Abstract
In contrast to myocardial infarction, stroke is a heterogeneous disease with multiple different causes. Determination of stroke mechanism is critical to choosing optimal therapy to prevent recurrence. This article reviews the diagnostic approach to stroke and prognostic and treatment implications of specific stroke mechanisms. Data on the role of antiplatelet and anticoagulant therapy in secondary stroke prevention, as well as the role of these agents and thrombolytic therapy in acute stroke treatment are reviewed. Situations of particular relevance to the practicing hematologist—stroke in the young, patients with multiple recurrent strokes, patients with abnormal hypercoagulable laboratory testing, and treatment of intracerebral hemorrhage following thrombolytic therapy—are discussed.
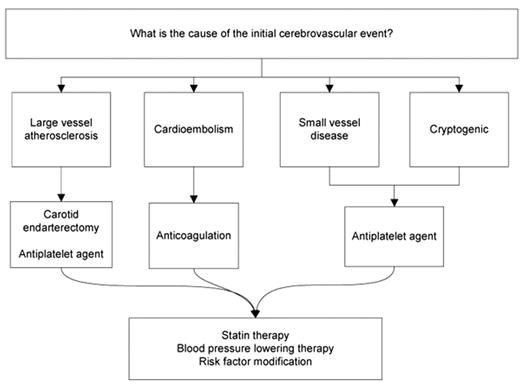
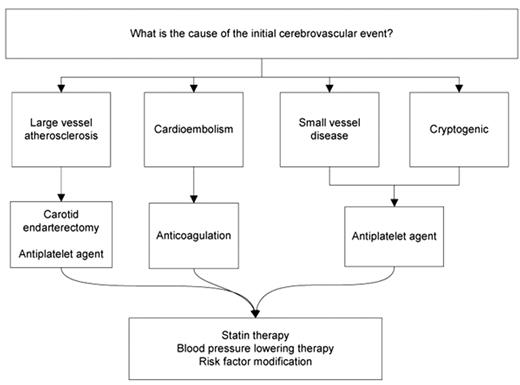
Unlike myocardial infarction, which uniformly occurs due to plaque rupture and local thrombosis of a coronary artery, ischemic stroke is a heterogeneous condition. Embolism to an intracranial artery may come from thrombus on an ulcerated plaque in the carotid artery or may be due to cardioembolism from atrial fibrillation. Large vessel atherosclerosis may progress to occlusion causing perfusion failure. Occlusion of the small perforating vessels deep in the brain may cause lacunar stroke. Less commonly, stroke may be caused by a venous clot, which paradoxically embolizes through a patent foramen ovale (PFO), by vasospasm of structurally normal vessels (for instance with sympathomimetic drug use), or by a hypercoagulable state, operating synergistically with any of these mechanisms or independent of them. A fundamental precept of stroke prevention is that determination of the underlying stroke mechanism is critical to choosing the optimal treatment to prevent recurrence (Figure 1 ). Most stroke patients will benefit from control of hypertension, statin therapy, and risk factor modification, but a discussion of these strategies is beyond the scope of this article.
The heterogeneity of stroke has two immediate consequences relevant to the consulting hematologist. First, when considering whether a hematologic cause of stroke is present, it is important to ensure that a complete diagnostic evaluation has been performed to identify the more typical stroke mechanisms, which are far more common causes of stroke. Second, the traditional dichotomy of separating thrombotic events into those that are arterial versus those that are venous should be applied cautiously to ischemic stroke. Beyond the obvious situation of paradoxical embolization through a PFO, other stroke mechanisms may be closer pathophysiologically to venous thrombosis than to “typical” arterial thrombosis. Atrial fibrillation, for instance, is associated with blood flow stasis in the left atrial appendage, markedly elevated D-dimer levels, and a much greater clinical response to warfarin compared with antiplatelet therapy.1,2
I find that a simple summary tool helps ensure a systematic diagnostic evaluation: (A) Is there a problem in the arteries? (B) Is there a problem in the blood? (C) Is there a cardiac source of embolism? (Table 1 ) Evaluation should begin with a thorough history and examination assessing for vascular risk factors, known vascular or cardiac disease, and contributors to a potential hypercoagulable state. Diagnostic testing should, at minimum, generally include cranial CT, imaging of the extracranial carotid arteries, transthoracic echocardiography and cardiac electrographic monitoring, and routine blood studies. A more comprehensive evaluation is appropriate for many cases in which the initial evaluation is unrevealing (Table 2 ). Despite extensive diagnostic testing, 20% to 30% of strokes will remain of undetermined cause (cryptogenic).
