Whether any of these interventions might affect the incidence of EBV+ HL awaits future investigations.
T-Cell Function in Patients with HL
Anergy in skin tests of delayed hypersensitivity was demonstrated more than 100 years ago. An increased incidence of shingles in patients with HL is also recognized. A role for suppressor T cells was suggested in the 1970s. Recently, regulatory T cells (Tregs) important in controlling the response to foreign antigens and in protecting against autoimmune disease have been described. Increasing evidence is suggesting a role for these cells in suppressing antitumor immune responses as well. In HL, a substantial percentage of the T-cell infiltrate consists of regulatory cells.16–18 The level of such cells as marked by FOXP3 is associated with the survival of patients with HL.19 These cells suppress interferon γ production by lymphocytes, including the CD8+ cells specific for EBV antigens expressed in HL. In the peripheral blood, Treg cells are consistently elevated in patients with newly diagnosed and recurring HL in contrast to cells from patients in remission and healthy controls.20 Note that the presence of Treg cells is characteristic of HL and not specifically associated with EBV+ HL. Underlying immune differences between EBV+ and EBV− HL have yet to be defined with certainty, but the increased incidence of EBV+ HL in people infected with HIV discussed below suggests that cellular immunity plays a complex role in pathogenesis.
HIV and HL
HL occurring in the setting of HIV has distinctive features. These include a nearly uniform association with EBV.21 In this regard, HL might be contrasted with BL. BL arising in patients with HIV in the U.S. and Europe lacks EBV more often than not. HL in patients with HIV tends to present at an advanced stage with associated B symptoms and extranodal involvement, and is most often a mixed cellularity disease. Whereas one of the defining characteristics of HL in other settings is contiguous spread, in patients with HIV “skip lesions” are common and bone marrow–only presentations are also recognized.
Early in the HIV epidemic, certain non-HL (NHLs) soared in frequency. Increased incidence of HL in other immunosuppressed hosts had been recognized (patients with congenital immunodeficiencies or following transplantation) but there was some uncertainty as to whether the incidence of HL was truly increased.
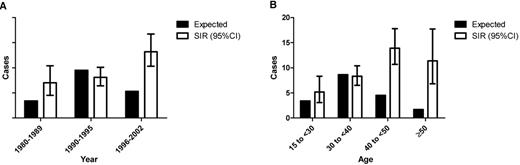
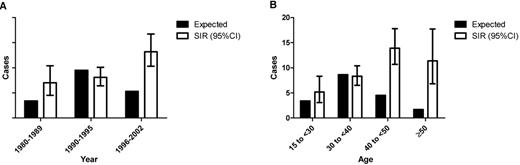
Linking AIDS and Cancer registries, Biggar and colleagues reported that the standardized incidence ratio (SIR) of HL in persons with AIDS has increased since the early days of the HIV epidemic (Figure 1A ).22 When highly active antiretroviral therapy came into widespread use in the U.S., CD4 counts at AIDS diagnosis rose, as did the SIR of HL. The risk of each of the individual subtypes of HL is lower in patients with the lowest CD4 counts, but the impact seems to be greatest on the incidence of nodular sclerosis HL. Nodular sclerosis HL was not observed in the group with the lowest CD4 counts. Most of the cases in patients with the lowest CD4 counts were of the mixed cellularity subtype. Model fitting suggested that for persons with AIDS with moderate immunosuppression (225 to 249 CD4 cells/μL) at the onset of AIDS, HL risk was 15-fold higher than in the general population. Higher and lower CD4 counts were associated with less risk. Whereas the SIR for AIDS-related NHL increases as CD4 counts fall, the risk of HL falls as CD4 counts fall. The increased incidence of HL was also difficult to recognize definitively because the young adult peak masked the increased incidence in patients infected with HIV (Figure 1B ). The increased risk is much more readily appreciated in older patients.
Why the increased incidence with increased CD4 count? Several possible explanations have been offered. Tumor cells may not be able to recruit lymphocytes required for tumor cell survival in the lymphodepleted host, the tumor may remain occult in the lymphodepleted host (perhaps to emerge with immune reconstitution), or lymphomagenesis may shift towards NHL in the absence of an inflammatory milieu.
