Abstract
This brief review aims to discuss the various cellular immunological aspects and related mechanisms of the use of specific components from traditional herbal medicines. We begin with lessons learned from thalidomide as an effective single drug with multiple mechanisms of action to treat multiple myeloma. Examples of “supplements” or integrative therapy will be drawn from arsenic trioxide, medicinal mushrooms including Coriolus vesicular and Ganoderma lucidum, followed by the discussion of beta-glucans affecting various immunological important cellular subsets. Different classes of compounds may enhance distinct immune cell populations that might contribute to a multi-targeted holistic effects on anti-cancer treatment. Finally, we conclude by highlighting an herbal formulation PHY906 as a potential adjunct to chemotherapy that might become one of the first US Food and Drug Administration (FDA) approved oral herbal medicines for anti-cancer adjunct treatment.
Supplements in Integrative Medicine Targeting Cancer
The term “supplement” in medicine typically refers to compounds that can be used together with another drug for treatment of disease leading to a better overall outcome or reduction of related undesirable side effects of the primary drug. In the context of cancer management, supplements may be desirable for the reduction of the hematological and non-hematological toxicities inherent with conventional chemotherapeutics. The same word, however, is also used to describe a wide range of commercially available over-the-counter health foods that allegedly lead to overall better health. Although these products do not come with substantial medical evidence to support these claims, many are advertised as “supplements” that are able to enhance normal physiological and immunological conditions. The critical reactions by many in the scientific and medical communities to the promotion and acceptance of supplements are often directed at the deficiency of evidence-based scientific support for their use.
The lack of readily available novel conventional therapies and a desire for self-empowerment has led many patients to explore the use of complementary and alternative medicines (CAM). In Australia, studies suggest that around 20% of cancer patients have used at least one kind of CAM, and that figure is thought to be increasing. A survey revealed that in 156 cancer patients (90% response rate), 52% were using at least one and 28% use 3 or more CAM treatments.1 In focus groups with 48 Chinese cancer patients and their relatives conducted by an Australian research group, a majority supported the use of Chinese medicine and believed it would assist in cure.2 In a survey of pediatric patients with cancers in Hong Kong, 42% of more than 200 children with cancer adopted herbal supplements while they were receiving chemotherapy. Interestingly, more than 60% of them did not inform their doctors and most users were children of young, educated parents.3
Within the context of hematological malignancies, this short review aims to explore the clinical evidence for some underlying immunological basis of a few selected adjunct therapies in conjunction with other standard chemotherapeutics for cancer management rather than describing the “health-product supplements” available in the market. While it has been shown that these adjunct therapies may act through multi-targeted mechanisms, this review will emphasize only the immunomodulatory aspects in relationship to the overall anti-cancer effect.
In this review, we begin with a discussion of “multi-targeted mechanism” as an alternative approach in contrast to the emerging trend of targeted therapies in cancer treatment. Accordingly, thalidomide can be viewed as a maintenance adjunct therapy together with standard chemotherapeutics in the treatment of multiple myeloma (MM).4,5 Thalidomide has been shown to be an effective anti-myeloma compound that can significantly enhance endogenous monoclonal CD8 T-cell expansion. We then go on to discuss the example of arsenic trioxide (As2O3), which originated from traditional medicines as a single agent and has emerged as an effective adjunct therapy in a few hematological malignancies. Traditionally herbal medicines, such as medicinal mushrooms Ganoderma lucidum and Coriolus vesicular, contain β-glucans and it has been suggested that these active compounds may have the immunomodulatory effects contributing to the overall anti-cancer property. A 4-herb traditional Chinese medicine called PHY906 has been developed at Yale University over the last 15 years as a model that differentiates adjunct therapies from “supplements” in cancer treatment. Encouraged by the favorable outcomes in a few phase 2 clinical trials, the study of PHY906 demonstrates a successful model for the study of poly-drug versus multiple targets principle. Thus it is very much desirable to expand the exploration of the use of other “supplements” for usage such as the reduction of serious side effects in conventional chemotherapeutic cancer management.
