Abstract
Hemochromatosis comprises a group of inherited disorders resulting from mutations of genes involved in regulating iron metabolism. The multicenter, multi-ethnic Hemochromatosis and Iron Overload Screening (HEIRS) Study screened ~100,000 participants in the US and Canada, testing for HFE mutations, serum ferritin and transferrin saturation. As in other studies, HFE C282Y homozygosity was common in Caucasians but rare in other ethnic groups, and there was a marked heterogeneity of disease expression in C282Y homozygotes. Nevertheless, this genotype was often associated with elevations of serum ferritin and transferrin saturation and with iron stores of more than four grams in men but not in women. If liver biopsy was performed, in some cases because of evidence of hepatic dysfunction, fibrosis or cirrhosis was often found. Combined elevations of serum ferritin and transferrin saturation were observed in non-C282Y homozygotes of all ethnic groups, most prominently Asians, but not often with iron stores of more than four grams. Future studies to discover modifier genes that affect phenotypic expression in C282Y hemochromatosis should help identify patients who are at greatest risk of developing iron overload and who may benefit from continued monitoring of iron status to detect progressive iron loading.
Hemochromatosis refers to a group of inherited disorders characterized by excessive dietary iron absorption, which in some cases can lead to severe iron overload.1–3 Five types of hereditary hemochromatosis are recognized, each caused by mutations in different genes involved in iron metabolism (Table 1 ). The most common form among Caucasian populations of northern European origin is related to mutations in the hemochromatosis (HFE) gene,4–6 and 80% to 90% of Caucasian patients diagnosed with hemochromatosis in the United States are homozygous for the HFE C282Y mutation (nt845G→A; cys282tyr).7,8 Another common mutation of HFE, H63D (nt187C→G; his63asp) rarely is a cause of iron overload in the homozygous state or in the compound heterozygous state with C282Y.2,5,9 Some hemochromatosis patients lack the C282Y/C282Y genotype or carry no known HFE mutation at all, and such patients may have one of the other disorders listed in Table 1 or have as-yet-unknown mutations in HFE or other genes involved in iron metabolism. The heterozygous C282Y carrier state, C282Y/wild type (C282Y/wt), found in ~10% of persons of northern European descent, is most often not associated with any significant increase in iron stores.
Mechanisms of Altered Regulation of Iron Metabolism in Hemochromatosis
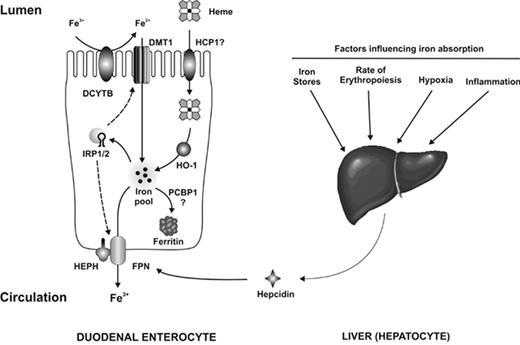
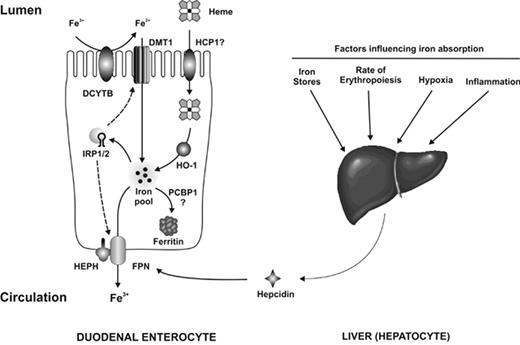
Recent advances in our understanding of hemochromatosis point to lack of hepcidin, or resistance on the part of ferroportin to hepcidin binding, as the central mechanism for the development of increased iron stores in all of the conditions listed in Table 1.10–12 Hepcidin, the product of the HAMP gene, is a liver-derived peptide that suppresses release of iron from enterocytes and macrophages by interacting with ferroportin (FPN), its cognate receptor on these cells, causing it to be internalized and degraded.13 The pathway of intestinal iron absorption across the duodenal enterocyte and its regulation are illustrated in Figure 1 .
Hepcidin expression is impaired in patients with HFE-related hemochromatosis and is inappropriately low for the level of body iron stores.15 This would be expected to lead to increased expression of FPN on the basolateral membrane of enterocytes and increased release of intestinal mucosal iron to plasma transferrin. Thus, lack of hepcidin (or, in some cases, resistance to it) appears to explain the earlier observation that the major determinant of increased iron absorption in patients with hemochromatosis is an increase in the rate constant for transfer of iron across the basolateral membrane of duodenal enterocytes to the systemic circulation,16 so that intestinal iron absorption is inappropriately high in relation to body iron stores.16,17
Lack of hepcidin or resistance to the molecule also leads to altered regulation of iron storage in macrophages, with excessive release of iron to the circulation.18 Thus, whereas macrophages normally have an important role in iron storage, the macrophages in patients with hemochromatosis paradoxically contain little iron despite the presence of iron overload in other tissues.19 The combination of excessive intestinal iron absorption and uncontrolled release of iron from macrophages results in increased plasma iron concentration and enhanced uptake of iron by parenchymal cells of the liver (hepatocytes) and a variety of other organs. (For further details on molecular mechanisms of iron homeostasis, the reader is referred to the accompanying article by A-S Zhang and CA Enns, beginning on page 207.)
