Abstract
The clinical hallmark of paroxysmal nocturnal hemoglobinuria (PNH) is episodic hemoglobinuria, and it was this feature that captured the attention of European physicians in the latter half of the 19th century, resulting in careful observational studies that established PNH as an entity distinct from paroxysmal cold hemoglobinuria and march hemoglobinuria. Curiosity about the etiology of the nocturnal aspects of the hemoglobinuria led the German physician Paul Strübing to develop the prescient hypothesis that the erythrocytes of PNH are abnormally sensitive to hemolysis when the plasma is acidified during sleep because of accumulation of carbon dioxide and lactic acid as a result of slowing of the circulation. Investigation of the intricate pathophysiology that underlies the abnormal sensitivity of PNH erythrocytes to hemolysis in acidified serum produced a number of remarkable scientific achievements that involved discovery of the alternative pathway of complement, identification of the membrane proteins that regulate complement, discovery of a novel mechanism for attachment of proteins to the cell surface, and identification of the genetic basis of the disease. These discoveries were made steadily over a period of more than 100 years, and each generation of physicians and scientists made important contributions to the field. The mysteries of PNH have been solved in a particularly satisfying way because the precision and orderliness of the solutions made clearly understandable what had seemed at the times prior to resolution to be problems of nearly insurmountable complexity. The history of PNH is an inspirational reminder of the elegant complexity of nature, the rewards of curiosity and the power and beauty of science.
Introduction
The Scientific Session of the first organizational meeting of the American Society of Hematology held in Boston in 1957 was divided into two parts, with “Paroxysmal Nocturnal Hemoglobinuria” sharing the program equally with “Preservation and Transplantation of Human Bone Marrow.” Why did such a rare disease play so prominent a role in the inaugural meeting of our Society? Coincidence, in part, explains the preeminent position of PNH because the alternative pathway of complement (APC) (known at that time as the properdin pathway) had been recently discovered by Louis Pillemer and colleagues at Case Western Reserve University.1 On the faculty of the Case medical school at the same time was the influential hematologist and medical educator Thomas Hale Ham (who would become the 7th president of the Society in 1965) (Figure 1 ). Learning of Pillemer’s discovery must have been an eureka moment for Ham as it explained his mysterious observations, published in 1939, showing that PNH erythrocytes are lysed in acidified serum by an antibody-independent system that required factors indistinguishable from complement.2 At the time of Ham’s original observations, only the antibody-dependent classical pathway of complement was known, but Ham’s rigorous studies showed that acidified serum lysis of PNH erythrocytes was an antibody-independent process. Studies by Hinz, Jordan and Pillemer3 and supported by Ham4 confirmed that the factors involved in the hemolysis of PNH erythrocytes in acidified serum were the same as those of the properdin pathway. One of the involved factors is magnesium5 that is required for binding together two of the components of enzymatic subunit of the C3 and C5 convertases of the APC, and the essential role of this divalent cation in the hemolysis of PNH erythrocytes in acidified serum was presented at the First Organizational Meeting of the Society by Hinz and discussed by Ham. These findings were novel and undoubtedly generated considerable excitement among academic hematologists as they demonstrated that the pathophysiology of a fascinating, but arcane, hematologic disease was linked to a newly discovered, but arcane, biological system.
Sharing the program on PNH with Hinz and Ham were two other hematologists, William Crosby and Robert C. Hartmann, who became distinguished members of the Society and who made important, lasting contributions to the study of PNH. In 1951, Crosby published a scholarly review of the early history of PNH that focused on the uncannily insightful observations of a young German physician, Paul Strübing.6 And, as a result of his astute personal observations and a detailed review of the published literature, Crosby (in a 43-page Blood paper) brought attention to the important role that thrombosis plays in the natural history of PNH, showing that thromboembolic complications are the principal cause of disease-associated mortality.7 While on the faculty at Vanderbilt University School of Medicine, Hartmann and colleagues (including David Jenkins) developed the sucrose lysis test (sugar water test)8 that, along with the acidified serum lysis test of Ham, was used as the standard diagnostic test for PNH until being supplanted in the mid-1990s by flow cytometry. Hartmann and colleagues also reported extensively on the clinical manifestations of the disease including the relationship between PNH and thrombosis, particularly the Budd-Chiari syndrome.9 Further, they discovered the deficiency of acetylcholinesterase on PNH erythrocytes,10 a finding that played an important role in elucidation of the cellular defect in PNH, as acetylcholinesterase was among the first membrane proteins shown to be anchored through glycosyl phosphatidylinositol.11
Although coincidence played a part in the prominence of PNH in the inaugural meeting of the Society, the fascination of hematologists with PNH has endured. I believe that the allure of PNH results from convergence of three factors: (1) the rarity of the disease and its protean clinical manifestations that make diagnosis challenging but particularly gratifying; (2) the elegant complexity of the disease, which is a manifestation of its intricate pathophysiology; (3) the captivating way in which the fundamental abnormalities have been elucidated systematically over many years. This historical overview illustrates how the study of PNH has rewarded persistence and vision and how serendipity is involved in many remarkable discoveries.
The Early History
Crosby,6 Rosse12 and Parker4 have undertaken scholarly studies of the early history of PNH. Although descriptions of patients with PNH were published by others (perhaps as early as the 1793), Paul Strübing of Greifswald, Germany clearly recognized PNH as an entity distinct from both paroxysmal cold hemoglobinuria and march hemoglobinuria, two syndromes known to 19th century physicians with features that overlap with PNH. (Strübing’s paper was published in 1882. In 1866, the renowned Victorian British physician, William Gull, published a noteworthy clinical description of a patient with PNH but failed to recognize PNH as an entity distinct from paroxysmal cold hemoglobinuria.) Strübing’s paper is a prototypical example of how insightful clinical observations can suggest mechanisms of disease and thereby provide direction for laboratory investigation.
