Abstract
The incidence and presenting features of chronic lymphocytic leukemia (CLL) have changed significantly over the last century. Routine diagnostic techniques can now detect very low levels of CLL phenotype cells. Monoclonal B-cell lymphocytosis (MBL) is a relatively recent diagnostic category encapsulating individuals with an abnormal B-cell population but not meeting the diagnostic criteria for a B-cell malignancy. This review focuses on CLL-type MBL, which represents the majority of MBL cases identified in diagnostic laboratories. CLL-type MBL has a phenotype identical to CLL and shares the same chromosomal abnormalities even at the lowest levels detectable. Recent evidence suggests that the immunoglobulin gene usage plays a key role in whether the abnormal cells will develop in significant numbers. In most cases, CLL-type MBL is a stable condition with only 1% per year among those presenting for clinical attention developing progressive disease requiring treatment, although suppressed immune function may have a more significant impact on outcome.
Changes in CLL Detection and Diagnosis
Chronic lymphocytic leukemia is (CLL) reported to be the most common leukemia among older adults in Western countries, accounting for approximately 30% of all leukemias and approximately 10% of hematological neoplasms.1 However, the incidence and presenting features of the disease have changed significantly over time.
In the 1950s only 10% of CLL cases were identified from a routine blood count.2 When the Rai staging criteria were introduced in the mid 1970s, individuals with stage 0 disease were required to have a lymphocyte count about 15,000 per mm3.3 Twenty years later the iwCLL criteria required a lymphocyte count above 10,000 and the National Cancer Institute (NCI) criteria above 5000 lymphocytes per mm3.4 During this time period, the incidence of disease doubled from 2.6 to 5.4 per 100,000 person-years5 and the median survival after diagnosis increased from 3 years to 7 years.6 However, the CLL mortality rate was relatively stable7 and the changes were primarily due to increased detection of early stage disease, rising from 15% of cases at Binet Stage A in the 1970s compared to 80% in the 1990s. The incidence of CLL stabilized where access to automated hematology analyzer technology is widespread and relatively inexpensive. However, the use of flow cytometry has the potential to significantly increase the sensitivity of detection of abnormal cells.
Short History of the Use of Flow Cytometry in the Diagnosis of CLL
The presence of abnormal antigens on CLL cells using a variety of techniques has been recognized since the early 1970s.8–11 By 1989 the French-American-British classification system proposed the use of flow cytometry or immunofluorescence to demonstrate strong expression of CD19 and CD5 with weak immunoglobulin and CD22/FMC7 expression and absence of CD10 expression to discriminate CLL from other chronic lymphoproliferative disorders.12 Expression of CD23 was also noted as helpful for distinguishing CLL from other CD5+ B-lymphoproliferative disorders,13 and the immunophenotype was noted as being adequate for a precise diagnosis in 1996 guidelines for diagnosis and treatment of CLL.4 Most diagnostic laboratories used 1- or 2-color flow cytometry, and the majority of lymphocytes would need to have a CLL phenotype before a diagnosis of CLL could be made. Therefore, the requirement for an absolute lymphocyte count above 5000 per mm3 with the lymphocytes demonstrating a CD19+CD5+CD23+ phenotype with monoclonal surface immunoglobulin expression was a reproducible and consistent threshold.
The diagnostic features were incorporated into minimal residual disease (MRD) assays that could distinguish CLL cells from a polyclonal normal B-cell background, first using the weak monoclonal immunoglobulin expression14 and then also incorporating CD5 expression.15–17 Multi-parameter flow cytometry with simultaneous analysis of CD19, CD5 and CD20/CD79b increased the sensitivity of detection to 1 CLL cell in 10,000 normal leucocytes.18–20
Small CLL-phenotype clones became easier to detect, and using only 3-color analysis to screen for perturbed kappa:lambda light chain expression on CD19+ B cells allowed identification of CLL-phenotype cells at the level of 100 per mm3 in occasional individuals presenting with a reactive T-cell lymphocytosis.21
This created a diagnostic problem because it would be inappropriate to report the results as normal, but classifying such cases as CLL could result in a significant psychological burden and financial implications for an individual with a low likelihood of developing progressive disease. In order to resolve this problem it became necessary to determine the prevalence of CLL phenotype cells in the general population as well as to perform a long-term outcome assessment.