The Hemochromatosis and Iron Overload Screening (HEIRS) Study
The major complications of iron overload in hemochromatosis patients can be prevented by phlebotomy therapy to remove excess iron, and patients treated before the onset of organ damage have a normal life expectancy.20 Although it is not known how many such patients will go on to develop organ damage if untreated, this observation has stimulated interest in early detection,3,21,22 and several screening studies recently have been conducted,23–27 including the Hemochromatosis and Iron Overload Screening (HEIRS) Study.28 The HEIRS Study evaluated the prevalence, genetic and environmental determinants, and potential clinical, personal, and societal impact of hemochromatosis and iron overload in a multi-ethnic, primary care-based sample of 101,168 adults enrolled over a 2-year period at four field centers in the US and one in Canada. Initial screening of participants included genotyping for the HFE C282Y and H63D alleles, measurement of serum ferritin (SF), iron and unsaturated iron-binding capacity (UIBC) levels, and calculation of transferrin saturation (TfS).28 The initial screening participants included 63,550 women (62.8%) and 37,618 men (37.2%). The median age was 50 years (range, 25 to 100). By self-identified race/ethnicity, the sample included 44% Caucasian participants, 27% African-American, 13% Asian, 13% Hispanic, 0.7% Pacific Islander, 0.7% Native American, and 2% mixed or unknown race. A follow-up clinical evaluation was offered to all HFE C282Y homozygotes and to all participants whose TfS and SF values exceeded study thresholds: TfS > 50% and SF > 300 μg/L for men; TfS > 45% and SF > 200 μg/L for women.28 In addition, to conduct family studies, relatives of probands were invited to participate in the clinical evaluation. In this presentation, we summarize insights into hemochromatosis that the HEIRS Study has provided.
Prevalence of HFE C282Y Homozygosity among Primary Care Patients in the HEIRS Study
Of 99,711 participants who did not learn about the study exclusively from a participating family member, 299 were homozygous for the C282Y mutation. The frequencies of each HFE genotype in different racial/ethnic groups are shown in Table 2 . The overall frequency of homozygosity for the C282Y mutation in non-Hispanic Caucasians was 4.4 per 1000,29 although there were some geographic differences in the frequencies of C282Y homozygotes among field centers.6 The estimated prevalence of C282Y homozygotes in non-Hispanic Caucasians was higher than in Native Americans, Hispanics, African Americans, Pacific Islanders, or Asians. The results indicate that the yield of HFE genotyping in identifying persons with C282Y homozygosity is low in racial/ethnic groups other than non-Hispanic Caucasians, which has important implications for screening strategies. These HEIRS Study results compare favorably to other studies, indicating that homozygosity for the C282Y mutation is found in 4–5 of every 1000 persons of northern European descent.5,26,30–34 A previous study had reported a frequency of homozygotes in Hispanics that was comparable to that in non-Hispanic Caucasians,35 but this was not confirmed by HEIRS or other studies.29,36
Elevations of Indirect Measures of Iron Status in Participants with or Without HFE C282Y Homozygosity
Elevations of Iron Tests in Participants with HFE C282Y Homozygosity or Other Genotypes
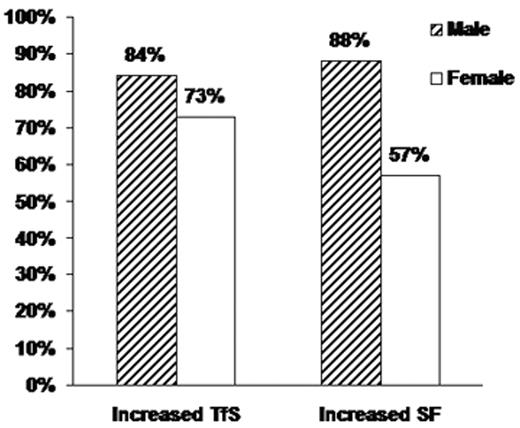
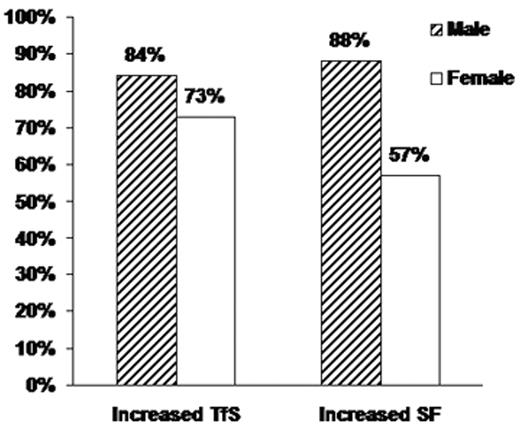
Among 98,529 participants who did not report a previous diagnosis of hemochromatosis or iron overload, data from non-Hispanic Caucasians indicated a strong association between HFE genotype and TfS subpopulations, with the highest mean TfS levels found in C282Y homozygotes.37 Moreover, only in C282Y homozygotes were the mean TfS and SF levels above the upper limits of the reference ranges.29 Among participants who were homozygous for the C282Y mutation but in whom iron overload had not been diagnosed (227 participants; 89 men and 138 women), the majority had elevated levels of TfS and SF (Figure 2 )29; both TfS and SF were higher in men than in women. Because TfS may become elevated in C282Y homozygotes before elevation of SF occurs, TfS has been proposed for screening, especially in younger persons. However, based on the thresholds for elevated TfS and SF used in the HEIRS Study, screening with TfS would have failed to identify 16% of male and 27% of female C282Y homozygotes in this population. Screening with SF would have failed to identify 12% of male and 43% of female homozygotes (Figure 2 ).29 Overall, the HEIRS Study identified 29 C282Y homozygotes and 335 other participants with SF > 1000 μg/L in whom iron overload had not previously been diagnosed.