Strübing studied a 29-year-old cart maker beginning in 1880.6 Based on the observation that after severe attacks the plasma was red, Strübing concluded that the red cells were dissolved within the vessels and not in either the kidney or the urine. (The astuteness of Strübing’s observation is underscored by the fact that, as late as 1911, Marchiafava believed that the hemolysis of PNH occurred in the kidney.4) Strübing used available technology to demonstrate that the discoloration of the urine was due to the presence of hemoglobin, and he described the characteristic morphological features of the urine sediment, although he did not recognize that hemosiderin accounted for the pigmentation of the cellular material. He also noted that erythrocytes were absent from the urine during an attack, thereby distinguishing hemoglobinuria from hematuria. (Even today, failure to distinguish hemoglobinuria from hematuria is a principal cause of delay in diagnosis of PNH, often leading an extensive urological evaluation before the distinction is made). Strübing concluded that sleep played a critically important role in the hemolytic process because only by awakening the patient during the night was the initial daily episode of hemoglobinuria observed at a time other than when the urine was first voided after awakening in the morning. Based on these observations, together with available background physiological information (likely contributed by his colleague Leonhard Landois),12 Strübing developed his visionary hypothesis that influenced research in the field for more than 50 years. Strübing reasoned that PNH red cells were destroyed during sleep because they were abnormally sensitive to an acid environment that resulted when carbon dioxide and lactic acid accumulated because of slowing of the circulation.
Strübing attempted to support his hypothesis with experimental data by giving the patient acid, but this treatment failed to induce hemoglobinuria, perhaps because the amount given was insufficient to adequately acidify the plasma. Fifty-five years later, Ham repeated this experiment by giving patients ammonium chloride, and under these experimental conditions, hemoglobinuria and hemoglobinemia increased. This in vivo observation provided the framework for Ham’s in vitro studies that lead to development of the acidified serum lysis test.
In 1889 Strübing became Director of the Nose and Throat Clinic at the University of Greifswald, and research in the field languished until 1911 when the brilliant Dutch physician A. A. Hijmans van den Berg built upon Strübing’s original observations. Hijmans van den Berg showed that the red cells of PNH hemolyzed in vitro in an atmosphere containing carbon dioxide when the cells were suspended in serum from the patient or from either of two blood group compatible normal subjects.12 He also demonstrated that red cells from normal volunteers did not lyse under the same experimental conditions. These studies were seminal because they demonstrated conclusively that the hemolysis of PNH is due to a defect in the red cell rather than to the presence of an abnormal plasma factor (as is the case with paroxysmal cold hemoglobinuria, in which hemolysis is induced by the Donath-Landsteiner antibody).
Hijmans van den Berg considered the possibility that the complement system, which had been identified by the pioneering work of Jules Bordet in 1894 and named as a component of the system of humoral immunity by Paul Ehrlich in 1899, mediated the hemolysis of PNH erythrocytes.4 At the time of Hijmans van den Berg’s study, the following two characteristics of complement were accepted: (1) activity was lost if the serum was heat inactivated; (2) the activity could be restored by adding a small amount of fresh serum. Consistent with a role for complement, Hijmans van den Berg observed that hemolysis of PNH erythrocytes in acidified serum was abrogated by heat treatment, but he concluded that hemolysis was caused not by the cytolytic activity of complement but by an abnormal fragility of the erythrocytes to carbon dioxide because the hemolytic activity of the heated serum was not restored by the addition of a small amount of fresh human or guinea pig serum. Only upon the discovery 43 years later of he APC by Louis Pillemer1 would the reason that small amounts of fresh serum failed to restore the hemolytic activity of heat-treated serum become apparent (unlike the activity of the classical pathway of complement that can tolerate dilutions of >1:100, the activity of the APC is lost following dilutions as low as 1:4 or 1:8).
Why Hijmans van den Berg’s rigorous studies did not lead to a diagnostic test for PNH is unknown, but he continued his interest in hemoglobinuria (and porphyria), and one of his students, F.L.J. Jordan, published an important paper on the role of complement in the hemolysis of paroxysmal cold hemoglobinuria and PNH in 1938,12 the same year that Hijmans van den Berg retired from Utrecht as Professor of Medicine.12 A year earlier, Thomas Hale Ham, then a 32-year-old physician working in the Thorndike Memorial Laboratory in Boston, published his first paper dealing with mechanism of the hemolysis of PNH in relation to acid-base equilibrium.13
In the 26-year interval between the seminal observations of Hijmans van den Berg and Ham, a number of clinical descriptions, including those of Marchiafava and his pupil Micheli, were published, and Marchiafava-Micheli disease was an eponym for PNH that was popular during the 1930s and 1940s. Although the eponym lingered into the 1960s, rightfully it has not endured because the contributions of Marchiafava and Micheli were too modest to warrant such recognition.14 In 1928, the Dutch physician J. Enneking was the first to use the term “paroxysmal nocturnal hemoglobinuria” (translated from the Latin) for what he described as a new form of intermittent hemoglobinuria.6,12,14
The acidified serum lysis test of Ham
As was Strübing, Ham was struck by the relationship between hemolysis and sleep in patients with PNH. This relationship lead him to postulate in his 1937 paper13 (just as Strübing had done in 1882, although Strübing’s paper was not cited by Ham) that “because of the elevation in carbon-dioxide content of the arterial blood and the decrease in pH known to occur during sleep, it was suspected that a change in acid-base equilibrium was related to the increased hemoglobinuria of the patients during sleep.” Ham challenged his hypothesis that acidification of the plasma induced the hemolysis by giving two of the three study patients sodium bicarbonate. He reported that this treatment caused the hemoglobinuria and hemoglobinemia to decrease. In a corollary experiment, Ham also reported that, following administration of ammonium chloride (to acidify the plasma), the hemoglobinemia and hemoglobinuria increased. (As noted above, Strübing undertook similar experiments. The results of those experiments were negative, however, presumably because of a difference in experimental design.)