Detection of CLL Phenotype Cells in the General Population
Several groups have performed population studies in the last twenty years. The data are summarized in Table 1 .
Hazardous Waste Sites
The earliest studies were performed by the National Center for Environmental Health in the US between 1991 and 1994 as part of a larger investigation into the potential health impact of living around hazardous waste sites.22 The study protocol included an immune biomarker panel with basic lymphocyte immunophenotyping but not B-cell kappa:lambda analysis. An abnormal B-cell phenotype was observed in 11 of 1926 participants (age range 47 to 72), of which 10 of 11 had an elevated total B-cell. Further tests confirmed an abnormal phenotype with light-chain restriction. Two additional cases with a B-cell clone were discovered during follow-up studies.23 By using this approach the overall prevalence was reported as 9 of 1499 (0.6%) of individuals. One case was diagnosed with CLL shortly after first follow-up and died with progressive disease two years after the abnormal B-cell population was detected. A second case was subsequently diagnosed with Waldenström macroglobulinemia and had stable disease at last follow-up.
Disease-specific Assays in the UK and Italy
Studies performed independently in the UK and Italy used high sensitivity flow cytometry to investigate the prevalence of abnormal B-cell populations in outpatient blood samples.24,25 In the study from the UK,24 samples from 425 male and 485 female hospital outpatients, median age 65 (range 40–90) with a normal blood count and no history or suspicion of cancer were tested by 4-color flow cytometry. Cells with a CLL phenotype were detected in 3.5% of total with a male:female ratio of 2.2:1. The prevalence increased with age, from 2.1% in the individuals between 40 and 60 years old to 5.0% for individuals over 60 (chi-square P = .01). The absolute numbers of CLL phenotype cells were low (median 13, range 3 to 1458 per mm3) and represented a minor proportion of total B-lymphocytes in most cases (median of 11%, range 3%–95%). In addition a CD5−monoclonal B-cell lymphocytosis (MBL) was detected due to a perturbation of kappa:lambda ratio in a further 9 (1.0%) of the 910 of individuals. These were mostly elderly individuals (median age 78 years old, range 49–88).24
An Italian study25 was conducted in a rural community outside Turin metropolitan area on samples from 269 females and 231 males (mean age 74, range 65–98 years) referred for common routine blood tests (eg, blood glucose, blood lipids). All individuals included in the study had a normal blood cell count, with no evidence of lymphocytosis and no history or suspicion of malignancy at the time of the routine blood test. Using a sensitive disease-specific protocol, CLL phenotype cells were detected in 5.5%, of individuals above 65 years of age, closely resembling the 5% frequency reported among the elderly (> 60 years) in the UK study.24 CLL phenotype cells represented a small proportion of total CD19+ B cells (mean 1.8%, range 0.7%–4%) The prevalence of monoclonal B-cells increased with age, being higher in individuals above 75 years of age and a male prevalence was manifest, especially among the latter age group. A CD5− monoclonal B-cell population was detected in 1.4%.
In spite of the different geographical origin and selection of patients (primary care vs hospital outpatients) both these studies reported very similar prevalence, age and gender distribution.
Further Population Studies
Both the UK and Italian groups have performed further studies in the general population with a focus on biological investigations, which are discussed below.21,26 Of note, the second Italian study used a higher sensitivity flow cytometry approach and identified a higher prevalence of monoclonal B-cell lymphocytosis (MBL) in the general population than in the primary care cohort. Studies from Alberto Orfao’s group in Salamanca utilized the highest sensitivity flow cytometry approaches available with analysis of 5 million events and identified a very high prevalence, with CLL phenotype cells detectable in more than 1 in 5 individuals over 60 years old.27 This study notes that the differences in reported prevalences are most likely due to the different sensitivity of the flow cytometry approach applied. The Salamanca group screened at least ten times more cells (5 million per case vs 200,000–500,000) and used 8-color staining panels versus 4-color25 or 5-color26 protocols. In the Salamanca study the percentage of aberrant/clonal B cells was below the maximum sensitivity of the latter approaches (< 0.01%) in more than half of the cases (62%). However, it should be noted that clonality was only confirmed by additional methods in 18 cases, all except one with > 0.01% aberrant/clonal B cells.