Implications of Specific Stroke Mechanisms
Artery Problems
Large Artery Atherosclerosis
In patients with a stroke or TIA and cervical carotid stenosis ≥ 70%, the risk of stroke over 2 years is reduced from about 25% to 10% with carotid endarterectomy.3 The benefit of surgery is substantially higher if performed within 2 weeks of the symptomatic event.3 For example, the absolute risk reduction for surgery performed within 2 weeks of symptoms is roughly 30%; at 4 weeks or later after symptoms, this decreases to about 10%. Older age should not preclude carotid endarterectomy in symptomatic patients; in fact, in the carotid endarterectomy trials, patients 75 years or older derived a much greater benefit than younger patients.4
The risk of recurrent stroke in patients with intracranial stenosis > 50% exceeds 20% at 2 years, and is not lowered with use of warfarin compared with aspirin.5 Based on this very high stroke risk, intracranial angioplasty and stenting is increasingly performed in clinical practice, particularly in patients with recurrent events on medical therapy, and appears to have a reasonable safety profile.6 Formal evaluation of the safety and efficacy of intracranial angioplasty and stenting is under evaluation in a large randomized trial.7
Arterial Dissection
Recurrent stroke from arterial dissection can occur either due to mural hematoma and thrombus serving as a proximal embolic source, or progressive thrombosis culminating in vessel occlusion. No randomized trials have assessed optimal antithrombotic therapy in arterial dissection, and non-randomized data and expert opinion vary as to whether antiplatelet or anticoagulant therapy is most appropriate.8,9 The risk of recurrent stroke appears to be quite low for most patients with arterial dissection, regardless of treatment.10 When anticoagulants are chosen, they are generally continued for only 3 to 6 months followed by antiplatelet therapy for the longer term.
Small Artery (Lacunar) Disease
Occlusion of the small penetrating arteries of the brain typically causes small (< 15 mm) deep infarcts. Micro-atheroma, lipohyalinosis, and fibrinoid necrosis have been identified as the major mechanisms causing disease in these vessels, although microembolism may also occur in some cases. Treatment is with antiplatelet agents, and the risk of recurrent stroke is generally lower than with other mechanisms.
Vasculitis
Inflammatory diseases affecting the cerebral vessels are a rare but important cause of stroke. In a study of 1000 young patients with stroke, 2% were felt to be due to vasculitis.11 Systemic involvement may or may not be present depending on the specific immune process. Infectious vasculitides with a predilection for the intracranial vessels include syphilis and herpes zoster.
Blood Problems
There are numerous specific hematologic conditions in which ischemic stroke can be a prominent sequelae. These include, but are not limited to, sickle cell anemia, hematologic malignancies, polycythemia, thrombocythemia, paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura, disseminated intravascular coagulation, and heparin-induced thrombocytopenia. Suspicion of these processes is usually triggered by abnormalities in routine laboratory studies. These conditions are quite rare causes of stroke, and a discussion of each is beyond the scope of this article.
More commonly raised is concern about a hypercoagulable state contributing to ischemic stroke. The yield of hypercoagulable testing in patients with stroke is low overall, but somewhat higher in patients with stroke of undetermined cause.12 The relationship between the more common hypercoagulable states and stroke has been extensively reviewed recently.13 To briefly summarize, data associating stroke with the factor V Leiden and prothrombin G20210A mutations, and protein C, protein S, and antithrombin III deficiencies are limited and conflicting, but on the whole do not demonstrate any clear association. In the case of homocysteine, the association with stroke risk is stronger, but evidence for a stroke preventative effect of homocysteine lowering is inconclusive.14 It should be noted that much of these data face methodologic problems, most prominently the inclusion of older stroke patients with typical atherosclerotic risk factors as well as patients with specific identified non-hypercoagulable stroke mechanisms. Those studies that have focused on young patients with stroke of undetermined cause, the group most likely to have stroke due to a hypercoagulable state, have generally had limited power to identify an association due to small sample sizes.
