Abstract
The hemoglobinopathies encompass a heterogeneous group of disorders associated with mutations in both the alpha-globin and beta-globin genes. Non-sickling disorders are found primarily in individuals of Mediterranean, Asian and Southeast Asian ancestry. With rapid growth in the Asian and Hispanic segments of the US population, the geographic distribution of hemoglobinopathies is expected to become significantly different from what it is today. The epidemiologic changes in the prevalence of non-sickling hemoglobin disorders have important implications for future public health programs, including newborn screening.
The purpose of newborn screening for hemoglobinopathies is to identify clinically significant disorders and provide early education and specialized care prior to the onset of clinical symptoms. Although newborn screening for sickle cell disease is mandated in all states, screening for non-sickling hemoglobinopathies is directed in only one state and limited to reporting of a presumptive diagnosis in most other states. Early delivery of comprehensive care, as well as new and potentially curative therapies, has significantly improved the prognosis for affected patients. This review will consider the increasing prevalence of once uncommon hemoglobinopathies in the US, highlighting the rationale for expanding newborn screening beyond sickle cell disorders.
Historical Perspective
In the US, newborn screening has evolved from isolated testing for a few metabolic disorders to universal screening for more than 30 different genetic disorders, including sickle cell disease and other hemoglobinopathies. All 50 states and the District of Columbia now require that every newborn be screened for sickle cell disease as recommended by the American College of Medical Genetics (ACMG) and endorsed by the March of Dimes.1 In most states, the purpose of the newborn screening for hemoglobin disorders is to identify infants with sickle cell–related conditions in order to prevent life-threatening infection through early initiation of penicillin prophylaxis.2 However, concurrent screening for non-sickling disorders, such as β thalassemia and Hb H disease, is not mandated and is often limited to making a presumptive diagnosis that requires confirmation outside of the newborn screening program. Confirmatory testing and follow up of these infants then becomes the responsibility of the infant’s primary care provider. The effectiveness of neonatal screening programs, when integrated into comprehensive follow-up services and coupled with parental education and support, has been clearly demonstrated in the US.3–6 Although many European countries advocate for prenatal screening as an effective means to prevent births with clinically significant hemoglobinopathies,7–9 studies in the US have failed to show widespread acceptance of prenatal diagnosis among pregnant women found to be at risk by prenatal screening.10,11 Some states have implemented a strategy combining prenatal and neonatal screening programs in an effort to optimize care through prenatal counseling, education and early diagnosis.12
Newborn screening programs that include testing for non-sickling disorders have coordinated systems in place to ensure that affected newborns are referred to a comprehensive care program or subspecialist for ongoing care. Many states have long-standing contracts with academic sickle cell and thalassemia centers to provide follow-up services. Education and counseling are an integral component of these services and ideally provided by a team of experienced health care professionals, including nurses, social workers, psychologists and genetic counselors. Multilingual and culturally sensitive resources are required for prenatal and neonatal education to be effective.13 Early diagnosis and referral of affected infants not only ensures prompt delivery of care, but permits screening for prediction of disease severity and intervention prior to the development of clinical complications. The availability of therapeutic and potentially curative interventions, such as stem cell transplantation, lends further support to the rationale for early diagnosis of thalassemia and other non-sickling hemoglobinopathies through newborn screening in California.