Armed with insights generated from his in vivo studies, Ham sought to identify the process that mediates the hemolysis of PNH, and, in 1939, he published two landmark papers that influenced the course of PNH research for the next 50 years.2,15 The finding of Ham’s first 1939 paper are remarkably similar to those of Hijmans van den Berg whose work was cited in both of Ham’s 1939 papers. As had Hijmans van den Berg, Ham reported the following: (1) rapid hemolysis of PNH erythrocytes was observed when serum or plasma was acidified by using either equilibration with CO2 or addition of lactic acid; (2) the effects of CO2 were neutralized by addition of sodium bicarbonate; (3) hemolysis was observed if serum or plasma from normal volunteers was substituted for patients’ serum or plasma; (4) Group O red cells from a volunteer donor were not hemolyzed when suspended in acidified serum or plasma from the patient.
The second of the two papers, coauthored with J. H. Dingle, is subtitled “Certain Immunological Aspects of the Hemolytic Mechanisms with Special Reference to Serum Complement,” and this publication suggested a mechanism by which the abnormal erythrocytes of PNH were lysed by a novel immunological system that was independent of antibody.2 As had Hijmans van den Berg, Ham and Dingle found that heat treatment inactivated the lytic substance but addition of a dilution of fresh serum did not restore the hemolytic activity. Unlike Hijmans van den Berg, however, the final conclusion of Ham and Dingle that “The serum factor essential for hemolysis was closely associated with, if not indistinguishable from complement or alexin (Buchner’s term for complement introduced in 1889) of human serum” suggests that the authors believed that the lytic substance of serum was indeed complement.4 But more than a quarter of a century passed before Pillemer’s discovery of the APC provided an explanation for their findings. The rigorous in vitro studies published in the two 1939 papers formed the basis for the acidified serum lysis test in which washed red cells from a patient suspected of having PNH are incubated in blood type–compatible acidified serum. Under these conditions, only PNH erythrocytes hemolyze, with the percentage of lysis being calculated based on spectrophotometric quantitation of hemoglobin in the supernatant fluid of the centrifuged sample. For more than 50 years, Ham’s test (along with Hartmann’s “sugar water test”) was the standard diagnostic test for PNH.
Discovery of the APC
The decade that followed publication of Ham’s studies produced limited progress toward defining the basis of the abnormal susceptibility of PNH erythrocytes to hemolysis in acid serum. As had Ham, others found evidence for involvement of complement, but deviations from the accepted characteristics of a complement-mediated lytic system were consistently observed as well. After reviewing the work of Ham and Dingle, Professor John Dacie of the Royal Postgraduate Medical School in London (Figure 2 ) made the following statement in a paper published in 194916: “There is thus some evidence for and some evidence against the participation of human serum complement in the hemolysis by serum of erythrocytes of patients with nocturnal hemoglobinuria, and in the present state of knowledge, any theory of the mechanism of hemolysis can only be speculative.” Five years later, Pillemer published his seminal observations that identified the APC,1 and the subsequent findings of Hinz, Jordan and Pillemer3 that were supported by Ham provided compelling evidence that this newly discovered component of the humoral immune system mediated lysis of PNH erythrocytes in acidified serum.
In an uncannily insightful review of PNH published in 1963, Dacie (Figure 2 ) stated that the conclusion that the properdin system was responsible for the lysis of PNH erythrocytes in acidified serum “seems generally to have been widely accepted.”14 Regrettably for Pillemer, however, doubt existed in the immunological community of the existence of the properdin pathway. The skepticism was in large part owing to the fact that preparations of purified properdin were found to have small amounts of natural antibodies to yeast and bacteria. In 1957, Pillemer died of a barbiturate overdose that was ruled a suicide. Apparently, he was depressed following a meeting at which other complement investigators were unwilling to acknowledge the existence of the properdin system. Ten years after his death, he was vindicated when others rediscovered the pathway, and by 1980 all of the components of the APC had been isolated and purified to heterogeneity.4 The discovery that the properdin system mediates lysis of PNH erythrocytes in acidified serum solved a problem that had challenged Hijmans van den Berg, Ham, Dacie and others. The role of Louis Pillemer in delineating the basis of the hemolysis of PNH erythrocytes is often overlooked, but his contributions were singular. And, likewise, studies into the mechanism of lysis of PNH erythrocytes played an important, but largely underappreciated, role in proving the existence of the APC.