Proposal of Diagnostic Criteria for Monoclonal B-cell Lymphocytosis
The rationale for a diagnostic category for individuals with low levels of CLL phenotype cells detectable in their peripheral blood was twofold. Firstly, it could provide a platform to investigate general outcome and risk of disease progression for the increasing number of individuals identified with the condition as an incidental finding during investigation of an unrelated disorder. Secondly, it would provide a uniform definition to facilitate epidemiological and biological studies of the condition. The diagnostic criteria for MBL were intended to identify individuals with an abnormal B-cell population but who did not meet the criteria for a currently defined B-lymphoproliferative disorder. An absolute B-cell count was used as a threshold rather than the lymphocyte count as many of the cases identified in a primary care or hospital setting had an absolute lymphocytosis but a monoclonal B-cell count within the range detectable in the general population. The criteria that were originally proposed29 are shown in Table 2 .
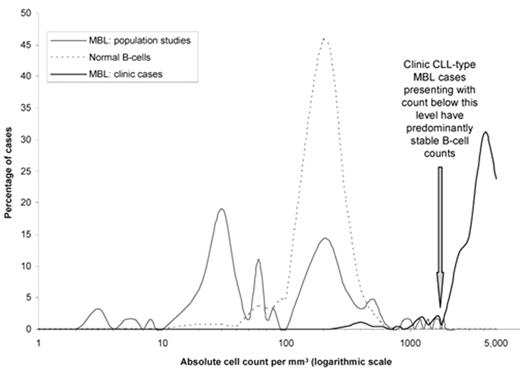
At the time that the criteria were published it was not clear whether individuals with CLL-type MBL, whether identified through scientific studies of the general population or routine diagnostic procedures within the healthcare system, had an increased risk of developing progressive CLL requiring treatment. The high prevalence of MBL in the general population, at least 100 times greater than the prevalence of CLL, indicates that if progressive disease were to develop it could only happen in a small proportion of MBL cases. It was also not clear whether there was a genuine biological relationship between CLL and MBL. An assessment of the distribution of absolute B-cell counts for CLL-type MBL detected in the general population compared to individuals in the healthcare system with a current or previous lymphocytosis is helpful in interpreting the biological data (Figure 1 ).
The data are taken from references.21,24 The normal B-cell count is typically around 200 cells per mm3 with 95% of polyclonal cases having a normal B-cell count between 50 and 500 per mm3. Approximately 40% of CLL-type MBL detected in the general population demonstrate an abnormal B-cell count below 50 per mm3, 45% within the same range as the normal B-cell count, ie, 50–500 per mm3, and the remaining 15% with a count between 500 and 1900 per mm3. For CLL-typeMBL cases identified in the healthcare system after investigation for a lymphocytosis there are also 15% with a count between 500 and 1900 per mm3 and 85% with an abnormal B-cell count above this level. Within the MBL with lymphocytosis group, the optimal cut-off point for predicting a low risk of developing CLL was 1900 per mm3.21
Biological Relationship Between MBL and CLL
Several studies have now focused on the biology of CLL-type MBL to determine the relationship with progressive CLL. Perhaps the strongest evidence comes from investigation of apparently unaffected individuals from families with a genetic predisposition to CLL.
Familial CLL
All epidemiological studies that have investigated the prevalence of CLL and other lymphoproliferative disorders in relatives of patients have reported elevated risks of CLL in relatives30–35 with the largest showing a 7-fold increase in risk.36 The first reports of MBL came from studies of apparently unaffected family members in the early 1990s.37 More recently studies in both the UK38 and US37 demonstrated a very high prevalence of CLL-type MBL in individuals from CLL families, with normal blood counts at 8 of 59 (13.5%) and 6 of 33 (18%), respectively. The UK study used the same methodology for familial CLL and general population studies and demonstrated at least a 4-fold increase in prevalence in families with a genetic predisposition to CLL. For young adults aged 16 to 40 years the relative risk is 17-fold38 and it is unlikely that this is an anticipation phenomenon,36 suggesting that in some families there is an inherited abnormality that increases susceptibility to development of CLL at a much earlier age than the general population, thus increasing the life-long risk of developing a clinically apparent CLL clone within the family as a whole.