Prevalence of Non-sickling Hemoglobinopathies in the US
The geographic distribution of non-sickling disorders, such as β thalassemia and Hb H disease, varies across the US and currently reflects both a growing Asian population as well as an aging cohort of Mediterranean patients.14 These shifts in age and demographic structure of the thalassemia population are largely a result of treatment advances that have increased survival of patients and historical events leading to increased Asian immigration over the past three decades. In 1979, Chinese immigration to the US jumped after diplomatic relations resumed between the US and China. The simultaneous influx of refugees fleeing the Communist governments of Vietnam, Cambodia and Laos as well as newcomers from other Asian countries such as India and Pakistan resulted in the entry of more than 2.5 million Asians into the US in 1980.15 In an effort to increase the diversity of immigrant flow, the Immigration Act of 1990 raised the total quota for admission of immigrants from “underrepresented” countries. More than 5 million Asian immigrants entered the US that year. In 2000, this figure was over 7 million. Census data show a 2000% increase in Asian immigration in the last three decades. This growth in the Asian population has been accompanied by a corresponding decrease in immigration from European and Mediterranean countries.16
In this historical context, the changing epidemiology of thalassemia and other non-sickling disorders in the US can be explained by the emerging demographic patterns observed in many states. The increased prevalence of certain thalassemic conditions, such as Hb E β thalassemia and Hb H disease, reflects a steady growth in the Asian-American population. At the same time, the β thalassemia population of Mediterranean origin is experiencing increased survival and decreased birth rates. The upshot is that Asian patients now account for over 50% of the US thalassemia population. Previously rare disorders such as Hb E β thalassemia and Hb H disease are now more common than classic β thalassemia major in many regions in the US.14 As a result of these shifts in demographics and increasing dispersal within the US population, the hemoglobinopathies have become a much more genotypically and phenotypically heterogeneous group of diseases.17
Comprising 13% of California’s population, Asians represent the fastest growing ethnic group in the state. Indeed, greater than 40% of the nation’s Southeast Asian population resides in California.18 The carrier frequency of the Hb E mutation among California Asians is almost equal to that of the Hb S mutation among African-Americans.19 In California, 25% and 11% of infants born to parents of Cambodian and Thai/Laotian descent, respectively, are Hb E carriers. Hb H disease, a clinically significant form of α thalassemia, is now the second most common hemoglobinopathy observed in California and has justified universal newborn screening for this condition.20
In California, an estimated 1 in 55,000 newborns is affected with β thalassemia (Table 1 ). While β thalassemia is more frequently observed in newborns of Asian Indian, Middle Eastern and Southeast Asian descent, with annual birth prevalence rates of 1/4000, 1/7000 and 1/10000, respectively, Hb E β thalassemia is exclusive to Southeast Asian newborns, with an estimated birth prevalence of 1 in 2600. Since implementation of newborn screening for non-sickling hemoglobinopathies in 1990, annual birth rates of β thalassemia major and Hb E β thalassemia in California have remained stable at 1.6/100,000.
Alpha thalassemia disorders are also prevalent in California newborns, observed in an estimated 1/9000 California births (Table 2 ). Most of these newborns are diagnosed with Hb H disease, but in our report spanning an 8.5-year period, we also identified 5 cases of Hb Bart’s hydrops fetalis, detected by initial screening showing a Hb Bart’s level >98%.19 Two of these infants subsequently received a stem cell transplant and are transfusion-independent. Since 2006, 3 additional cases of Hb Bart’s hydrops fetalis have been identified through California’s newborn screening program.
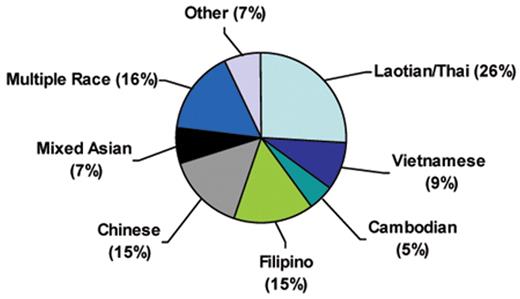
In a recent analysis examining the frequency of Hb H genotypes in California newborns, we reported 131 Hb H and 3 Hb Bart’s hydrops fetalis cases. All of these cases were due to α thalassemia mutations commonly found in Southeast Asian and Southern Chinese populations, consistent with the ethnic distribution of these newborns (Figure 1 ). The majority of cases of Hb H disease occurred in Southeast Asian (40%) infants, followed by Chinese (15%) and Filipino (15%) infants (Kidd J et al. IJLH, accepted for publication, 2009). The clinically more severe Hb H Constant Spring was observed primarily in newborns of Laotian/Thai origin and accounted for 10% of Hb H cases, mirroring the high allelic frequency of the Hb Constant Spring mutation in this ethnic group.
Hb H disease is not exclusive to California’s Asian population. In our study, almost 16% of newborns diagnosed with Hb H were classified as non-Asian or mixed race. A previous study of universal newborn screening for sickle cell disease in California found that 10% of affected newborns would be missed if screening were limited to certain racial and ethnic groups.21 These data, along with the cost and questionable accuracy of determining race and ethnicity in the newborn nursery, argue against targeted screening in states with extensive population admixture and support the rationale for universal screening in California22 where 5% of live births are multiracial.23
Alpha thalassemia is becoming increasingly recognized outside of California. Although most state neonatal screening programs detect and report increased Hb Bart’s, second-tier confirmatory testing for Hb H disorders is not routinely performed. As a consequence, many state NBS programs refer newborn samples with elevated Hb Bart’s to our laboratory for definitive testing. In the 3-year period between 2006 and 2008, 42 cases of Hb H disease (10 with Hb H Constant Spring) were identified by our laboratory among 1163 samples referred by outside state newborn screening programs. Carriers of the Hb CS mutation were also identified in 35 of the total samples referred to our laboratory. While prevalence figures could not be estimated for this set of selected samples, the large number referred for testing suggests that Hb H disease is becoming a greater public health concern in regions outside of California. These data, together with the genetic complexity of the thalassemias, underscore the need for regional or centralized reference laboratories with the capacity to rapidly and accurately test for causative mutations.