The association between antiphospholipid antibody (APLA) syndrome and stroke offers an example of how such data must be interpreted cautiously. Prospective observational studies provide convincing evidence of an association between APLA syndrome and ischemic stroke in younger patients, and patients with APLA syndrome have a high rate of recurrent thrombotic events without anticoagulation.13,15,16 However, a large study of APLA nested within a randomized trial of warfarin compared with aspirin (APASS: The Antiphospholipid Antibodies and Stroke Study) found no difference in recurrent thrombotic events between those with and without APLA, and no benefit to warfarin compared with aspirin in patients with APLA.17 In the APASS study, APLA were measured only one time (and were present in 41% of the 1770 patients enrolled) and the population enrolled was older (mean age 62) with a high prevalence of typical vascular risk factors. Extrapolation from this data to younger patients meeting diagnostic criteria for APLA syndrome is probably unwise.
In my experience, the two most common hypercoagulable conditions associated with ischemic stroke are cancer, in the elderly, and oral contraceptive (OC) use, in the young. In stroke patients with cancer, marantic endocarditis is found in up to 20%, and an additional 10% have other definite embolic sources consistent with a hypercoagulable state on transesophageal echocardiography (TEE).18 Low-molecular weight heparin is the preferred treatment strategy for patients with venous thrombosis associated with cancer, and this may also be applicable to patients with cancer-associated stroke, particularly if mediated by marantic endocarditis.19,20 In a large meta-analysis, oral contraceptive use was found to be associated with 1 additional ischemic stroke per year for every 24,000 women using OC.21 While this risk is low in absolute terms, given the widespread use of OC the population attributable risk from OC is significant.22 Hormone replacement therapy is also associated with an increased risk of ischemic stroke.23
Cardiac Problems
Atrial Fibrillation
In patients with TIA or stroke due to atrial fibrillation, warfarin is substantially more effective than aspirin at preventing recurrent stroke. A meta-analysis of 12 trials involving almost 13,000 patients demonstrated that warfarin was associated with a nearly 40% relative risk reduction compared with antiplatelet therapy, with only a modest increased risk of bleeding complications.2 Another recent meta-analysis has shown that, because stroke risk increases with age, the absolute benefit of warfarin over aspirin increases as patients get older.24 The efficacy and safety of warfarin in the elderly population was recently confirmed in the BAFTA trial, which enrolled nearly 1000 patients aged 75 or older, and demonstrated significantly fewer thromboembolic events with warfarin with no significant difference in bleeding risk compared to aspirin.25
Combination antiplatelet therapy with aspirin and clopidogrel has been proposed as an alternative to warfarin; however, this is not supported by current data. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W) randomized 6706 patients to warfarin or the combination of aspirin and clopidogrel.26 Vascular events were significantly less frequent in the warfarin arm (warfarin: 3.9% per year; aspirin: 5.6% per year, P = .0003). Major bleeding was not significantly different between the two groups, and total bleeding was in fact more frequent in the aspirin and clopidogrel group. A related but separate trial (ACTIVE A) compared aspirin and clopidogrel with aspirin alone in patients who were felt to be unsuitable candidates for warfarin.27 There was a modest reduction in vascular events with combination therapy but this was counterbalanced by a significant excess of major bleeding.
Aortic Arch Atherosclerosis
A frequently overlooked cause of stroke is severe aortic arch atherosclerosis, typically best identified with TEE. In a cohort of 331 patients with ischemic stroke, the risk of recurrent stroke in patients with > 4 mm aortic arch plaque was nearly 12% per year.28 This extremely high rate of stroke recurrence is similar to that of patients with atrial fibrillation or symptomatic carotid stenosis. Optimal treatment of these patients remains unclear, with some limited data suggesting a benefit to warfarin over antiplatelet therapy.29
Patent Foramen Ovale
A PFO is present in about 40% to 50% of young patients with unexplained stroke compared with about 25% of normal healthy controls.30 In the individual stroke patient, it can be challenging to determine whether PFO is an innocent bystander or the conduit for a paradoxical embolus to the cerebral circulation, especially given that lower extremity ultrasonography has a surprisingly low yield (< 5%) in identifying deep vein thrombosis (DVT) in patients with suspected paradoxical embolism.31 The risk of recurrent stroke in young patients with PFO and unexplained stroke is extremely low, < 1% per year in a large prospective study of 581 subjects, similar to patients without PFO.32 Recent data from a Spanish cohort of 486 subjects have confirmed this low risk of recurrent stroke and also found no association between PFO size and stroke risk.33 The association of atrial septal aneurysm with PFO had been considered a factor significantly increasing the risk of recurrence; however; more recent data have called this into question.32,33 Current management guidelines emphasize the lack of data to support anticoagulation or PFO closure, as opposed to antiplatelet therapy, in treating patients with unexplained stroke and PFO.34 Several large trials are underway that will better define the risk of recurrent stroke, impact of hypercoagulable testing, and role of PFO closure in management of these patients.35
Other Cardiac Sources of Embolism
A variety of other cardiac sources of embolism are important causes of stroke and are generally considered to benefit from warfarin, though clinical trial data are more limited than with atrial fibrillation. These include severe congestive heart failure, left ventricular or left atrial appendage thrombus, valvular disease, and akinetic left ventricular segment post myocardial infarction. Infective endocarditis and cardiac tumors (atrial myxoma, fibroelastoma) are cardioembolic sources that require specific treatment other than warfarin.