Protein and mRNA Expression Studies
The majority of MBL cases show the same expression pattern for the routine diagnostic markers as typical CLL. More extensive analysis of 18 B-cell associated markers, with routine diagnostic markers CD5, CD20, CD23 and CD79b excluded to minimize bias, was performed in 9 CLL-type MBL, 35 B-lymphoproliferative disorders and 7 control cases. Unsupervized k-means clustering analysis identified two major clusters, the first containing CLL and CLL-type MBL cases with the second containing normal samples and other B-lymphoproliferative cases with only CLL case. This demonstrates an extremely close association in the extended protein expression profile of CLL-type MBL and CLL.39 Similar evidence was obtained by Brian McCarthy and colleagues using microarray analysis. They previously identified a selected panel of genes comprising FMOD, CKAP4, PI3Kc2b, LEF1, PFTK1, Bcl2 and GPM6a in order of receiver operating characteristic (ROC) concordance. The panel was then used to categorize and predict the relationship of RNA expression between normal donors, MBL cases and CLL patients. Using this approach the group could readily distinguish MBL from normal B cells, and the results suggest that LEF1 expression is a common feature of CLL at all stage of disease.40
Chromosomal Abnormalities
There is no specific chromosomal translocation associated with CLL, but a 13q14 deletion is detected in the leukemic cells from over 50% of CLL patients.41 As a sole abnormality, deletion 13q14 is associated with a good prognosis. Trisomy 12 and deletion of 11q and/or 17p are also common in CLL; the latter two abnormalities confer a poor prognosis.41 Reports of chromosomal abnormalities in CLL-type MBL are summarized in Table 3 . All studies detect a deletion of 13q14 in a proportion of cases, although in most cases not all the abnormal cells have the abnormality. The proportion of cells with a deletion of 13q14 in the Leeds outpatient normal-count MBL group was 5% to 90% of total B cells.21 Markers associated with poor prognosis (deletion of ATM or P53) were rarely detected in MBL, and then only in cases with a lymphocytosis. When detected in CLL-type MBL with a lymphocytosis (2/33 ATM deletion and 1/33 P53 deletion) the abnormalities were present in less than 20% of the MBL cells. The studies from Salamanca27 and Duke University42 are important as the individuals in these studies had very low counts, including one case from Salamanca with only 0.007% CLL phenotype cells.
Several studies have focused on the IGHV mutation status and in most a higher proportion of CLL-type MBL cases have more than 2% IGHV mutation than is seen in clinical CLL. The results, summarized in Table 4 , demonstrate that in cases where there are few residual normal B cells, the CLL-phenotype cells use similar IGHV genes to clinical disease. However, when the CLL-phenotype cells represent a small proportion of total B cells the IGHV repertoire is different.
In the Italian general population and Salamanca primary care group, the numbers of abnormal cells were typically below 100 cells per mm3. The Duke University study investigated cases with a possible genetic predisposition to developing CLL. The Leeds study used a clonality approach for screening, and IGHV analysis was only performed in cases where direct sequencing was possible, ie, the abnormal cells represented the majority of total B cells.
These findings support the hypothesis proposed by Paolo Ghia and colleagues26 that the IGHV repertoire may be different in MBL patients with very low counts and that cases using certain IGHV families are at a higher risk of evolving into clinical disease.
Clinical Outcome for Individuals with CLL-type MBL
There is very limited information on outcome for individuals identified as having a CLL-type MBL from general population studies with low abnormal B-cell counts because ethical and logistic considerations limit the possibility for follow-up. It has been demonstrated by Ola Landgren and colleagues that virtually all cases of CLL have been preceded by CLL-type MBL several years prior to diagnosis.45 However, the risk for an individual with CLL-type MBL to develop CLL is likely to be low, because such a large proportion of the general population has an abnormal B-cell clone. The key question is how to manage the individuals identified to have an abnormal B-cell population after investigation of a lymphocytosis. This important issue has been studied by five groups.