Thalidomide as a Useful Adjunct Therapy/Supplement in Myeloma Treatment
Thalidomide, despite its being retracted as a result of teratogenic effects in humans, is an example where a drug was revived as an anti-cancer treatment after a successful clinical trial on relapsed and refractory myeloma patients.4–8 This was followed by extensive studies into its mechanism of actions.9–14 The multi-targeted mechanisms of actions of thalidomide for its anti-cancer activities, including direct cytotoxic effect on myeloma cells, its effects on modifying the microenvironment of the cancer site, and its enhancing effect on patients’ anti-cancer immunity.
Thalidomide seems to be a single drug but possesses multiple activities. This phenomenon might be due to the fact that it exists as twelve hydrolysis products when dissolved in water.15 Thus the study of thalidomide represents a model of a poly-drug eliciting multi-targeted mechanisms of actions that research on traditional Chinese medicines (TCM) can be drawn upon.
As2O3 as Single-Compound Adjunct Therapy
Arsenic trioxide has long been used as a medicine in China. Recorded in an ancient Compendium of Materia Medica (Ben Cao Gang Mu, 1596 AD), it was principally used for treatment of parasites, strokes and goiters. Its modern-day application in cancer treatment demonstrates an evidence-based “old drug/new use” approach.
As2O3 has potential therapeutic benefits to patients with APL as well as a variety of other hematological malignancies. Similar to thalidomide, the underlying mechanism is pleiotropic involving apoptosis induction, anti-proliferation, angiogenesis inhibition and immunomodulation.16–19 However, disparity in response patterns was noted among different types of hematological malignancies, and the intrinsic genetic differences among leukemia subtypes may account for such variation.
As2O3 as a single agent did not produce any significant responses in advanced MM patients in one report.20 In contrast, the combination of As2O3 with other anti-cancer therapeutic agents showed promising results for MDS as well as MM in phase II studies.21–24 As2O3 in clinically relevant doses generally does not induce myelosuppression, which is a good complement to conventional cytotoxic agents. This feature, together with the multiple molecular pathways targeted by arsenic, supports the potential role of As2O3 as an adjuvant with other chemotherapy. Whether such a combination strategy can result in enhanced anti-tumor activity without significant additive side effects has yet to be confirmed.
For non-APL myeloid leukemias, T-ALL and lymphoma, the results of using As2O3 as a single agent are disappointing.25 Again, the combination of As2O3 with selected targeted therapeutic agents may enhance the sensitivity of resistant blast cells to As2O3. Studies to further investigate the relatively poor cytotoxic response to As2O3 in these hematologic malignancies and to reverse the underlying resistant mechanisms will help to develop new therapeutic targets. Studies suggest that the expression of Aquaporin-9 may affect the entrance of As2O3 into the cells, which may account for the differences in response.26 Since most previous trials are single-arm studies with small sample sizes, well-designed larger randomized prospective clinical trials are needed to verify these pilot study results and determine the best combination strategy of As2O3 for different diseases types in the future.
Preclinical studies have demonstrated an immunological mechanism for the therapeutic effects of As2O3 on myeloma cells. As2O3 induced a marked increase in lymphocyte-activated killer–mediated killing, possibly through the up-regulation of the CD38/CD31 and CD11a/CD54 receptor-ligand systems, which increase recognition, adhesion, and lysis of targeted myeloma cells.27 These data suggest that As2O3 has a role in the management of relapsed or refractory multiple myeloma via immunomodulatory mechanisms.
Natural killer cells are important effector cells against tumor cells, and their activation is mediated through specific receptors and ligands. It is now known that induction of NKG2D ligands on tumor cells by various stresses will render them more sensitive to NK cell–mediated killing. As2O3 can up-regulate NKG2D ligands on CML, APL and breast cancer cells and increase their susceptibility to NK cell–induced lysis.28 This increase in cytotoxicity was abolished by the addition of a blocking NKG2D mono-clonal antibody, indicating that the action is mediated through up-regulation of NKG2D ligands. This study further supports that the immunomodulatory property of As2O3.