Overview of Antithrombotic Therapy for Stroke Prevention
Antiplatelet Therapy
Aspirin reduces the risk of recurrent vascular events in patients with stroke or TIA by about 20%, such that for every 1000 patients treated for about 2.5 years, 36 events will be prevented.36 Higher-dose aspirin (300 to 1500 mg) is not more effective than low-dose aspirin but is associated with a greater incidence of side effects.36,37 The combination of aspirin and extended-release dipyridamole (ER-DP) is more effective than aspirin alone for secondary stroke prevention. Based on data from the European Stroke Prevention Study-2 (ESPS-2), about 30 strokes would be prevented for every 1000 patients treated for 2 years with combination aspirin and ER-DP compared with aspirin alone.38 The bleeding risk of combination aspirin and ER-DP is not significantly different than that with aspirin alone. A subsequent trial (ESPRIT) showed similar results.39
Clopidogrel is more effective than aspirin at preventing recurrent vascular events. Based on data from The Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial, about 10 vascular events would be prevented for every 1000 patients treated for 2 years with clopidogrel instead of aspirin.40 The side-effect profile of clopidogrel is comparable to aspirin. In the Management of Atherothrombosis with Clopidogrel in High-risk Patients (MATCH) trial, the combination of clopidogrel and aspirin was no more effective than clopidogrel monotherapy for secondary stroke prevention but was associated with a significant excess of life-threatening and major hemorrhage (4.5% vs 1.9%; P < .001).41 Based on these data, the combination of clopidogrel and aspirin should be avoided in most patients with cerebrovascular disease.
Recently, the largest antiplatelet trial for stroke prevention yet conducted, the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study, has been completed.42 PRoFESS enrolled 20,332 patients with a history of stroke and compared the combination of aspirin and ER-DP to clopidogrel monotherapy. The two agents performed nearly identically in terms of recurrent stroke (9.0% aspirin + ER-DP vs 8.8% clopidogrel, HR 1.01, 95% CI 0.92–1.11) and the composite of stroke, MI, vascular death (13.1% in both groups, HR 0.99, 95% CI 0.92–1.07). There was slightly more major bleeding in the aspirin + ER-DP compared to clopidogrel arm (4.1% vs 3.6%, HR 1.15, 95% CI 1.00–1.32). A summary of the role of antiplatelet therapy is provided in Table 3 .
Anticoagulant Therapy
For unselected patients with non-cardioembolic stroke, clinical trial data show no aggregate benefit to anticoagulation compared with antiplatelet therapy.43 It is important to recognize that these trial data are based on mostly older patients with typical vascular risk factors, and thus lack generalizability to young patients with stroke or patients with stroke due to unusual mechanisms.
Treatment of Acute Ischemic Stroke
Unlike the situation with secondary stroke prevention, at present the selection of treatment for acute stroke is not generally mechanism specific. This may change as technologies to rapidly determine stroke mechanism mature.
Antiplatelet Agents
Early treatment with aspirin reduces death and disability following acute ischemic stroke, although the effect size is small. In a pooled analysis of roughly 40,000 patients in the two largest studies of acute aspirin use (the International Stroke Trial and the Chinese Acute Stroke Trial), aspirin reduced recurrent ischemic stroke by 7 per 1000 treated (P < .0001) and mortality by a further 4 per 1,000 treated (P = .05). There was an increase in intracranial bleeding of 2 per 1000 treated, for an overall net benefit of 9 per 1000.44 There are very limited data on use of antiplatelet agents other than aspirin in the acute setting. A small pilot trial of combination aspirin and clopidogrel in 400 patients with TIA or minor stroke failed to demonstrate superiority of combination therapy compared with aspirin alone, although a trend toward reduction in recurrent stroke was found (7.1% vs 10.8%; P = .19).45 However, a significant excess of symptomatic bleeding events was seen with combination therapy (3.0% vs 0%; P = .03).