First, Sarah Fung and colleagues reported on the outcome for patients with CLL-type MBL (n = 54) compared with those with Rai stage 0 CLL (n = 112). MBL patients have a low probability of early progression, with no patients requiring treatment or dying of CLL-related causes after a median 2.5 year follow-up. There was a trend towards improved progression-free survival compared to Rai stage 0 CLL, but overall survival was very similar between MBL and stage 0 CLL.46
The Leeds group reported a series of 185 CLL-type MBL cases with a median follow-up of 6.7 years. The lymphocyte count increased to more than twice the presentation count and remained at this level or increased at subsequent assessments in 51 cases (28%) with a lymphocyte count above 30,000 per mm3 occurring in 31 cases (17%). Progressive CLL, characterized by lymphadenopathy, splenomegaly, anemia, thrombocytopenia, lymphocyte doubling time < 6 months, persistent infection, or drenching night sweats, developed in 15% (28 of 185) and chemotherapy was required in 13 (7%). There were 62 deaths among the subjects with CLL phenotype MBL and lymphocytosis during follow-up, of which 13 had progressive CLL, but this was noted as a cause of death in only 4.21
Stephen Mulligan and colleagues reported on the outcome for a series of 220 CLL-type MBL cases with a median 4.1 years follow-up of which 76 cases (35%) showed an increasing lymphocyte count with 18 cases (8%) reaching a count above 30,000 per mm3.47
Tait Shanafelt reported the Mayo Clinic data on 459 cases identified as Rai stage 0 between January 2000 and December 2007, of which 190 (41%) would be re-classified as CLL-type MBL according to the 2009 diagnostic criteria.48 The outcome data for individuals with MBL as defined in the current criteria were similar to the Leeds data. They report a significant difference in treatment-free but not overall survival for CLL-type MBL compared with Rai 0 CLL43 defined according to the 2009 criteria,48 but stress that a higher B-cell cut-off of 11,000 cells per mm3 is a better predictor of treatment-free and overall survival, discussed below.49
Most recently Rossi and colleagues reported on a series of 123 MBL, which showed a better clinical course and lower infection risk compared to 154 Rai stage 0 cases. B-cells counts below 1200 per mm3 or above 3700 per mm3 predicted a significantly lower or higher risk, respectively, of developing CLL or SLL. Standard biological markers IGHV mutation, CD38 expression, ZAP-70 and chromosomal abnormality predicted treatment-free survival, although FISH status was the only independent prognostic factor. The risk of requiring treatment appears higher in this study than in others at 4% per year in the first 7 years, with a high proportion developing SLL.50 The follow-up for patients is significantly longer in the Rossi study, and the difference in outcome may reflect less extensive screening for lymphadenopathy in the cases diagnosed many years ago.
The majority of individuals with CLL-type MBL will not develop progressive disease over a 5-year period. Approximately 75% will be alive with a stable lymphocyte count and between 1% and 4% per year will develop progressive disease requiring treatment. The rate of progression is similar to that seen from MGUS to myeloma.
Approaches to Monitoring
The available outcome data relate almost wholly to individuals presenting with levels of abnormal cells above 500 cells per mm3. There are no published outcome data for individuals with a CLL phenotype cell count below 50–100 cells per mm3, and there is some evidence that the cells in this setting are biologically different to CLL26; therefore, there is no clear rationale for monitoring individuals with such a low-count MBL if they are detected through investigation of a lymphocytosis. However, such individuals are rarely detected in a healthcare setting and are usually identified through scientific studies in the general population.
The Leeds outcome data indicate that more than 90% individuals presenting with a CLL cell count below 1900 per mm3 will have a stable lymphocyte count over a 5-year period. Mulligan and colleagues stress that some of these individuals do develop CLL47; in our experience, a given individual will usually develop progressive disease requiring treatment if a CLL counts rise from such a low count measured at initial presentation. It may be beneficial to perform at least one follow-up flow cytometry assessment within 6 to 12 months to ensure that the B-cell count remains stable.