Data on the use of As2O3 on children with non-APL hematological malignancies are very limited. In our in-vitro study, non-APL leukemic cells often required higher concentration of As2O3 to achieve satisfactory cytotoxic effects. The serum concentration should be in the range of 1 to 2 μM/mL. However, in an pharmacokinetic study done on 13 children with refractory solid tumors by 0.15 mg/m2 daily intravenously, most children only achieved a peak drug concentration of <1 μM/mL (range 0.6-1 μM/mL) (GCF Chan, unpublished data). That partly explained why the clinical effects were suboptimal in previous trials.
The Use of Coriolus Versicolor in Cancer Management
The dried fruiting body or mycelia of Coriolus versicolor, also known as Polyporus versicolor, Polysticutus versicolor and Trametes versicolor, is used medicinally throughout Asia. In China, it is called Yun Zhi. In Asia, C versicolor extract is available as a health supplement and can be purchased without a prescription. It is generally believed that the anti-cancer effects of the medicinal mushroom are related to the immunomodulatory effector functions of the polysaccharide component of Ganoderma, or the polysaccharide protein fractions in Coriolus, which is commercially available as Polysaccharide Krestin (PSK) in Japan or Polysaccharide Protein (PSP) in China.29
A Japanese study of PSK as an adjuvant to chemotherapy in 28 patients found that leukemia remission and survival were significantly prolonged for patients who received PSK plus chemotherapy compared with that of those who received chemotherapy alone.30 In another multicenter trial from Japan of 67 patients with acute non-lymphocytic leukemia in remission, those who received maintenance chemotherapy plus PSK tended to have longer survival over the control group that received chemotherapy alone, though without statistical significance.30
There is evidence from animal studies that the anti-tumor action of mushroom polysaccharides is mediated through immunomodulatory regulation rather than by direct cytotoxicity toward tumor cells. For instance, PSK has been shown to regulate cytokines and activate the complement system and natural killer cells.31 It has also been reported that PSP effectively stimulates the generation of IFN-α and markedly improves the production of IFN-γ.32 The latter is of special interest to us as our laboratory has previously reported that peripheral blood dendritic cells from myeloma patients were defective in their ability to up-regulate the expression of CD80 when ligating with CD40-ligand partly due to the presence of cytokines of IL-10 and TGF-β.33 We have subsequently demonstrated that such defects can be reversed by treatment with IL-12 and IFN-γ.34 It would be important to examine whether the IFN-γ induced by PSP actually would be able to correct the defective blood dendritic cells in cancer patients.
This immunomodulatory hypothesis for mushroom polysaccharide effects is supported by results from an in vivo study showing restoration of suppressed immunological responsiveness by PSP in cancer patients but no significant immunological effects on a normal host, reflecting the potential real-life situation in cancer patients or those having chemotherapy.35 PSP has also been shown to counteract the myelo- and immuno-suppressive effects of cyclophosphamide, a commonly used chemotherapeutic drug for the treatment of lymphoma and leukemia, by increasing the number of white blood cells and IL-2 production, that included the improvement in lymphocyte proliferation, NK-cell functions, and the growth of spleen and thymus.36,37
PSP can affect the cellular immunity as suggested by its ability to induce proliferation of human T cells and increased the CD4/CD8 ratio in vitro.37 However, it is important to note that it remains unclear whether the change of the various T cell subsets has any direct relationship with overall anti-tumor effects or merely reflects a lymphocytotic process due to cytokine profile changes.