Anticoagulants
Despite a long tradition of use of parenteral anticoagulation in acute stroke treatment, data from multiple trials has not supported this treatment strategy. A meta-analysis of trial data of heparin or low-molecular-weight heparin showed no evidence of a net benefit of acute anticoagulation in ischemic stroke.46 Some uncertainty remains as to whether particular stroke mechanisms might benefit from acute anticoagulation, with some studies suggesting a benefit in patients with stroke due to large vessel stenosis but not in stroke due to other mechanisms.47,48 For the most part, studies of acute anticoagulants in stroke have been focused on neurologic outcome. However, prevention of venous thromboembolism is an additional therapeutic target of anticoagulant use. Low-dose subcutaneous heparin (5000 U BID) has been shown to be safe in acute ischemic stroke patients, and possibly to be clinically beneficial.49 Recently, a large trial comparing this regimen to enoxparin (40 mg subcutaneously once daily) for prevention of venous thromboembolism in stroke demonstrated significantly greater efficacy for enoxaparin with similar bleeding risk.50 Graduated compression stockings and pneumatic compression devices are often used in addition to anticoagulants for prevention of venous thromboembolism in stroke patients. The former strategy has been shown in a large randomized trial to be ineffective and is not recommended at this time.51 There are very limited data on pneumatic compression devices in stroke patients, although these have been shown to be effective supplements to anticoagulants in post-surgical patients, and their use in stroke is common.52 A large trial to evaluate their role specifically in stroke patients is underway.53
Thrombolytic Therapy
Acute stroke therapy was dramatically altered in 1995 with the publication of the pivotal National Institute of Neurologic Disorders and Stroke (NINDS) tPA trial, which demonstrated the benefit of intravenous tPA in patients with acute ischemic stroke when given within 3 hours of symptoms onset.54 Treatment with tPA was associated with an increase of about 120 patients with minimal or no disability for every 1000 patients treated. A subsequent pooled analysis of six randomized controlled trials of t-PA in acute ischemic stroke confirmed this benefit and also suggested a benefit up to 4.5 hours after symptoms onset.55 Recently, a randomized trial (ECASS-III) specifically enrolling patients with acute ischemic stroke in the 3 to 4.5 hour window was completed and confirmed a significant benefit to thrombolytic therapy in this group.56
The major side effect of thrombolytic therapy for stroke is symptomatic intracerebral hemorrhage, which occurs in about 6% of patients treated. Bleeding complications from thrombolytic therapy may represent the effects of fibrinogen depletion, platelet dysfunction due to circulating fibrin degradation products, and/or reperfusion injury.57 In the NINDS trial, an algorithm for management of post-tPA hemorrhage recommended measurement of coagulation studies including fibrinogen levels, and treatment with 6 to 8 units of cryoprecipitate and 6 to 8 units of platelets. Others have suggested that cryoprecipitate be dosed based on fibrinogen levels, with a goal of achieving levels > 100 mg/dL, that 10 units of cryoprecipitate be given or that fresh frozen plasma be given in addition to cryoprecipitate.58,59 Anti-fibrinolytic agents such as E-aminocaproic acid have also been suggested to have a role, particularly if bleeding occurs during the duration of action of the infused thrombolytic (< 1 hour for t-PA); however, these are rarely used in clinical practice in acute stroke.57 A practical consideration in management of post-tPA hemorrhage is that intracerebral hemorrhage growth appears to occur very rapidly, providing a very short window for intervention and limiting the ability to use laboratory tests to guide therapy. In centers where thawed plasma is immediately available, this may provide an initial time advantage in initiating replacement therapy, and can be followed by additional agents as appropriate. Factor VIIa has been shown to reduce hemorrhage expansion in spontaneous intracerebral hemorrhage (though without clear impact on clinical outcome) and has a rapid onset of action; however, it is also associated with an increased risk of arterial thrombosis, making its use in ischemic stroke patients probably inadvisable until data specific to post-thrombolytic hemorrhage become available.60 It is worth noting, as well, that the prognosis of patients with symptomatic ICH is poor; in the NINDS trial, 75% of patients with a symptomatic ICH were dead at 3-month follow-up.61
Common Clinical Scenarios Relevant to the Practicing Hematologist
Stroke in the Young