For individuals presenting with a count above 1900 per mm3, Kaplan–Meier curves of disease progression show no plateau over time, indicating that, as with MGUS, indefinite periodic monitoring is indicated for subjects with CLL phenotype MBL presenting with a lymphocytosis. The majority of individuals are likely to be elderly with coexistent health issues; therefore, any monitoring should ideally be provided in a setting convenient to the patient. We use a postal system with local phlebotomy and central hematologist review of laboratory parameters and symptoms identified by a patient self-assessment questionnaire for MGUS, MBL and early-stage CLL cases.51 Flow cytometry is performed at each assessment as it is easier to monitor increases in B-cell and decreases in T-cell numbers which are often not evident from the lymphocyte count alone. Alternatively, a yearly blood count and brief physical assessment are sufficient to exclude disease progression.
Factors Predicting Requirement for Treatment
For individuals with higher B-cell counts, between 5000 and 11,000, the normal CLL prognostic markers of IGHV mutation status, FISH, ZAP-70 and CD38 expression, apply.43,49,50 For cases with lower counts the prognostic impact of such markers is less clear. CD38 expression was predictive of outcome in the Mayo49 and Italian50 series but not the Leeds series.21 The Italian study reports that the IGHV mutation status is predictive of outcome, but a higher number of CLL-type MBL cases developing progressive disease have mutated IGHV than unmutated.21,50 This is likely to reflect the fact that the majority of CLL-type MBL cases are mutated and outnumber all the unmutated IGHV cases when they progress. Because disease progression is rare for CLL-type MBL, predicting the risk from presentation features is likely to be inaccurate.
Factors Predicting Mortality
In the Leeds series, age above 68 years and hemoglobin level below 12.5 g per deciliter were the only independent prognostic factors for mortality. By definition, anemia at the time of diagnosis of CLL phenotype MBL was not autoimmune or due to bone marrow infiltration by CLL. Therefore, individuals with CLL-type MBL and anemia should undergo further investigations to determine the cause of anemia. Cases presenting with a higher B-cell count had a significantly poorer overall survival in both Leeds21 and Mayo Clinic43,49 series, whereas those presenting with a higher T-cell count had a significantly better overall survival. The development of progressive disease is not the major cause of mortality in CLL-type MBL, but there is an inverse correlation between CLL cell numbers and residual normal B-cell numbers (unpublished observation), suggesting that immune suppression may be a significant factor. The inter-relationship between lymphocyte subsets makes it difficult to identify independent variables in multivariate analysis. The Mayo Clinic report notes that “as B-cell count increased, survival decreased; as T and NK counts increased, survival increased making the ALC summary measure less accurate than the prognostic value of its components.”49
Bone Marrow Investigation
CLL-type MBL may be detected as an incidental finding in bone marrow samples taken for lymphoma staging investigation. Using sensitive screening techniques, this is likely to approach the prevalence seen in the general population at this age, ie, up to 5% of samples. The presence of a small population of CLL-phenotype cells should not be considered evidence of bone marrow involvement unless a clonal relationship between the CLL phenotype cells in the bone marrow and the monoclonal cells in the tissue biopsy can be demonstrated. It should not be assumed that the detection of CLL-phenotype cells at low levels in a peripheral blood or bone marrow eliminates the need for lymph node biopsy in individuals undergoing evaluation for lymphadenopathy.
In an individual presenting with cytopenia, the detection of CLL-type MBL does not eliminate the need for further investigation to identify the underlying cause of cytopenia. Less than 5% of individuals without lympadenopathy and with fewer than 5000 circulating CLL cells per mm3 have extensive bone marrow involvement with CLL. There are limited unpublished data available, but this suggests that in most individuals with CLL-type MBL who require a bone marrow biopsy for investigation of cytopenia the underlying cause is another condition, such as metastatic carcinoma or myelodysplastic syndrome, which is unrelated to CLL.