Clinical Significance of Non-sickling Hemoglobinopathies
In addition to sickle cell disorders, newborn screening programs have historically focused on identifying infants with classic β thalassemia major. However, clinically significant forms of α-thalassemic disorders, Hb H and Hb H Constant Spring, as well as Hb E disorders are now becoming increasingly recognized in western states.14,19 β thalassemia major refers to individuals who are homozygous or compound heterozygous for β0 thalassemia mutations and who are transfusion dependent as a result of both ineffective erythropoiesis and hemolytic anemia. Without aggressive chelation therapy, transfusion-associated hemosiderosis inevitably ensues, manifested by endocrine, hepatic and cardiac dysfunction.
The phenotype of β thalassemia major can also result from compound heterozygosity for a β0 thalassemia mutation and Hb E, and from coinherited α gene duplications. Hb E β thalassemia presents in infancy as a variably severe anemia with a clinical phenotype ranging from a complete lack of symptoms to transfusion dependence. Osteoporosis, iron overload, growth failure, and pulmonary hypertension are commonly reported complications in both transfused and nontransfused patients.24,25
Patients presenting with a thalassemia intermedia phenotype during childhood often become transfusion dependent as adults due to worsening anemia and fatigue. Consequently, patients with Hb E β0 thalassemia may also develop severe transfusional iron overload and, like other patients with β thalassemia major, eventually succumb to cardiac failure.26 A β0 thalassemia mutation coinherited with alpha gene triplication may also result in a thalassemia intermedia phenotype from globin chain imbalance. In many cases, the β thalassemia genotype may not explain the observed clinical phenotype, reflecting the complex genetic interactions between β-globin genes, β-globin gene production controlling regions, and a growing list of other potential genetic modifiers.
Similarly, Hb H disease has been described as a relatively benign condition, but some variants are more severe than originally considered.27 Since most state newborn screening programs do not include α thalassemia disorders in their screening panel, the diagnosis of Hb H is usually made after clinical complications, such as cholelithiasis, exacerbations of severe anemia, or splenomeglay arise. Up to 50% of patients with deletional Hb H disease require intermittent transfusions.
Hb Constant Spring is the most common nondeletional α thalassemia mutation associated with Hb H disease. Laboratory diagnosis is difficult because the messenger RNA is highly unstable, and there is less than 1% of the total protein output of a wild-type gene. As a result, the Hb H Constant Spring phenotype is moderate to severe, with a majority of patients requiring repeated transfusions in infancy.28 Fifty percent of patients require splenectomy with an associated risk of portal vein thrombosis.29 Older patients may become transfusion independent but still require intermittent transfusion during illness. Increased iron absorption from the gastrointestinal tract results in iron overload in most patients.
Laboratory Methods to Detect Clinically Significant Non-sickling Disorders
In the US, testing for hemoglobinopathies is incorporated into existing programs that screen for other inherited conditions, such as phenylketonuria and congenital hypothyroidism. A capillary blood sample is obtained from newborns by heelstick and preserved as a dried blood spot on filter paper, or “Guthrie card.” Although the recent introduction of tandem mass spectrometry (MS/MS) to newborn screening has enabled multiplexing procedures for simultaneous identification of more than 30 additional medical conditions from a single “Guthrie card,”30 the hemoglobinopathies are not identifiable by MS/MS and still need to be independently screened using analyte-specific assays.