The other EBV-associated lymphomas arising in patients with AIDS, such as primary central nervous system lymphoma, occur with increased frequency in patients with lower CD4 counts. NHLs occurring in patients with AIDS with higher CD4 counts such as BL tend to be EBV− tumors. Why does HIV specifically predispose to EBV+ HL; and is there any link with the time of primary EBV infection?
Association with Inflammation
Several lines of evidence suggest that the inflammatory response may play an important role in the pathogenesis of virus-associated HL and other virus-associated malignancies. Thus, as noted above, it may be that symptomatic primary EBV infection is associated with higher risk than asymptomatic primary infection. There is the intriguing observation that early-stage disease in cervical nodes is more likely to be associated with EBV HL than early-stage disease involving inguinal nodes.23 And, of course, these are the nodes that are enlarged in infectious mononucleosis. The association of HL with patients with HIV who have relatively high CD4 counts also suggests that an immune response, albeit dysregulated, may play a role in the pathogenesis of the disease. Inflammation is recognized to play a role in pyothorax-associated lymphoma.24 Patients treated for tuberculosis in the pre-antibiotic era with iatrogenic pneumothorax maintained with ping pong balls (practiced in many parts of the world but especially in Japan) developed chronic pyothorax. EBV-associated lymphomas developed in some of these patients beginning approximately 2 decades later. Insofar as EBV-associated B-cell lymphoma presenting as solid tumors in the pleural space is otherwise virtually unheard of, this is evidence that the ping pong balls or the chronic suppuration associated with the procedure promoted tumorigenesis in this setting.
The Role of the Virus
The specific contribution of EBV to any of the associated malignancies is not well understood, and present evidence suggests that its contribution is likely to be different in different tumors. Data have been accumulating that suggest the role in HL may be distinctive. In B-cell lymphopoiesis, expression of immunoglobulin is required so as to generate signals that prevent apoptosis. In general, B-cell progenitors will undergo apoptosis when immunoglobulin gene rearrangements or somatic hypermutation lead to crippling mutations. In some instances in HL, but not in the majority of cases, tumor cells carry these crippled immunoglobulin genes.25 In almost every instance, tumors in which these crippling mutations have been found are EBV associated. One EBV gene expressed at very high levels in HL, LMP2A, carries an immunoreceptor tyrosine-based activation motif (ITAM) that resembles that carried by immunoglobulin molecules. When expressed at the cell membrane, LMP2A provides a tonic signal that inhibits apoptosis.26 In RAG knockout mice that do not generate immunoglobulins because they do not undergo immunoglobulin gene rearrangement, there is no B cell population (as would be recognized by other phenotypic markers). However, when LMP2 is expressed, a B-cell population survives, presenting evidence suggesting that the ITAM motif of LMP2A (a membrane protein) substitutes for the missing immunoglobulin ITAM tonic signal. Thus EBV infection with LMP2A expression may allow the survival of cells otherwise destined to undergo apoptosis.27–29 In other cases of HL (EBV+ or EBV−) immunoglobulins are not expressed—but the coding regions are not crippled. Instead there is an absence of transcription, at least in some instances reflecting an absence of B-cell–specific transcription factors.
EBV and Clinical Outcome
Several studies have suggested that the presence or absence of virus has no association with disease-free or overall survival, while others have suggested an association in very young and in very old patients.30–32 These studies have not defined particular modalities of therapy that are more or less likely to be effective in the treatment of virus-associated tumor.
Monitoring
EBV copy number is widely used to monitor immunosuppression in organ transplant recipients, and is sometimes useful in guiding diagnosis.33 Several studies suggest that plasma or serum levels of virus DNA in patients with EBV HL may have utility as a guide to therapy; there is precedence for this in nasopharyngeal carcinoma, where viral copy number in cell-free blood has proven to be a very useful tumor marker.34,35 No role has been suggested for classic serology in monitoring such patients.