The Modern History
Phenotypic mosaicism
In discussing the acidified serum lysis test of Ham, Dacie commented in his 1963 review of PNH,14 “Even if the serum is changed several times, there appear to be always some cells which resist hemolysis.” This observation suggested the existence either of one population of cells that varied in sensitivity to lysis or of two populations, one abnormally sensitive to lysis and the other of normal sensitivity. In the early 1960s, the pioneering work of Manfred Mayer at Johns Hopkins University made possible quantitative analysis of complement activity.4 To analyze the complement sensitivity of PNH erythrocytes, Wendell Rosse (who would become the 28th President of the Society and who was on assignment from the NIH to work with Dacie in London in 1963) modified the technique that Mayer had developed to assay serum complement. The results of this complement lysis sensitivity assay of Rosse and Dacie (Figure 3 ) demonstrated for the first time the magnitude of the difference in complement sensitivity between PNH and normal erythrocytes and clearly separated PNH erythrocytes into two quantitatively definable populations. The seminal studies of Rosse and Dacie, published in 1966,17,18 demonstrated that the erythrocytes of PNH are a mosaic. The insightful interpretation of their studies was a major conceptual advance that defined many of the fundamental characteristics of PNH and greatly influenced future investigation. In particular, the recognition that the basic defect underlying the disease was represented in the complement-sensitive population provided a means for assessing whether a particular observation is PNH-specific. According to this paradigm, if a process is specific for PNH erythrocytes, it should be observed in the complement-sensitive population but not in the insensitive population.
Using an assay modeled on the complement lysis sensitivity assay of Rosse and Dacie, Aster and Enright, in 1969,19 demonstrated that both PNH platelets and neutrophils are abnormally sensitive to complement-mediated lysis, providing evidence that the PNH defect arose in a primitive hematopoietic stem cell (the existence of which was an issue of debate at the time). In 1959 Auditore and Hartmann10 had reported that PNH erythrocytes are deficient in acetylcholinesterase, and in 1969 Kunstling and Rosse20 demonstrated that deficiency of erythrocyte acetyl-cholinesterase was restricted to the complement-sensitive population. These findings suggested that the acetylcholinesterase deficiency was a fundamental part of the pathophysiology of PNH, but more than 25 years would pass before the connection became apparent as acetylcholinesterase was among the first proteins to be shown to use glycosyl phosphatidylinositol as a membrane anchor. In 1970, Luzzatto and colleagues,21 by using glucose-6-phosphate dehydrogenase isotyping, showed that the complement-sensitive population is monoclonal while the complement-insensitive population is polyclonal. These studies established the clonal nature of PNH and provided experimental support for Dacie’s hypothesis that somatic mutation accounts for the phenotypic mosaicism of PNH.14
Functional basis of the abnormal sensitivity of the erythrocytes of PNH to complement-mediated lysis
In 1973, Gerald Logue and Wendell Rosse reported that PNH erythrocytes bound more C3 when complement was activated by either the classical or the alternative complement pathways.22 They also observed that for a given amount of C3 bound, PNH erythrocytes lysed to a much greater degree than normal cells. The accurate interpretation of these laborious, technically challenging experiments provided the conceptual framework for subsequent studies aimed at delineating the aberrant interactions of complement with PNH red cells.
Over the next 12 years, the molecular basis of the aberrant interactions of complement with PNH erythrocytes were defined at the molecular level.4 A major technical advance that made these studies possible was the development of methods for purifying to homogeneity the proteins of the complement cascade. By the early 1980s, it was possible to assemble the entire cascade from the individual isolated components. By using these techniques, along with radio-labeled monoclonal antibodies that were particularly useful for quantitative studies, investigators at Duke University23–25 and Rochester University26 showed that the abnormal sensitivity of PNH erythrocytes to complement-mediated lysis is due to defects at two distinct sites within the complement cascade. Aberrant regulation of the C3 convertase accounted for the greater binding of activated C3 to PNH erythrocytes, while unregulated formation of the membrane attack complex accounted for the greater efficiency of lysis of PNH erythrocytes when complement is activated.
Identification of the erythrocyte membrane proteins that regulate complement
Normal human erythrocytes are markedly resistant to complement-mediated lysis. Seeking to understand the basis of this resistance, Douglas Fearon of Harvard University isolated a protein from normal human erythrocytes that inhibited the activity of the C3 convertase of the APC and showed that this inhibitory factor was the membrane receptor for activated C3.27 Fearon reported his findings in 1979, and the protein that he discovered was called complement receptor 1 (CR1, CD35). Although CR1 was found to be uninvolved in the pathophysiology of PNH (CR1 is a transmembrane protein), isolation and characterization of the protein was nonetheless a watershed event in the history of PNH because those studies confirmed the existence of discrete membrane constituents that function specifically as complement regulators. Further, Fearon’s studies demonstrated that membrane-bound complement regulatory factors could be purified to homogeneity while retaining functional activity.
A decade earlier (1969), Edward Hoffmann of the University of Florida had published the results of a series of rigorous experiments showing that an extract prepared from human erythrocyte membranes contained a factor or factors that inhibited complement-mediated hemolysis.28,29 He also showed that a portion of the active material possessed the capacity to bind to the indictor cell and retain functional activity. Hoffmann used the name decay accelerating factor (DAF) to describe the functional property of the extract because the material enhanced the rate at which the complement C3 convertase diminished over time. In 1982, one of the complement regulatory proteins contained in the extracts described by Hoffman was purified to homogeneity by Anne Nicholson-Weller,30 who was a colleague of Douglas Fearon at Harvard. This protein was originally named decay-accelerating factor of stroma (DAF-S) to distinguish the membrane-derived factor from serum-derived proteins with decay-accelerating activity such as factor H, but the designation did not endure, and the protein quickly became know simply as DAF.