UIBC appeared to be at least as useful as TfS in detecting C282Y homozygotes and had a greater area-under-the-curve for the receiver operating curve in comparison with TfS.38 Also, because UIBC is a single automated test, it is somewhat less expensive than TfS. Neither TfS nor UIBC proved more reliable in detecting C282Y homozygotes using morning fasting specimens at the time of the follow-up clinical examination than with the random samples obtained at initial screening.39 Based on these findings, the results of the HEIRS Study do not support a superiority of fasting versus non-fasting TfS or UIBC for screening for hereditary hemochromatosis or a superiority of these tests versus SF for this purpose.
Variations in Elevations of Iron Tests in Different Racial/Ethnic Groups
Among all racial/ethnic groups in the HEIRS Study, Pacific Islanders and Asians had the highest geometric mean levels of SF and mean TfS despite having the lowest prevalence of C282Y homozygotes.40 The biological basis and clinical significance of higher levels among Asians and Pacific Islanders are unclear. In African Americans, overall mean TfS and percentages of participants with elevated TfS were significantly lower than in Caucasians, and overall mean SF and percentages of participants with elevated SF were significantly greater than in Caucasians.41 The lower TfS and higher SF levels in African-American participants in the HEIRS Study may have implications for the approach to phenotypic screening for iron overload in African Americans. Approximately 7% of the adult African Americans had elevation of SF in combination with TfS in the highest-quartile (TfS > 29% for women and > 35% for men).42
Increased Iron Stores Revealed by Direct Measurements in C282Y Homozygotes and Non-C282Y Homozygotes
Liver Biopsy
Follow-up clinical evaluation was performed in 302 of 333 HFE C282Y homozygotes and 1375 of 1920 nonhomozygotes with SF > 300 μg/L (men), > 200 μg/L (women) and TfS > 50% (men), > 45% (women). Liver biopsy was not part of the protocol for the HEIRS Study. However, a small number of participants had diagnostic liver biopsies performed by their personal physicians as part of their clinical care, and specimens were available to the study from 22 of 302 HEIRS C282Y homozygotes and 64 of 1375 non-C282Y homozygotes who presented for follow-up clinical evaluation.43 The indications for liver biopsy were not exclusively to document iron overload and included examinations performed to assess viral hepatitis and to evaluate causes of increased ALT or AST levels. Liver iron concentration measurements were available in 34 participants, including 14 C282Y homozygotes. Twelve of the 14 C282Y homozygotes and 7 of 20 non-C282Y homozygotes had elevated hepatic iron concentrations,43 including 3 (of 8) Asians and 1 (of 2) African Americans. Three of the 7 non-C282Y homozygotes with increased liver iron concentration had a diagnosis of hepatitis B or C or nonalcoholic steato-hepatitis, and 4 had no diagnosis of liver disease, including 1 Caucasian compound heterozygote (C282Y/H63D) with a mildly increased liver iron concentration, 1 Caucasian C282Y heterozygote, and 2 Asians; 1 was heterozygous for H63D and one had no C282Y or H63D mutation.43
Quantitative Phlebotomy