Imaging
There is the possibility that the presence of virus in tumors might ultimately be useful for imaging tumors in patients. The viral thymidine kinase (TK) phosphorylates nucleoside analogs such as acyclovir and 2′-fluoro-2′-deoxy-beta-D-5-iodouracil-arabinofuranoside (FIAU).36 The latter molecule is readily imaged if labeled with an isotope of iodine. However, present evidence suggests that the enzyme is not expressed or is expressed only at very low levels in virus-associated tumors. However, expression of this enzyme and various other enzymes can be activated by various chemotherapy agents, most notably bortezomib. Thus, in murine xenograft models of human EBV-associated tumors in severe combined immunodeficiency (SCID) mice, treatment with bortezomib followed by [125I]FIAU allows imaging. Models studied to date do not include EBV+ HL because the available HL cell lines, even when they harbor virus, do not mirror the pattern of viral expression characteristic of clinical tumors.
Adoptive Immunotherapy
A variety of strategies have been investigated for treating EBV-associated tumors specifically, and several of these have generated some intriguing successes. Adoptive immunotherapy with T cells targeting EBV antigens has been explored, particularly by the Baylor group.37–40 In the autologous peripheral stem cell transplantation setting, it has been possible to expand EBV-specific T cells and reinfuse. In some instances, regression or stabilization of disease has followed. The antigens targeted may have limited the impact of such therapy. Thus, the preparation of T cells by expansion using autologous EBV lymphoblastoid cell lines as stimulators leads to polyclonal T-cell populations predominantly targeting viral antigens not expressed in EBV-associated HL. Recent advances in expansion technology allow more targeted expansion of T cells and will likely be associated with more effective therapies.
Pharmacologic Approaches
A second approach is pharmacologic rather than immunologic. As noted above, the EBV TK is not expressed in most tumors. However, this enzyme and another viral enzyme with a similarly restricted expression pattern do phosphorylate ganciclovir.41 Ganciclovir, when phosphorylated, inhibits the cellular DNA polymerase, leading to apoptosis in cycling cells. In a phase 2 clinical trial, butyrate has been combined with ganciclovir in the hope that butyrate would upregulate these viral enzymes and that ganciclovir would kill EBV-associated tumors.42 Early results are encouraging. Other parallel strategies include the use of bortezomib and rituximab to upregulate these enzymes.
The Future
It seems very likely that continued progress in adoptive T-cell immunotherapy will offer more effective salvage options for some patients with recurring HL. Viral enzyme activation strategies might have a similar role but are much less investigated. Monitoring viral copy number in plasma or serum may prove useful as part of an algorithm for response-adapted therapy in patients with EBV tumors. The association with symptomatic primary infection and the emerging understanding of the cellular immune correlates of this association suggest the possibility that it might ultimately be possible to reduce the incidence of HL with interventions to prevent or modulate primary viral infection. An effective vaccine that prevented primary infection might reduce the incidence of HL in the same way that hepatitis B vaccines appear to be lowering the incidence of hepatoma in Taiwan, and now in other parts of the world. Even if such a vaccine did not prevent primary infection but only modulated its course, the risk of HL might be altered. Other interventions, such as the use of valacyclovir, anti-inflammatory agents, and perhaps others, might similarly change the consequences of primary infection. Whereas cytotoxic chemotherapy and radiation are mature as modalities, with present debate focused on the acceptable balance between relapse risk and iatrogenic complications, interventions targeting viral infection either in established tumors or before there is tumor are still in their infancy but hold promise.
Estimated incidences of HL if there were no increased risk associated with AIDS are shown in the filled bars. Standardized incidence ratios with 95% confidence intervals are shown in the hollow bars. (A) Incidence of HL by year. Note that the incidence has increased in the era of effective antiretroviral therapy. (B) Incidence of HL by age. Note that the young adult peak is masked by the increased incidence in patients with AIDS. Both figures are adapted from Biggar et al22 (Table 1).
Estimated incidences of HL if there were no increased risk associated with AIDS are shown in the filled bars. Standardized incidence ratios with 95% confidence intervals are shown in the hollow bars. (A) Incidence of HL by year. Note that the incidence has increased in the era of effective antiretroviral therapy. (B) Incidence of HL by age. Note that the young adult peak is masked by the increased incidence in patients with AIDS. Both figures are adapted from Biggar et al22 (Table 1).
Johns Hopkins School of Medicine, Baltimore, MD