A hypercoagulable state is often suspected in young patients with stroke. Before considering this, however, the priority should be to ensure that a comprehensive evaluation has been performed looking for alternative causes. Common overlooked causes of stroke in the young include arterial dissection, use of sympathomimetic drugs or other drugs of abuse, and the interaction of migraine, oral contraceptive pills, and smoking. In a large study of over 1000 young patients (aged 15–49 years) with stroke, 15% were due to arterial dissection.11 Identification of dissection requires, at minimum, imaging of both the extracranial and intracranial circulation with either magnetic resonance (MR) or computed tomography (CT) angiography. Ultrasonography is insufficient to evaluate patients for dissection.62 Many dissections will heal spontaneously within 1 to 2 months, so if imaging is delayed normal findings do not rule out the possibility that dissection may have accounted for the stroke. The association of sympathomimetic drugs with stroke in the young is well established, but not always considered, especially if patients are not specifically questioned about over-the-counter medications such a nasal decongestants or herbal supplements containing ephedra-like compounds.63 Migraine, particularly migraine with aura, approximately doubles the risk of stroke; women with migraine who take oral contraceptive pills have an eightfold increase risk of stroke.64 Smoking likely further increases this risk.
Patients with Multiple Recurrent Strokes
As noted above, the rate of recurrent cerebrovascular events is particularly high in patients with severe aortic arch atherosclerosis, intracranial stenosis, and atrial fibrillation. These are also mechanisms that may not be identified as part of the basic stroke evaluation. For instance, aortic arch atherosclerosis is commonly only identified by TEE, and intracranial stenosis by MR or CT angiography of the intracranial circulation. In the case of intracranial stenosis, disease in the intracranial portion of the carotid artery may be quite difficult to diagnose given frequent artifactual findings on MR- and CT-based studies in this anatomical region; catheter angiography may be required for an accurate diagnosis. Identification of paroxysmal atrial fibrillation (PAF) may be a particular challenge. A recent systematic review of published studies found that standard Holter monitoring (24–72 hours) detected new AF in 4.6% of patients.65 More prolonged monitoring using event loop recorders detected AF in an additional 5.7% to 7.7% of patients in whom Holter monitoring was unrevealing. Many patients with recurrent stroke will be found to have one of these mechanisms once a comprehensive diagnostic evaluation is completed, potentially obviating speculation about the role of a hypercoagulable state.
Patients with Abnormal Hypercoagulable Testing
All too often stroke patients without obvious indications are given a “screening” battery of hypercoagulable tests, a positive result is discovered, and the question then becomes what to do with the information. In such situations, a reasonable first step is to evaluate whether a more obvious stroke mechanism is present (for example, a cardioembolic source requiring anticoagulation), which might render the question clinically irrelevant. Second, the results should be confirmed to be truly abnormal. For instance, low protein C or S levels drawn shortly after hospitalization for acute stroke, or in patients on warfarin therapy, are uninformative and should not be considered indicative of a hypercoagulable state. A final step is assessing whether the abnormal test results are truly likely to represent a contributor to stroke in this particular patient. For example, as noted earlier, the prevalence of a positive APLA test in older patients with stroke and typical vascular risk factors exceeds 40% and does not have prognostic or treatment implications. In general, if the patient is older and/or has vascular risk factors, abnormal hypercoagulable testing is less likely to have clinical implications; conversely, if the patient is younger without vascular risk factors, a higher level of clinical suspicion of the significance of such test results is appropriate.
Conclusion
Ischemic stroke is a major cause of death and disability, and efforts to prevent recurrence in stroke survivors are critically important. A systematic approach to diagnostic evaluation will identify a specific cause in most patients with stroke, and optimal treatment strategies in these patients are increasingly well-defined based on data from clinical trials. The role of hypercoagulable states in stroke remains an area of considerable uncertainty and is fertile ground for further investigation. Such research may be most successful if focused specifically on patients with cryptogenic stroke, particularly younger patients.
Disclosures Conflict-of-interest disclosure: The author receives honoraria from BMS-Sanofi. Off-label drug use: This paper discusses the use of t-PA for acute ischemic stroke outside of a 3-hour time window.
References
Author notes
Department of Neurology, University of Pennsylvania Medical Center, Philadelphia, PA