Available data from clinically indicated phlebotomy treatment performed by health care providers made it possible to quantify the amount of iron removed to achieve iron depletion in some of the participants. Thus, it was possible to assess the degree of iron overload by phlebotomy in a larger sample of HEIRS participants than by liver biopsy. C282Y homozygotes and nonhomozygotes with persistently elevated SF at clinical evaluation were advised to consider a phlebotomy program. The recommended phlebotomy regimen consisted of removal of one unit of blood weekly until the SF decreased to < 50 μg/L. An increase in body iron stores was prospectively defined as > 2.0 g (2–4 g, mildly increased; 4–10 g, moderately increased; 10–20 g, substantially increased; and > 20 g, severely increased). Quantitative phlebotomy was conducted in 122 of 175 C282Y homozygotes. These homozygotes may or may not have had elevated SF and TfS at screening. The estimated prevalence in the Caucasian population of C282Y homozygotes with SF > 900 μg/L at subsequent clinical evaluation, regardless of SF and TfS at initial screening, was 20 per 10,000 men and 4 per 10,000 women; this constellation was predictive of iron stores > 4 g in men and > 2 g in women. This prevalence is consistent with other primary care-and population-based studies.25,26
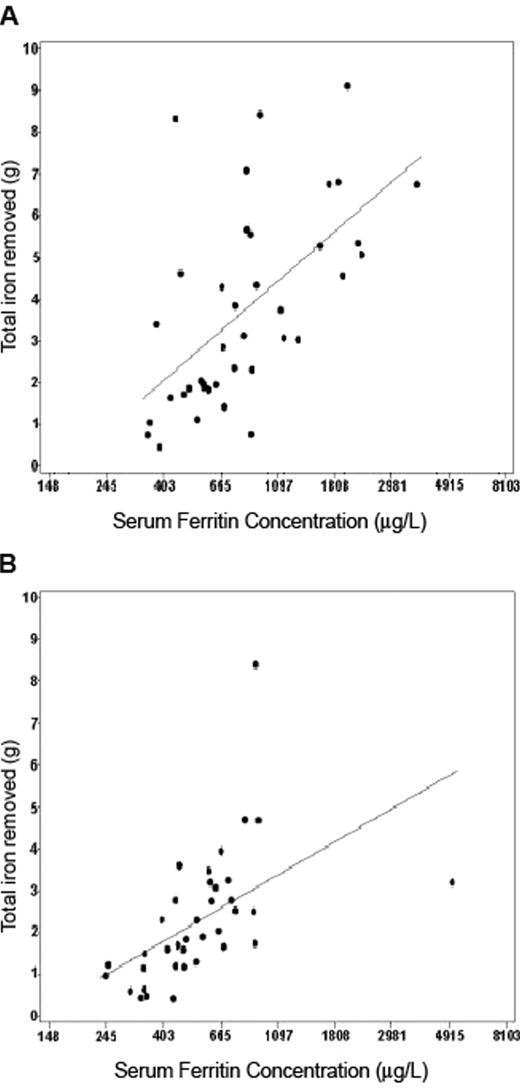
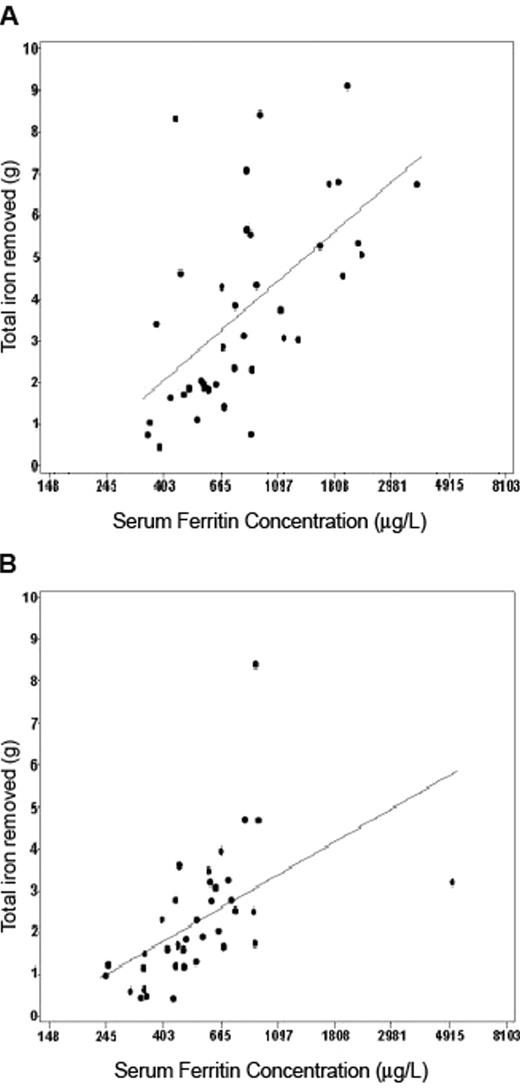
Quantitative phlebotomy was conducted in 122 of 1102 non-homozygotes with non-transfusional SF elevation at clinical evaluation. All of these non-homozygous participants had elevated SF and TfS at initial screening. The estimated prevalence per 10,000 of non-C282Y homozygotes with SF > 900 μg/L at clinical evaluation was 7 among Caucasians, 13 among Hispanics, 20 among African Americans, and 38 among Asians and Pacific Islanders, and this constellation was predictive of iron stores > 2 g but < 4 g.44 Only 1 non-C282Y homozygote was observed to have iron stores > 4 g on the basis of quantitative phlebotomy. Similar to the phlebotomy study of Beutler et al published in 2002,45 there were fairly robust correlations between serum ferritin and mobilizable iron stores among male and female HEIRS HFE C282Y homozygotes (Figure 3 ) and also significant correlations among Caucasian, African-American, Hispanic, and Asian and Pacific Islander non-homozygotes.
In conclusion, among multi-ethnic primary-care patients in the United States and Canada, SF > 900 μg/L in C282Y homozygotes or following initial elevations of SF and TfS in non-homozygotes is highly predictive of body iron stores > 2 g regardless of HFE genotype, ethnicity, gender, or elevations of ALT, AST, or CRP. SF > 900 μg/L is highly predictive of body iron stores > 4 g in male C282Y homozygotes but not in female C282Y homozygotes or in male or female non-homozygotes. SF levels in the ranges of 200 to 900 μg/L (women) or 300 to 900 μg/L (men) in C282Y homozygotes or following initial elevations of SF and TfS in non-homozygotes are associated with iron stores > 2 g in about one half of C282Y homozygotes and about one third of non-homozygotes. Interestingly, our results predict that about 40% of primary-care male C282Y homozygotes with SF concentrations between 300 μg/L and 900 μg/L have iron stores > 4 g.