The functional properties of DAF suggested that its absence from erythrocytes would lead to greater C3 deposition on the cell surface, and therefore, deficiency of DAF could account for the observed differences between PNH and normal erythrocytes. In 1983, using immunohistochemical techniques, Nicholson-Weller and colleagues, reported that PNH erythrocytes are deficient in DAF.31 They also showed that PNH erythrocytes of intermediate sensitivity to complement (the PNH II phenotype) were partially deficient in DAF.31 Simultaneously, Michael Pangburn and colleagues at the Scripps Institute presented both functional and immunohistochemical evidence of DAF deficiency in PNH.32 The discovery of DAF and its deficiency in PNH were milestones in the journey towards understanding the basis of the hemolysis of PNH that began with the observations of Strübing, Hijmans van den Berg and Ham.
Deficiency of DAF provided a logical explanation for the greater binding of C3 to PNH erythrocytes, and a causal role for DAF in the pathophysiology of PNH was readily accepted. But compelling evidence had indicated that regulation of membrane attack complex (MAC) formation was also abnormally regulated on PNH erythrocytes,24–26 and subsequent studies demonstrated that DAF had no MAC inhibitory activity, implying that a discrete MAC regulatory factor must also be deficient in PNH. In 1989, a protein from normal erythrocytes that inhibited reactive lysis (a process in which the MAC is deposited onto bystander cells by a fluid-phase C5 convertase) of PNH erythrocytes was isolated in the laboratory of Dr. Charles Parker of the University of Utah School of Medicine.33 The protein was called membrane inhibitor of reactive lysis (MIRL, CD59). Immunohistochemical studies showed that PNH erythrocytes are deficient in MIRL (Figure 4 ) and that isolated MIRL bound to the membrane of PNH cells and protected them against reactive lysis. Subsequent quantitative studies demonstrated that the most complement-sensitive PNH III erythrocytes, which are susceptible to reactive lysis, are completely deficient in MIRL (Figure 4 ), while the intermediately sensitive PNH II erythrocytes, which are resistant to reactive lysis, have about 10% of the normal amount of MIRL thereby explaining the molecular basis of the difference between the two PNH erythrocyte phenotypes.34
The cellular defect
Working at the recently founded UCLA Medical School, William Beck and William Valentine (both of whom became distinguished members of the Society) reported in 1951 that neutrophils from patients with PNH (and chronic myelogenous leukemia) are deficient in alkaline phos-phatase.35 This observation appears to have been serendipitous as PNH leukocytes were included along with white cells from other “miscellaneous hematological conditions” in a study dealing mainly with chronic lymphocytic leukemia and acute leukemia. As noted above, Auditore and Hartmann in 1959 reported that PNH erythrocytes are deficient in acetylcholinesterase,10 and Kunstling and Rosse subsequently demonstrated (in 1969) that the acetylcholinesterase deficiency was restricted to the complement-sensitive population.20 Other studies reported deficiency of 5′ nucleotidase on PNH lymphocytes. The discovery that the hematopoietic cells of PNH are deficient in these three proteins was not a consequence of hypothesis-driven research but rather arose from descriptive studies. Despite the archival nature of the observations, deficiency of alkaline phosphatase, acetylcholinesterase and 5′ nucleotidase continued to resonate with investigators interested in PNH. That the hematopoietic cells of PNH were deficient in at least three membrane constituents argued that the underlying basis of the deficiency was not a consequence of mutations affecting the structural genes that encoded the individual proteins but that the deficient proteins share a common post-translational modification that affects their membrane expression and that this modification is defective in PNH. Therefore, any hypothesis that attempted to explain PNH had to account for deficiency of multiple proteins.
In 1975, while working with phosphatidylinositol-specific phospholipase C (PI-PLC) at the University of Birmingham, Martin Low observed that, when added to cells, the enzyme caused them to release alkaline phosphatase into the medium.36 Although Low and colleagues were investigating other properties of the enzyme, they found that the enzyme clipped several additional proteins from the cell surface, including acetylcholinesterase and 5′ nucleotidase. Low hypothesized that these proteins are attached to the membrane because they are covalently linked to the inositol ring of phosphatidylinositol.37 Although initially Low’s hypothesis was largely disregarded because it was generally accepted that cell-surface proteins are anchored by a short sequence of hydrophobic peptides, by 1985 it was becoming clear from the work of other investigators that a number of membrane proteins from diverse cell types, including the murine lymphocyte Thy-1 antigen and the variable surface protein of trypanosomes, are attached to the membrane by phosphatidylinositol.
A brief summary of the observations of some of the early contributors to the phosphatidylinositol anchor hypothesis was published as a News and Views article in Science in 1985 (Figure 5 ).36 A sentence from the report reads as follows: “But Low’s enzyme cleaved phosphatidylinositol and released three unrelated proteins—acetylcholinesterase, 5′ nucleotidase and alkaline phosphatase.” To an investigator familiar with the deficiency of these same three proteins in PNH, reading this summary would certainly have resulted in one of those rare eureka moments when suddenly everything makes sense. Among those who recognized the importance of the findings were members of Dr. Victor Nussensweig’s laboratory at New York University. By using enzyme provided by Dr. Low, those investigators reported that DAF is released from the cell membrane by PI-PLC.38 As a consequence of those observations, the following hypothesis was proposed for PNH: all proteins that are deficient on the hematopoietic cells of PNH are GPI anchored; (and the corollary of this argument) all GPI-anchored proteins that are expressed by hematopoietic cells are deficient in PNH. This hypothesis was quickly accepted because it identified the post-translational modification that explained the deficiency of multiple proteins in PNH. Deficiency of leukocyte alkaline phosphatase, erythrocyte acetylcholinesterase, and lymphocyte 5′ nucleotidase account for none of the clinical manifestations of PNH, but description and awareness of these abnormalities lead directly to discovery of the basis of one of the most remarkable characteristics of the disease and provided the insight needed to identify the genetic abnormality that underlies PNH.