Screening Donors for Blood Transfusion and Allogeneic Transplantation
As noted above, a CLL-type MBL population is detected in approximately 1 in 1000 blood donors but should have no implication for irradiated or leukocyte-depleted blood products.28 The implications for allogeneic transplantation are significantly more complicated when the transplant procedure is for a CLL patient. In addition, there is a reasonable probability that a relative considered to become a donor could have a CLL-type MBL population. Studies of prognostic factors in CLL families indicate that different prognostic groups are found within the same families, ie, the genetic predisposition is to develop CLL and not a specific prognostic group of CLL.52 Given that the majority of CLL-type MBL is unlikely to progress, there is no reason to predict that CLL-type MBL cells would have an impact on overall survival after transplantation. However, at the current time there are no published data; the issues are discussed in a report by Hardy et al.53
CD5– Monoclonal B-cell Lymphocytosis
CD5- MBL is detected in approximately 1% of individuals in the general population.24,25 There are limited data concerning the outcome for individuals with CD5− MBL either detected in population screening or through investigation of a lymphocytosis. As there is no specific immunophenotype to discriminate CD5− MBL cells from other B cells, by definition normal B-cell levels must be significantly reduced in order to detect CD5− MBL. In addition, CD5− B-lymphoproliferative disorders are likely to have more extensive bone marrow and nodal involvement than peripheral levels suggest. Therefore the detection of a circulating CD5− MBL warrants the work up for non-Hodgkin lymphoma.
Practical Aspects of Diagnosing CLL-type MBL
In most respects a diagnosis of CLL-type MBL has similar implications as a diagnosis of MGUS. It is important that individuals are aware that their risk of requiring treatment for leukemia is low but increased compared to the general population. The risk is sufficiently high that periodic checking of the blood count is indicated, and individuals should be informed of the key symptoms to monitor themselves, particularly enlargement of lymph nodes and persistent tiredness. Many individuals falling within the MBL category will previously have been given a diagnosis of CLL. In such cases it may help to inform the individual that their risk of developing progressive disease is lower than for people with CLL. The approach to monitoring CLL-type MBL will usually be the same as for individuals with early stage CLL involving a yearly blood count and physical assessment. The monitoring may be provided using an outreach approach (see above), in a primary care setting or at a hematology clinic.
Approximately 20% to 25% of CLL-type MBL cases have CD38 expression, ZAP-70 expression, and/or unmutated IGHV.21,43,49,50 Identifying the prognostic relevance of these markers in CLL-type MBL will require longer follow-up. There is no consensus yet on how to use prognostic information in CLL-type MBL and it is reasonable assess the same markers tested locally for CLL. As with CLL, there is no indication to initiate treatment based on the presence of adverse prognostic markers.
There is no discrete international classification of diseases (ICD) code for CLL-type MBL and in the WHO classification the disorder is grouped with CLL.54 However, placing such individuals within the CLL diagnostic category can limit their ability to obtain loans or insurance. US clinicians may code the disorder as “lymphocytosis” (eg, ICD-9-CM 288.61) to reflect the fact that mortality or morbidity associated with progressive CLL is rare. In some countries, particularly those with a universal health care system, it may be necessary to use a CLL code to enable the costs of monitoring to be provided. In such cases it will be helpful if individuals with CLL-type MBL can access supporting documentation to demonstrate the low risk of developing progressive disease should there be issues with financial institutions. Ideally an improved coding system that reflects both the underlying pathology and low risk of disease progression can be identified in the future.
Many groups are attempting to improve the identification of cases likely to develop progressive disease and separate these from cases that could be considered part of the normal range, which do not require monitoring. Identifying the differences in biology between CLL-type MBL and CLL is likely to play a crucial role in understanding the mechanisms of disease progression.
Frequency distribution of absolute B-cell or CLL-phenotype cell counts in polyclonal and CLL-type MBL cases in the general population and CLL-type MBL in individuals referred for investigation of a lymphocytosis.
Frequency distribution of absolute B-cell or CLL-phenotype cell counts in polyclonal and CLL-type MBL cases in the general population and CLL-type MBL in individuals referred for investigation of a lymphocytosis.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
HMDS, Department of Haematology, St. James’s Institute of Oncology, Leeds, United Kingdom