Other approaches to the assessment of tissue iron overload, beyond the scope of the HEIRS Study and this article, are reviewed separately in the accompanying article by R Fischer and PR Harmatz, beginning on page 215.
Clinical Manifestations in C282Y Homozygotes
Symptoms and Signs
To assess the prevalence of clinical manifestations of hemochromatosis in C282Y homozygotes, HEIRS participants were asked at the time of initial screening before receiving the results of any genetic test whether they had a history of liver disease, diabetes, arthritis, congestive heart failure, impotence, or infertility. Among men, C282Y homozygotes and C282Y/H63D compound heterozygotes were more likely to report a history of liver disease than were participants without HFE mutations (wt/wt).29
To examine the prevalence of clinical manifestations in greater detail, C282Y homozygotes and control subjects were examined further at the subsequent follow-up clinical evaluation. The evaluation included a participant-completed questionnaire addressing medical history and a focused physical examination of the heart, liver, spleen, skin, and metacarpophalangeal joints.28 In addition, a morning fasting blood sample was obtained for confirmation of genotyping results for the C282Y and H63D alleles,7,46 repeat TfS and SF determinations,28,38 and measurements of serum glucose and insulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), and C-reactive protein (CRP). Controls were frequency-matched for age and gender to cases seen at each Field Center. The medical history questionnaire and physical examination were designed to document the prevalence of the kinds of symptoms and clinical conditions that have been reported in previous studies to be associated with hemochromatosis and iron overload.1–3,21,47,48 Participants also completed the SF-36 General Health scale.49 Altogether, 36 outcome variables (symptoms, clinical conditions, and physical signs) were included in this evaluation. Non-Hispanic Caucasians who had complete data available for evaluation (N = 282) were compared to controls who carried neither the C282Y nor H63D HFE alleles (wt/wt) and had SF and TfS levels between the 25th and 75th percentiles of gender-specific distributions (N = 364).50 Regression analysis revealed that previously diagnosed C282Y homozygotes and newly-diagnosed homozygotes with elevated SF had higher prevalences of chronic fatigue, hyperpigmentation and swelling or tenderness of the second and third metacarpophalangeal joints than control subjects. Joint stiffness was also more common among newly diagnosed C282Y homozygotes with elevated SF than among control subjects.50 Although in the HEIRS Study the observed prevalences of most other outcomes, such as self-reported symptoms and signs of liver disease, heart disease, diabetes, and other major clinical manifestations of hemochromatosis, were also higher in previously diagnosed C282Y homozygotes and to a lesser extent in newly diagnosed homozygotes with elevated SF, than in controls, there were no statistically significant differences for these outcomes after adjustments for gender, age, and 36 multiple comparisons. The increased reports of fatigue and evidence of arthropathy in HEIRS C282Y homozygotes are consistent with a recent longitudinal study from Australia, a study which also found that male C282Y homozygotes with an SF higher than 1000 μg/L were more likely to report a history of liver disease than men without HFE mutations.51
Hematologic Values
Recent studies have found higher MCV and hemoglobin levels in C282Y homozygotes with increased TfS at the time of diagnosis than in patients with other HFE genotypes or control subjects.35,52 This is likely attributable to increased iron uptake by erythroid precursors. Among HEIRS C282Y homozygotes, the adjusted mean MCV and hemoglobin levels in women, but not in men, were higher than in HFE wt/wt controls. This effect was observed even for female C282Y homozygotes with SF in the reference range, suggesting that an as-yet-unidentified influence of the HFE C282Y/C282Y genotype is a significant determinant of MCV and hemoglobin in women, in addition to the effects of elevated TfS and SF.53
Assessment of Thyroid Function, Serum Lipids, and Pancreatic and Liver Function
The prevalences of hypothyroidism and hyperthyroidism in HEIRS C282Y homozygotes and controls were comparable, suggesting that routine measurements of thyroid-stimulating hormone or free thyroxin levels are not necessary as part of screening programs for iron overload.54
In the HEIRS Study, total serum cholesterol and low-density lipoprotein (LDL) levels were lower in C282Y homozygotes than in HFE wt/wt controls.55 It remains to be seen whether lower total and LDL cholesterol levels in C282Y homozygotes are related to excess iron, a direct effect of HFE gene mutations, or other genes in linkage disequilibrium with the HFE locus.
The serum glucose, insulin, AST, GGT and CRP levels in C282Y homozygotes and controls were comparable. ALT was higher in previously diagnosed homozygotes and in newly diagnosed homozygotes with elevated SF than in newly diagnosed homozygotes with normal SF (P < .001).