An interesting mouse study showing that PSP works as an adjuvant in enhancing a simultaneous T cell–dependent B-cell response.38 In this study, PSK was found to induce significantly elevated antibody titers against keyhole limpet hemocyanin with the highest activity among botanicals of Coriolus, PSP, Maitake mushroom, a 10-herb herbal formula H-48, Echinacea species, turmeric from Curcuma longa, Astragalus species, and yeast β-glucans. This study has clearly demonstrated that the medicinal mushroom coriolus extract can effectively up-regulate a simultaneous T cell–dependent B-cell response (such as those against tumor antigens) while causing a general polyclonal antibody response against the polysaccharide protein itself. However, the quality of such antibody responses is not clear. For instance, it is important to question whether PSK can enhance the affinity maturation and class switching of ongoing B-cell responses or T-cell–independent type-1 and type-2 pathways. It is also important to note that PSP did not exert a direct cytotoxic effect on tumor cell lines but rather stimulated the phagocytic function of macrophages,39 which was consistent with the in vivo mouse results.32
β-glucans Derived from Ganoderma Lucidum as Supplements for Immunomodulatory Anti-tumor Effects in Hematological Malignancies
Ganoderma lucidum is popular among patients with cancer in China, Korea, and Hong Kong.40–42 In recent years, the scientific investigations of the biological activity of beta-glucans have focused on how our immune system reacts to fungal infection.43,44 β-glucans are mainly glucose polymers with a β 1→ 3 backbone and side branches extended by either 1→4 or 1→6 linkages, and they are components of bacterial or fungal cell walls. Studies have found that β-glucans can be taken up in the upper gastrointestinal tract and trigger various immune response via a group of membrane receptors such as Dectin-1, CR-3, SIGNR1, TLR-2/6 and 4.43 However, there should be caution in interpretation these data because some of these studies used non-purified β-glucans so the responses may not reflect β-glucans–specific phenomena.
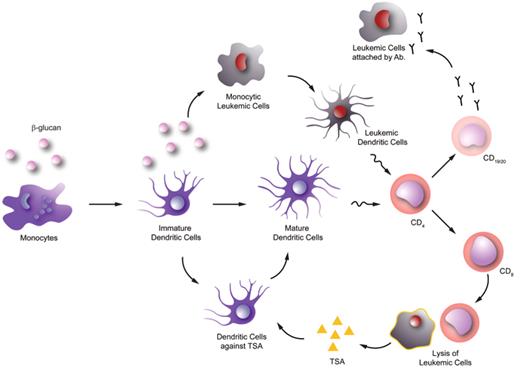
Other studies have shown that Ganoderma-derived β-glucans can enhance monocyte, macrophage, dendritic cell, neutrophil and NK cell function44 (Figure 1 ). These cells have significant roles in anti-cancer immunity; therefore, it is very tempting to postulate that β-glucans have anti-cancer function. Some studies even suggested that Ganoderma-derived extract has direct cytotoxic effects on cancer cells, but many of these studies used Ganoderma spore extract, which has a high triterpene content.45 The direct cytotoxic effect is unlikely related to the β-glucans component as we did not find any direct cytotoxic effects induced by purified β-glucans on cancer cells in-vitro.
Furthermore, Ganoderma-derived β-glucans might induce selected monocytic leukemic cell lines to differentiate into leukemic-derived dendritic cells. These leukemia-derived dendritic cells are immunopotent and can induce allogeneic T-cell responses. These findings suggest that in certain kinds of leukemia (especially monocytic lineage) β-glucans may serve as a differentiating agent, turning leukemic cells into functional dendritic cells.46
There are very few, if any, published clinical trials reporting the anti-cancer effects of purified β-glucans in cancer patients. Similar to the PSP in vivo studies, most related clinical studies investigated the change in the patients’ immune systems by measuring selected surrogate markers such as leukocytes counts or cytokine profiles instead of assessing tumor responses. Furthermore, almost all these studies used non-purified β-glucans or even crude extracts; therefore, the actual anti-cancer effects specifically related to β-glucans remains to be verified by future well-designed clinical trials.
PHY906 for Anti-cancer Treatment in Patients with Colon, Liver, or Pancreatic Cancer
The current paradigm of mainstream pharmaceutical discovery can be described as a reductionist approach aiming to “bioprospect” and identify a single compound that can regulate a given target associated with a disease. It is argued that such a reductive single-chemical approach is related to incremental improvement in many cases; thus, a “holistic” polychemical approach such as the multiple herbal medicinal formula of traditional clinical usage should be considered in the strategies for development of cancer drugs. It will be extremely unlikely to find a single drug that can treat the majority of cancers originally caused by multiple reasons with defined targets. In addition, it will be difficult to find one drug to relieve all the side effects that come with a given treatment. Furthermore, it is important to consider the host factor including the immunological baseline, which differs significantly among patients in any specific treatment method.