The genetic basis of PNH
By 1987, the complex structure of the GPI anchor had been fully elucidated. Clearly the metabolic pathways necessary for synthesis of the anchor, post-translational modification of the proteins that are GPI-anchored and expression of the GPI-anchored constituents onto the membrane surface would require multiple enzymes and cofactors. Hypothetically disruption of any component of this intricate pathway occurring in a pluripotent hematopoietic stem cell would result in the PNH phenotype. But PNH had another surprise in store for investigators, as subsequent studies revealed that all cases of PNH result from mutation of a single gene.
A wealth of information about the genetics and biochemistry involved in the synthesis of the GPI anchor accrued from the careful study of Thy-1− mutants derived from a murine lymphoma cell line by Robert Hyman at the Salk Institute.39–41 The mutants were developed in the early and mid-1970s to investigate the molecular events involved in the expression of cell surface molecules. Serendipitously, the membrane protein selected for study by Hyman was Thy-1. He isolated Thy-1− mutants and rigorously characterized by cell fusion experiments nine discrete complementation groups (designated A-I). In 1985, Thy-1 was shown to be GPI-anchored, and subsequent studies by several different groups identified the biochemical defect in most of the mutant cell lines. That Thy-1 mutants might be informative reagents for the study of PNH was a pivotal concept that pointed straight to the genetic origin of PNH.
In 1993, Taroh Kinoshita and members of his laboratory (particularly Junji Takeda) in Osaka, Japan made a remarkable series of conceptual and technical leaps. Those investigators took advantage of the mosaicism in PNH to develop paired GPI+ and GPI− cell lines derived from patients. In a series of technically demanding experiments, the Osaka group observed complementation of GPI-anchor protein expression in each of the GPI− set of cells by all complementation groups except class A, and they then demonstrated that the GPI− cells, but not the GPI+, had the same defect in mannolipid biosynthesis as the class A Thy-1− mutants.42 These studies indicated that at least some cases of PNH are a consequence of mutation of the same gene that is defective in the complementation class A Thy-1− mutants originally developed by Hyman.39 The gene that is defective in complementation class A was identified by expression cloning by Kinoshita and colleagues and given the clever name PIGA (for phosphatidylinositol glycanclass A).43 Armed with a PIGA cDNA expression vector and with knowledge of the sequence of PIGA, the Osaka group went on to show the following: (1) complementation of GPI-anchor protein expression in GPI− PNH cell lines by transfection with PIGA cDNA (Figure 6 );44 (2) discrete, functionally significant PIGA mutations in the GPI- but not the GPI+ cell lines (proving that the mutations are somatic rather than germ line);;44 (3) neutrophils have the same PIGA mutations as those found in the GPI− cell lines of the PNH patients from whom the lymphoblastoid cell lines were derived (proving that the mutations arose in a pluripotent hematopoietic stem cell);44 (4) PIGA is on the X-chromosome [providing a plausible explanation for why the PNH phenotype is always a consequence of PIGA mutation (assuming all other GPI-anchor pathway genes are autosomal) and why males and females are equally affected by PNH].44
The discovery by Kinoshita and colleagues that PNH is a consequence of mutant PIGA was another watershed event in the history of PNH. To date, a somatic genetic basis for PNH other than mutant PIGA has not been reported. On the other hand, more than 150 unique mutations in PIGA have been reported in patients with PNH.45 Characterization of the genetic basis of PNH also made possible studies that defined the etiology of the phenotypic mosaicism, showing that PNH phenotype is determined by PIGA genotype,46 and that demonstrated the oligoclonal nature of PNH.46
Conclusions and the Future
The molecular basis of the abnormal complement sensitivity of PNH erythrocytes has been characterized in great detail with these results leading to new, effective therapy in the form of eculizumab.47 We know the cellular basis of PNH (GPI-anchor protein deficiency), we know that PNH is a hematopoietic stem cell disorder and we know the genetic basis of the GPI-anchor protein deficiency. But mutant PIGA does not explain why the affected cells expand and, in many cases, dominate hematopoiesis. Further, we don’t understand how (or if) deficiency of GPI-anchored proteins provides a selective advantage in the setting of immune-mediated bone marrow injury. In this sense, Dacie’s ultimate problem—”the etiology of the disease and its relationship to marrow failure”14—remains unanswered. And the molecular basis of the thrombophilia of PNH remains enigmatic. These issues are the subjects of ongoing investigations and challenges for the future that will undoubtedly be recounted in some subsequent reviews of this alluring disease that has played a unique role in the history of the Society.