Histological Evaluation of Liver Biopsies
Of 11 C282Y homozygotes in the HEIRS Study with elevated SF (497 μg/L to 5200 μg/L) who underwent liver biopsy, fibrosis was present in 8 cases, including 1 with cirrhosis.43 Thus, some C282Y homozygotes had previously undiagnosed liver damage that was revealed by biopsy. This illustrates the fact that hepatic fibrosis and cirrhosis in hemochromatosis patients can be silent.56,57
Evidence for Other Genetic Modifiers of Clinical Manifestations in C282Y Homozygotes and Non-homozygotes
Heritability Studies
Heterogeneity in the severity of iron overload in patients with hemochromatosis and the variability of clinical manifestations has long been recognized,58 and it is now known that some of this heterogeneity is related to the different genetic basis of the various kinds of hemochromatosis (Table 1 ). However, the reasons for the heterogeneity among C282Y homozygotes remain largely unknown, and this is one of the most important questions extant in the field. In the HEIRS Study, a self-reported history of hemochromatosis or iron overload in the participant’s family was a predictor of the risk of iron overload in the participant.59 Analysis of the joint distribution of TfS and SF in groups of HEIRS Study participants, including African Americans, Asians, Hispanics, and non-Hispanic Caucasians, identified different components with successively increasing means for TfS and SF in each population.60 In a further analysis of HEIRS probands and their relatives, the heritability of serum iron measures was formally estimated to assess the proportion of the observed variation among participants and family members that is attributable to hereditary factors. Probands were HFE C282Y homozygotes or non-C282Y homozygotes with elevated TfS (TfS > 50%, men; TfS > 45%, women) and SF concentration (SF > 300 μg/L, men; SF > 200 μg/L, women). Heritability (h2) was estimated by variance component analysis of TfS, the natural logarithm (ln) of SF, and unsaturated iron-binding capacity (UIBC). For HFE C282Y homozygote probands and their family members, excluding variation due to HFE C282Y and H63D genotype and measured demographic and environmental factors, the residual h2 (SE) was 0.21 (0.07) for TfS, 0.37 (0.08) for ln SF, and 0.34 (0.08) for UIBC (all P < .0004 for comparisons with zero). For the non-C282Y homozygote proband group, residual h2 for ln SF was 0.64 (0.26), which was also significant (P = .0096). The results indicate that serum iron measures have significant heritability components, after excluding known genetic and non-genetic sources of variation.61
Less-common HFE Alleles and Mutations in Genes Other than HFE as Potential Modifiers of Iron Phenotype
Occasional reports of patients with HFE-related hemochromatosis who also have mutations in other genes, such as HJV and HAMP, have suggested that such potential genetic modifiers may account for some of the observed heterogeneity of iron phenotype in patients with C282Y homozygosity. However, these cases are rare and explain only a small proportion of the variability in the disorder.62 In addition, HFE mutations other than C282Y and H63D may in some cases be associated with iron overload. For example, the less-common HFE mutation S65C (nt193A→T; ser65cys) was associated with an increased risk of hemochromatosis in C282Y/S65C compound heterozygotes in Australia,63 although in a recent screening study this allele was not associated with elevations of TfS or SF in ethnic Danish men.64 In recent years, genes classically linked to the immune system have emerged as modifiers of iron overload.65
To identify quantitative trait loci (QTL) that influence iron-related phenotypes, the first genome-wide linkage analysis in a human population for this purpose was conducted using microsatellite markers as part of the HEIRS Family Study.66 The strongest evidence of linkage for TfS, UIBC, and SF was to the chromosome 6p region containing HFE. After adjustment for HFE genotype and other covariates, the strongest linkage relationship was between SF and chromosome 16p. In addition, there was linkage of UIBC to chromosome 17q and to chromosome 5q. The gene heme oxygenase 2 (Hmox2) maps to chromosome 16p13.3, but it is not clear how mutations in Hmox2 might modify SF. The specified regions of chromosomes 16p, 17q, and 5q are candidates for fine mapping to more precisely localize QTL that contribute to variability of iron status markers SF and UIBC.
To examine the influence of candidate genes on iron status, HEIRS Study participants who previously had been genotyped for C282Y and H63D were assigned to groups of C282Y homozygotes according to TfS and SF levels (high TfS/SF or low TfS/SF) and controls, selected without constraint regarding TfS and SF. Denaturing high-performance liquid chromatography (DHPLC) was used to screen 20 regions of HFE, ferroportin (SLC40A1), HAMP, HJV, TFR2, and ferritin light chain (L-ferritin, FTL) in each participant. Non-C282Y homozygotes, grouped by race/ethnicity as either high TfS or SF or controls, were also screened. The DHPLC analyses were successful in 99.3% of participants and detected 117 different mutations. Mutations other than HFE C282Y and H63D reported to be pathogenic were infrequently associated with high TfS/SF phenotypes. In a previous study of selected HEIRS C282Y homozygotes, no deleterious non-HFE mutations had been identified in any of these genes.67 However, in the current study, the frequencies of two mutations, HJV c.-6C>G and FTL L55L, were greater in Caucasians with high TfS/SF than in controls (0.0811 vs. 0.0200, P = .0144; 0.5743 vs 0.4400, P = .0204, respectively). The results indicate that genetic regions in linkage disequilibrium with HJV c.-6> G and FTL L55L could partly explain high TfS/SF phenotypes in Caucasians.68 One Hispanic woman without iron overload was heterozygous for the HAMP promoter mutation nc.-153C>T.69 The nc.-153C>T mutation was previously reported in a man with C282Y homozygosity and severe iron overload,70 but it is rare.69,70
In an HEIRS sub-study, African-American men with elevated SF had a significantly higher frequency of SLC40A1 Q248H than the African-American men who were controls (17% of 106 men with elevated SF versus 5% of 60 controls, P = .047), but this finding did not apply to women.71 The HEIRS investigators concluded that SLC40A1 Q248H is probably a relatively minor contributor to increased SF and increased iron stores in African Americans.