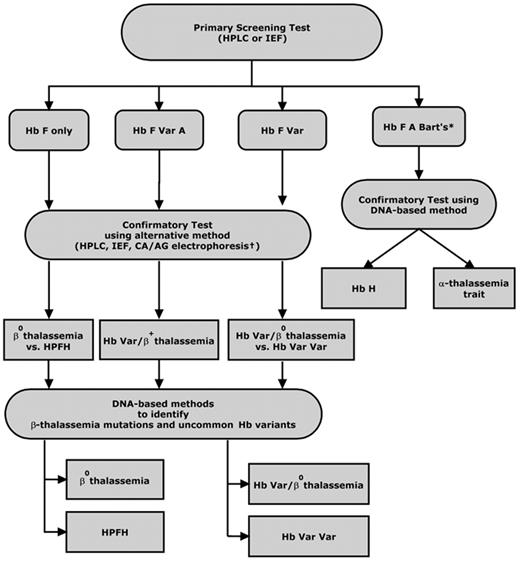
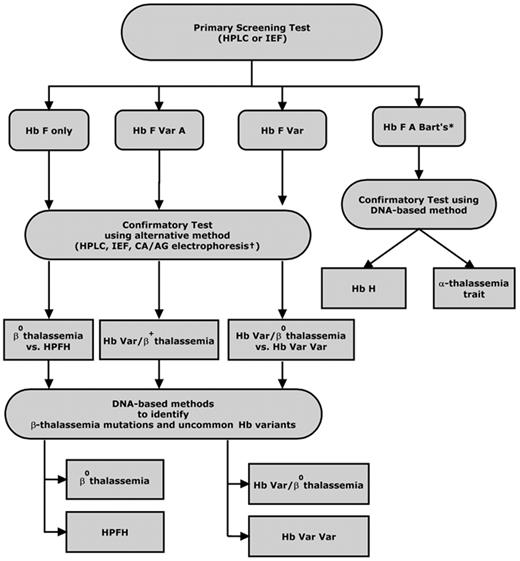
Initial screening methods differ between states, but most newborn screening programs employ high-performance liquid chromatography (HPLC) or isoelectric focusing (IEF) as the preferred first-line technique to make a presumptive diagnosis of a clinically significant hemoglobinopathy (Figure 2 ). These methods are preferred over electrophoretic techniques such as cellulose acetate and citrate agar electrophoresis because they are more sensitive, less labor intensive, and better suited for high-throughput population screening. In addition, HPLC permits quantification of Hb variants. This is particularly advantageous when testing for Hb H disorders, as an accurate quantification of the percentage of Hb Bart’s can be used to identify newborns whose levels exceed a specified threshold and require further follow-up testing. While the sensitivity of HPLC and IEF are excellent, results and interpretation can be confounded by extreme prematurity or previous blood transfusion.31 Ideally, samples should be tested within 72 hours of collection, as hemoglobin on the blood spot will degrade with time, leading to variation in baseline HPLC chromatograms.
Second-tier testing using a complementary method on a second filter blot sample or a liquid blood sample collected from the infant is performed to confirm the initial screening test result. Policies and practices for confirmatory testing of hemoglobinopathies, particularly non-sickling disorders, vary by state. As most states are only mandated to test for sickle cell disease, diagnostic testing for non-sickling disorders is most often directed by the primary care practitioner or a pediatric hematology referral center.
Ethnicity information and parental studies are extremely helpful in guiding the sequence of diagnostic tests for specific hemoglobinopathies. Parental testing to identify carriers for the purpose of defining an infant’s diagnosis and providing genetic counseling typically includes a complete blood cell count and hemoglobin separation by IEF and/or HPLC. Carriers of β thalassemia mutations show a decreased mean corpuscular volume (MCV) and increased levels of Hb A2 and/or Hb F. Thus, accurate quantitation of Hb F and Hb A2 is needed if the MCV is decreased.32
In many cases, only a provisional diagnosis of a clinically significant hemoglobin disorder is made, and most newborn screening programs recommend further confirmation and clarification of the clinical significance of the hemoglobin variants detected when the infant is more than 6 weeks old. However, some newborn screening programs include molecular diagnostic testing as part of second-tier testing to definitively distinguish β thalassemia major from Hereditary Persistence of Fetal Hemoglobin (HPFH), Hb E β thalassemia from Hb EE, and Hb H disease from α thalassemia trait. DNA derived from bloodspots can be amplified by PCR and then genotyped for second-tier confirmation of hemoglobinopathies, particularly thalassemia and Hb H disease.33 More than 200 β thalassemia mutations have been characterized, the majority of which are point mutations or very short deletions/insertions.34 These mutations are regionally specific and their frequencies have been determined for most at-risk populations. The often-used strategy for identifying these mutations is to first test a panel of variants common to the population being screened, with further testing for rare mutations as indicated by clinical suspicion, family history and ethnic background.