Thomas Hale Ham (1905–1987). In 1939, while working in the Thorndike Memorial Laboratory, Harvard Medical Service, Boston City Hospital, Ham reported evidence that complement mediates the abnormal lysis of PNH erythrocytes in acidified serum.2,15 These landmark studies influenced research in the field for the next 50 year, and led to development of a specific diagnostic test for PNH (the acidified serum lysis test or the Ham Test) that was the standard clinical assay until it was supplanted by flow cytometry in the early to mid-1990s. Fortuitously, Ham was on the faculty at Case Western Reserve University at the same time as Louis Pillemer, who discovered the alternative pathway of complement in the 1950s. Colleagues of Ham’s at Case collaborated with Pillemer to demonstrate that hemolysis of PNH erythrocytes in acidified serum is mediated by the alternative pathway. Some of this work was presented at the 1st Organizational Meeting of the American Society of Hematology in 1957. Dr. Ham became the 7th President of the American Society of Hematology. Reproduced from
Thomas Hale Ham (1905–1987). In 1939, while working in the Thorndike Memorial Laboratory, Harvard Medical Service, Boston City Hospital, Ham reported evidence that complement mediates the abnormal lysis of PNH erythrocytes in acidified serum.2,15 These landmark studies influenced research in the field for the next 50 year, and led to development of a specific diagnostic test for PNH (the acidified serum lysis test or the Ham Test) that was the standard clinical assay until it was supplanted by flow cytometry in the early to mid-1990s. Fortuitously, Ham was on the faculty at Case Western Reserve University at the same time as Louis Pillemer, who discovered the alternative pathway of complement in the 1950s. Colleagues of Ham’s at Case collaborated with Pillemer to demonstrate that hemolysis of PNH erythrocytes in acidified serum is mediated by the alternative pathway. Some of this work was presented at the 1st Organizational Meeting of the American Society of Hematology in 1957. Dr. Ham became the 7th President of the American Society of Hematology. Reproduced from
John V. Dacie (1912–2005). Professor Dacie made many important contributions to the clinical and laboratory description of PNH during his tenure on the Pathology faculty of the Royal Postgraduate Medical School, Hammersmith Hospital, London. Among them was the connection between PNH and aplastic anemia. From this observation, he developed the hypothesis that PNH arises in the setting of bone marrow hypoplasia as the result of somatic mutation because in this setting “the abnormal cells must have some, as yet not understood biological advantage.”14 Although he retired in 1975, Professor Dacie’s remarkably clear vision continues to influence PNH research today. Professor Dacie was 87 years old when this photograph was taken. Reproduced from Rosse W. A Brief History of PNH. PNH and the GPI-Linked Proteins. San Diego: Academic Press; 2000:1–20 by copyright permission of Academic Press.
John V. Dacie (1912–2005). Professor Dacie made many important contributions to the clinical and laboratory description of PNH during his tenure on the Pathology faculty of the Royal Postgraduate Medical School, Hammersmith Hospital, London. Among them was the connection between PNH and aplastic anemia. From this observation, he developed the hypothesis that PNH arises in the setting of bone marrow hypoplasia as the result of somatic mutation because in this setting “the abnormal cells must have some, as yet not understood biological advantage.”14 Although he retired in 1975, Professor Dacie’s remarkably clear vision continues to influence PNH research today. Professor Dacie was 87 years old when this photograph was taken. Reproduced from Rosse W. A Brief History of PNH. PNH and the GPI-Linked Proteins. San Diego: Academic Press; 2000:1–20 by copyright permission of Academic Press.
Quantitation of complement sensitivity and demonstration that paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes are a mosaic. Rosse and Dacie developed the complement lysis sensitivity assay to quantify the complement sensitivity of PNH erythrocytes. In the case shown, both complement-sensitive and complement-insensitive populations were identified. RBCs from a patient with PNH (closed circles) and from a normal volunteer (open triangles) were incubated with antibody in excess and incremental (limiting) dilutions of serum as the complement source. Subsequently, hemolysis was quantified. The inflection point of the curve that marks the end of lysis of the complement-sensitive cells and the beginning of lysis of the complement-insensitive cells is indicated by the solid lines. The dashed lines mark the dilution of serum required to produce 50% lysis of normal RBCs and of the complement-sensitive and complement-insensitive PNH cells. These studies demonstrated that PNH erythrocytes are approximately 25 times more sensitive to complement-mediated lysis than normal erythrocytes and that the peripheral blood of patients with PNH is a mosaic of normal and abnormal cells. Reproduced from