PHY906, a 4-herb formula originally recorded in Chinese literature in 400 BC, consists of Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch, Paeonia lactiflora Pall and Ziziphus jujube Mill. PhytoCeutica Inc, an initiative led by Professor Yung-Chi Cheng of Yale University, aims to develop traditional Chinese botanical formulations into FDA-approved drugs for cancer and cancer supportive care. PHY906 was traditionally used for treatment of diarrhea, abdominal spasms, vomiting, nausea and fever—all common adverse effects in cancer chemotherapy. Based on the LC/MS analysis, structures for 64 bioactive compounds in PHY906, including flavonoids, triterpenes, saponins, and monoterpene glycosides, have been revealed.47 Subsequent carefully planned animal experiments attempted to demonstrate the effects of various combinations of the four herbs in formulation on anti-tumor activity, therapy-induced body weight loss and the prevention of death caused by Camptothecin-11 (CPT-11). CPT-11 is a commonly used chemotherapeutic agent for solid tumors with significant gastrointestinal side effects. The protective potential of PHY906 against CPT-11–induced toxicity led to a series of multi-center international clinical trials.48 This formulation could be useful in humans decrease non-hematological side effects and enhance the anti-tumor activity of chemotherapeutic agents. PHY906 has the potential to be the first oral herbal medicine approved by the FDA.
Conclusion and Future Directions
In summary, compounds derived from traditional herbal medicines may induce a wide spectrum of immunological and anti-cancer effects. In particular, we used arsenic trioxide and medicinal mushrooms as examples to illustrate these multi-targeted mechanisms of action. We also highlighted that even multiple herb–containing herbal formulations such as PHY906 can be used as potential adjuncts to chemotherapy.
The non-profit organization Consortium for Globalization of Chinese Medicine (http://www.tcmedicine.org) was established in 2003 and now has over 100 academic member institutes and industrial members. A few traditional adjunct therapeutic formulas are now going through the process of gathering substantial scientific and clinical evidence for their future usage in cancer management. Within the past two decades, many international and national collaborative clinical trials, in vivo animal studies and in vitro biological and chemical studies have been conducted to work towards the fulfillment of the basic regulatory requirements: 1) consistency or batch-to-batch preparations; 2) evidence-based clinical efficacy; 3) safety; and 4) knowledge of its action that comprises sites and mechanism of actions, active ingredients and interactions with other drugs. With collaborative efforts of multidisciplinary expert teams, we may witness some “supplements” soon evolving into FDA-approved adjunct therapies to standard chemotherapy to contribute to overall improved treatment of hematologic malignancies.
The potential role of β-glucan in leukemia. In vitro data suggested that β-glucan can enhance monocyte-derived dendritic cell maturation. This can possibly facilitate the leukemic tumor-specific antigen (TSA) recognition by T-helper cells (CD4) and subsequently leads to activation of cytotoxic T cells (CD8) and leukemic cell lysis. More TSA will then be released for adaptive immune response thereafter. In addition, in vitro data also showed that β-glucan can help to induce dendritic cell differentiation from selected monocytic leukemic cell lines. These leukemia-derived dendritic cells are immunopotent and may trigger anti-leukemic T-cell responses by presenting the leukemic antigen repertoire. This can lead to activation of B cells (CD19/20) and production of anti-leukemic antibodies.
The potential role of β-glucan in leukemia. In vitro data suggested that β-glucan can enhance monocyte-derived dendritic cell maturation. This can possibly facilitate the leukemic tumor-specific antigen (TSA) recognition by T-helper cells (CD4) and subsequently leads to activation of cytotoxic T cells (CD8) and leukemic cell lysis. More TSA will then be released for adaptive immune response thereafter. In addition, in vitro data also showed that β-glucan can help to induce dendritic cell differentiation from selected monocytic leukemic cell lines. These leukemia-derived dendritic cells are immunopotent and may trigger anti-leukemic T-cell responses by presenting the leukemic antigen repertoire. This can lead to activation of B cells (CD19/20) and production of anti-leukemic antibodies.
Disclosures Conflict-of-interest: The authors declare no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Department of Health Technology and Informatics, The Hong Kong Polytechnic University, HKSAR, China
Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, HKSAR, China