The unexpectedly high prevalence of elevated TfS and SF among Asians in the HEIRS Study led the investigators to hypothesize that this observation might be explained by the presence in the population of persons carrying a novel HFE IVS5+1 G/A splice site mutation previously reported in a Vietnamese man with iron overload.72 A group of 200 HEIRS Vietnamese participants from southern California with TfS and SF values above the 75th percentile and 149 Vietnamese controls were genotyped. The allele frequencies of the splice site mutation in the two groups were comparable (2.32% vs 2.04%, respectively, P > .05), suggesting that the mutation is not the major explanation for the high prevalence of elevated iron status tests in HEIRS Asian participants.72,73 Severe iron overload recently has been shown to be associated with novel mutations in HJV, HAMP, and FPN in Asian families from Pakistan, Bangladesh, Sri Lanka, and Thailand. None of the patients in these families had mutations in HFE.74
Another molecule possibly involved in iron absorption is heme carrier protein 1 (HCP1), first identified as an intestinal heme transporter. In the HEIRS Study, the entire coding region of the HCP1 gene was examined using DHCLP in C282Y homozygotes, non-homozygotes with elevated TfS and SF, and controls. Although eight HCP1 variants were identified, these were infrequent, occurring only in the heterozygous state and usually in a single participant. A disproportionate number of participants with non-synonymous coding-region mutations had elevated TfS.75
In summary, despite the discovery of numerous mutations of HFE and other genes of iron metabolism, their individual or cumulative allele frequencies do not account for most iron phenotype heterogeneity in C282Y homozygotes, indicating that routine screening to detect unusual HFE or non-HFE mutations would have a low yield in population- or primary care-based screening for hemochromatosis and iron overload. Future gene discovery studies could provide further insight into modifiers of HFE-related hemochromatosis and other clinical disorders that are characterized by perturbations of iron metabolism.
Social, Ethical, and Legal Issues (ELSI) Related to Screening in the HEIRS Study
To evaluate the possible personal impact of screening for and/or receiving a diagnosis of hemochromatosis or iron overload, the HEIRS Study included surveys and questionnaires designed to assess participants’ perceptions of a variety of social, ethical, and legal issues (ELSI). A majority of participants did not express concern about the possibility that genetic testing might cause difficulty in obtaining or keeping health insurance, although there were disparities among different racial/ethnic groups, with African Americans and Asians being much less likely and Hispanics being more likely to have this concern.76 However, few participants surveyed one year after screening reported insurance or employment problems.77
Participants with HFE mutations or elevated TfS and SF of uncertain significance were more likely to report diminished general health and mental well-being than controls, and they had more health worries78; this was particularly so for C282Y homozygotes with non-sustained elevations of TfS or SF, who were significantly more likely to have such concerns.79 These results may have important implications for screening studies in which information about genotype and phenotype are communicated to participants. A number of factors predicted lower understanding of test results among participants, including lower education levels, older age, and being non-white and/or non-English speaking80; C282Y homozygotes had the best understanding of genetic results. Thus, explaining aberrant TfS and SF test results and genotypes, and communicating recommendations for further evaluation and the need for screening relatives, require culturally appropriate strategies.
Summary
Based on the results, the HEIRS Study Investigators have drawn several key conclusions and made certain recommendations.81 First, although genetic testing is well accepted and associated with a minimal risk of discrimination, generalized population screening in a primary care population as performed in the HEIRS Study is not recommended. Elevations of serum ferritin are common, particularly in Asians, Pacific Islanders, and African Americans. However, in the absence of homozygosity for HFE C282Y, this finding usually does not reflect an increase of iron stores more than 4 grams. HFE C282Y homozygosity is not reliably detected by TfS, which limits its role as a screening test. Most clinical manifestations typically associated with hemochromatosis are no more common in HFE C282Y homozygotes identified by screening in primary care populations than in control subjects lacking HFE mutations. An increase in iron stores > 4 g occurs most commonly in male Caucasian HFE C282Y homozygotes. Thus, there may be a role for focused screening in Caucasian men. There is no consensus at this time whether to screen with genotyping for HFE mutations, followed by phenotyping with TfS and SF, or to perform phenotyping first, followed by genotyping for persons with elevated levels.