Quantitation of complement sensitivity and demonstration that paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes are a mosaic. Rosse and Dacie developed the complement lysis sensitivity assay to quantify the complement sensitivity of PNH erythrocytes. In the case shown, both complement-sensitive and complement-insensitive populations were identified. RBCs from a patient with PNH (closed circles) and from a normal volunteer (open triangles) were incubated with antibody in excess and incremental (limiting) dilutions of serum as the complement source. Subsequently, hemolysis was quantified. The inflection point of the curve that marks the end of lysis of the complement-sensitive cells and the beginning of lysis of the complement-insensitive cells is indicated by the solid lines. The dashed lines mark the dilution of serum required to produce 50% lysis of normal RBCs and of the complement-sensitive and complement-insensitive PNH cells. These studies demonstrated that PNH erythrocytes are approximately 25 times more sensitive to complement-mediated lysis than normal erythrocytes and that the peripheral blood of patients with PNH is a mosaic of normal and abnormal cells. Reproduced from
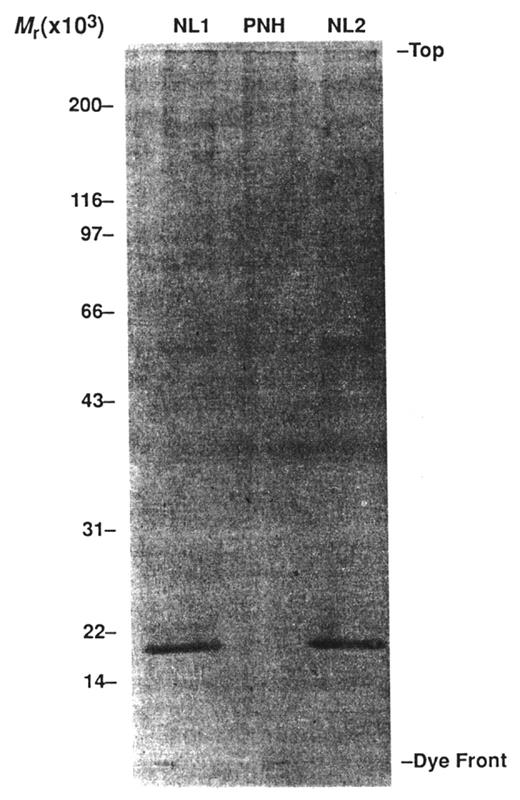
Demonstration that paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes are deficient in MIRL (CD59), the glycosyl phosphatidylinositol (GPI)–anchored protein that inhibits formation of the membrane attack complex of complement. Illustrated is analysis by western blot of normal (NL) and PNH erythrocyte membrane proteins using anti-MIRL as the primary antibody. A band representing a protein with a molecular mass of ~18 kilodaltons is present in the lanes containing normal erythrocyte membrane proteins (NL1 and NL2). A band with this mass is absent in the lane containing the PNH erythrocytes membrane proteins (PNH). Deficiency of MIRL (CD59) and DAF (CD55) accounts for the abnormal sensitivity of PNH erythrocytes to complement-mediated lysis. Reproduced from
Demonstration that paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes are deficient in MIRL (CD59), the glycosyl phosphatidylinositol (GPI)–anchored protein that inhibits formation of the membrane attack complex of complement. Illustrated is analysis by western blot of normal (NL) and PNH erythrocyte membrane proteins using anti-MIRL as the primary antibody. A band representing a protein with a molecular mass of ~18 kilodaltons is present in the lanes containing normal erythrocyte membrane proteins (NL1 and NL2). A band with this mass is absent in the lane containing the PNH erythrocytes membrane proteins (PNH). Deficiency of MIRL (CD59) and DAF (CD55) accounts for the abnormal sensitivity of PNH erythrocytes to complement-mediated lysis. Reproduced from
The glycosyl phosphatidylinositol anchor. This illustration, drawn by Dr. Martin Low, accompanied a News and Views article published in Science in 1985.36 The figure legend, authored by Dr. Low, but excluded from the article, read as follows: “A protein molecule is covalently attached to a phosphatidylinositol molecule situated in the membrane. Cleavage of the phosphatidylinositol by phospholipase C releases the protein from the membrane.” The publicity from this article lead to discovery that all of proteins that are deficient in PNH are anchored to the cell surface by means of this glycosyl phosphatidylinositol moiety. Reproduced from
The glycosyl phosphatidylinositol anchor. This illustration, drawn by Dr. Martin Low, accompanied a News and Views article published in Science in 1985.36 The figure legend, authored by Dr. Low, but excluded from the article, read as follows: “A protein molecule is covalently attached to a phosphatidylinositol molecule situated in the membrane. Cleavage of the phosphatidylinositol by phospholipase C releases the protein from the membrane.” The publicity from this article lead to discovery that all of proteins that are deficient in PNH are anchored to the cell surface by means of this glycosyl phosphatidylinositol moiety. Reproduced from
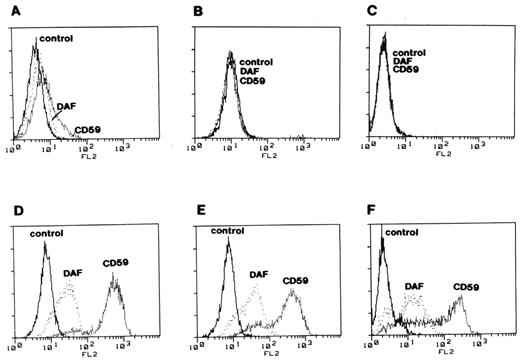
Complementation of deficient surface expression of DAF and MIRL (CD59) on paroxysmal nocturnal hemoglobinuria (PNH) cells. Three glycosyl phosphatidylinositol (GPI)–anchor-deficient cell lines derived from B lymphocytes of patients with PNH (panels A, B & C) were stained with anti-DAF and anti-MIRL (CD59) and analyzed by flow cytometry. The same cell lines transfected with PIGA cDNA (panels D, E & F) were analyzed under the same conditions as the controls. These experiments implied that PIGA is the gene that is mutant in PNH. That mutations in PIGA account for the deficiency of GPI-anchored proteins in PNH was demonstrated by Dr. Junji Takeda, Dr. Taroh Kinoshita and colleagues in this same landmark paper. Reproduced from
Complementation of deficient surface expression of DAF and MIRL (CD59) on paroxysmal nocturnal hemoglobinuria (PNH) cells. Three glycosyl phosphatidylinositol (GPI)–anchor-deficient cell lines derived from B lymphocytes of patients with PNH (panels A, B & C) were stained with anti-DAF and anti-MIRL (CD59) and analyzed by flow cytometry. The same cell lines transfected with PIGA cDNA (panels D, E & F) were analyzed under the same conditions as the controls. These experiments implied that PIGA is the gene that is mutant in PNH. That mutations in PIGA account for the deficiency of GPI-anchored proteins in PNH was demonstrated by Dr. Junji Takeda, Dr. Taroh Kinoshita and colleagues in this same landmark paper. Reproduced from
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Division of Hematology and Bone Marrow Transplantation, University of Utah School of Medicine