Two general PCR-based approaches, allele-specific oligonucleotide hybridization (ASO) and allele-specific priming, are used to detect both β thalassemia and α thalassemia point mutations.35,36 PCR-based assays are amenable to multiplexing and have been adapted for simultaneous detection of the hemoglobin variants and thalassemia mutations relevant to the screened population. The most commonly used methods are reverse dot blot analysis or primer-specific amplification, using a set of probes or primers complementary to the mutations of interest. The sensitivity of the various methods used is variable depending on the mutations included in the panel and the individual’s ethnicity. If the mutation remains unidentified, direct DNA sequence analysis is performed. The sensitivity of DNA sequencing is 99%. Gap PCR methods are employed to detect common α thalassemia deletion mutations, α gene duplication, and other deletional variants such as Hb Lepore and HPFH. Novel methods, such as multiplex ligation–dependent probe amplification (MLPA), have recently been developed to screen for unknown globin gene deletions by determining copy number variation in genomic sequences.37
Genomic microarrays have recently been advocated as a potential diagnostic tool for newborn screening.38 The advantages of this technology include the ability to simultaneously screen for many genetic and metabolic conditions with one test, thereby improving efficiencies of scale. Carriers, as well as affected individuals, could be identified using this approach. In the setting of hemoglobinopathies, microarray analyses to detect disease-modifying genes as well as causative globin gene mutations would enhance prediction of disease severity and lead to improved treatment, planning and counseling.39,40 Such risk-based approaches to treatment could be applied, for example, when assessing an individual’s eligibility for stem cell transplantation versus standard therapies.
Integration of Newborn Screening with the Medical Home Model
Integration of newborn screening with comprehensive care and public health services is an essential component of newborn screening programs. State newborn screening follow-up programs ensure that infants identified by newborn screening enter a seamless system of care that includes consultation with specialists as well as routine health maintenance. The American Academy of Pediatrics has further defined a model, or “medical home,” as comprehensive care that is accessible, family-centered and culturally effective.41 In this context, newborn screening programs could provide the initial steps toward long-term comprehensive follow-up and medical management through collaboration with state-sponsored programs for special health care services for children.
Ideally, neonatal and prenatal screening programs are integrated, so that parents may make informed choices based on their risk for having a child with a hemoglobinopathy and receive education on the prevention and treatment of complications should they have an affected child. Linguistic isolation and socioeconomic barriers, often associated with immigrant populations, need to be overcome by trained counselors and translators. A multi-disciplinary approach that addresses the changing epidemiology and treatment of thalassemia and other hemoglobinopathies will ensure improved quality of life and survival.
Future Directions in Newborn Screening
With the increasing prevalence of hemoglobinopathies in the US, new approaches to newborn screening for these disorders are needed to ensure appropriate and ongoing delivery of health care to affected individuals. Expansion of newborn screening to integrate clinically relevant non-sickling hemoglobinopathies into the model established for sickle cell disease will require additional resources as well as continued efforts by federal-state partnerships to develop uniform guidelines for testing and follow-up.
The success of expanded screening relies on well-developed surveillance and data-tracking systems that will enable continuous evaluation and improvement. Surveillance for hemoglobinopathies by NBS programs may serve a dual purpose by providing a basis for both observational and experimental research in addition to case tracking for clinical follow up. Understanding the phenotypic expression of the various hemoglobin disorders, many of which are not well characterized even in their countries of origin, will be essential for optimizing treatment for patients in the future. Monitoring the natural history and health outcomes of affected children will accelerate research in this area and inform practical application of needed services. Expansion of newborn screening programs to include non-sickling hemoglobinopathies may provide the first steps towards a comprehensive care model that ensures long-term follow-up and the provision of necessary treatment and services to all affected patients across their lifespan.
Laboratory testing for non-sickling hemoglobinopathies in newborns. *In California, Hb Bart’s is quantified by HPLC; a level > 25% requires confirmatory testing for Hb H disease. †CA indicates citrate agar electrophoresis; AG, agarose gel electrophoresis; Var, variant hemoglobins (eg, Hb S, C, E, D, O-Arab).
Laboratory testing for non-sickling hemoglobinopathies in newborns. *In California, Hb Bart’s is quantified by HPLC; a level > 25% requires confirmatory testing for Hb H disease. †CA indicates citrate agar electrophoresis; AG, agarose gel electrophoresis; Var, variant hemoglobins (eg, Hb S, C, E, D, O-Arab).
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Children’s Hospital & Research Center Oakland, Oakland, CA