The absorption of dietary iron. Iron in the diet is present as either heme iron or non-heme iron. Most dietary non-heme iron is in the form of Fe3+, which must first be reduced to Fe2+ before it can be transported across the brush border membrane by DMT1. This reduction step is likely catalyzed by the brush border reductase Dcytb, although other reductases may also be involved. Once inside the enterocyte, the newly absorbed iron enters the intracellular iron pool. If the iron is not required by the body it is loaded onto the iron storage protein ferritin, a process possibly mediated by the iron chaperone PCBP1. Iron required by the body is transferred across the basolateral membrane by FPN. The export of iron also requires the ferroxidase hephaestin (HEPH), although the precise role of this protein is not known. The uptake of heme iron by enterocytes is not as well understood. HCP1 can transport heme; however, its principal role appears to be the uptake of folate and its role in heme absorption remains unclear. Once heme has been transported into the enterocytes the iron is released from the porphyrin ring by heme oxygenase 1 (HO-1), after which it enters the intracellular iron pool. Iron absorption is regulated both by systemic signals and by local iron levels. Systemic factors influencing body iron requirements are detected in the liver and affect the expression of hepcidin, which binds to FPN and induces its internalization and degradation, thereby reducing absorption. Local iron concentrations alter IRP RNA-binding activity, which in turn may affect the levels of DMT1 and FPN. These changes serve to maintain enterocyte iron levels within defined limits despite changes in dietary iron intake. Reprinted with permission from Anderson GJ, et al. Curr Opin Gastroenterol. 2009;25:129–135.14
The absorption of dietary iron. Iron in the diet is present as either heme iron or non-heme iron. Most dietary non-heme iron is in the form of Fe3+, which must first be reduced to Fe2+ before it can be transported across the brush border membrane by DMT1. This reduction step is likely catalyzed by the brush border reductase Dcytb, although other reductases may also be involved. Once inside the enterocyte, the newly absorbed iron enters the intracellular iron pool. If the iron is not required by the body it is loaded onto the iron storage protein ferritin, a process possibly mediated by the iron chaperone PCBP1. Iron required by the body is transferred across the basolateral membrane by FPN. The export of iron also requires the ferroxidase hephaestin (HEPH), although the precise role of this protein is not known. The uptake of heme iron by enterocytes is not as well understood. HCP1 can transport heme; however, its principal role appears to be the uptake of folate and its role in heme absorption remains unclear. Once heme has been transported into the enterocytes the iron is released from the porphyrin ring by heme oxygenase 1 (HO-1), after which it enters the intracellular iron pool. Iron absorption is regulated both by systemic signals and by local iron levels. Systemic factors influencing body iron requirements are detected in the liver and affect the expression of hepcidin, which binds to FPN and induces its internalization and degradation, thereby reducing absorption. Local iron concentrations alter IRP RNA-binding activity, which in turn may affect the levels of DMT1 and FPN. These changes serve to maintain enterocyte iron levels within defined limits despite changes in dietary iron intake. Reprinted with permission from Anderson GJ, et al. Curr Opin Gastroenterol. 2009;25:129–135.14
Prevalences of elevated transferrin saturation (TfS) and serum ferritin (SF) in C282Y homozygotes in the HEIRS Study. Vertical bars represent the proportion of HFE C282Y homozygotes whose TfS and SF values exceeded study thresholds: TfS > 50% and SF > 300 μg/L for men (N = 89); TfS > 45% and SF > 200 μg/L for women (N = 138). Adapted with permission from Adams PC et al. N Engl J Med. 2005;352:1769–1778.29
Prevalences of elevated transferrin saturation (TfS) and serum ferritin (SF) in C282Y homozygotes in the HEIRS Study. Vertical bars represent the proportion of HFE C282Y homozygotes whose TfS and SF values exceeded study thresholds: TfS > 50% and SF > 300 μg/L for men (N = 89); TfS > 45% and SF > 200 μg/L for women (N = 138). Adapted with permission from Adams PC et al. N Engl J Med. 2005;352:1769–1778.29
Scatter plots of total iron removed versus serum ferritin (SF) concentration. SF values are those at clinical evaluation, with linear regression line among (a) the C282Y homozygote men who completed the quantitative phlebotomy program (N = 39, R2 = 0.35, P < .0001) and (b) C282Y homozygote women who completed the quantitative phlebotomy program (N = 37, R2 = 0.29, P = .0006). Reprinted with permission from Gordeuk VR et al. Am J Hematol. 2008;83:618–626.44
Scatter plots of total iron removed versus serum ferritin (SF) concentration. SF values are those at clinical evaluation, with linear regression line among (a) the C282Y homozygote men who completed the quantitative phlebotomy program (N = 39, R2 = 0.35, P < .0001) and (b) C282Y homozygote women who completed the quantitative phlebotomy program (N = 37, R2 = 0.29, P = .0006). Reprinted with permission from Gordeuk VR et al. Am J Hematol. 2008;83:618–626.44
Disclosures Conflict-of-interest disclosures: GDMcL receives research funding from Novartis Pharmaceuticals Corp. VRG declares no competing financial interests. Off-label drug use: Exjade (deferasirox), an iron-chelating drug used for treatment of iron overload.
Acknowledgments
The authors thank the HEIRS Study Investigators for their contributions. The HEIRS Study was initiated and funded by NHLBI, in conjunction with NHGRI. The study was supported by contracts N01-HC-05185 (University of Minnesota), N01-HC-05186 (Howard University), N01-HC-05188 (University of Alabama at Birmingham), N01-HC-05189 (Kaiser Permanente Center for Health Research), N01-HC-05190 (University of California, Irvine), N01-HC-05191 (London Health Sciences Centre), and N01-HC-05192 (Wake Forest University). Additional support was provided by the General Clinical Research Center, School of Medicine, University of California, Irvine, with funds provided by the National Center for Research Resources, 5M01RR 00827-29, U.S. Public Health Service; GCRC grant #M01-RR00032 (University of Alabama at Birmingham); and the Howard University General Clinical Research Center (GCRC) grant, M01-RR10284 and from the NHLBI and the Office of Minority Health UH1-HL03679-05.
References
Author notes
Hematology/Oncology Section, Veterans Affairs Long Beach Healthcare System, Long Beach, and Division of Hematology/Oncology, Department of Medicine, University of California, Irvine, CA
Division of Hematology/Oncology, Howard University, Washington, DC