Abstract
The last four years have seen an explosion in our understanding of the myeloproliferative neoplasms. Important and often unexpected insights into the molecular mechanisms responsible for these disorders have been accompanied by the development of new diagnostic tests and by an improved understanding of the relationship between the different disease entities. This review will focus on recent developments in the pathogenesis and management of essential thrombocythemia with a particular emphasis on its phenotypic overlap with polycythemia vera and primary myelofibrosis.
Essential thrombocythemia (ET), a clonal stem cell disorder characterized by an isolated thrombo-cytosis and thrombo-hemorrhagic complications, shares phenotypic and pathogenetic similarities with other myeloproliferative neoplasms (MPN), particularly polycythemia vera (PV) and primary myelofibrosis (PMF). Although first recognized as a specific disease entity in the 1930s, relatively little was known about the pathogenesis of this disorder until 2005, when an acquired mutation in JAK2 (V617F) was reported in around 50% of patients with ET, along with half of those with PMF and the vast majority with PV.1,2 More recently, mutations in MPL were reported in around 4% of those with ET or PMF,3–5 and mutations in TET2 have been observed in a variety of myeloid malignancies including JAK2 V617F–positive and –negative ET.6
Pathogenesis
Relationship of ET to PV and PMF
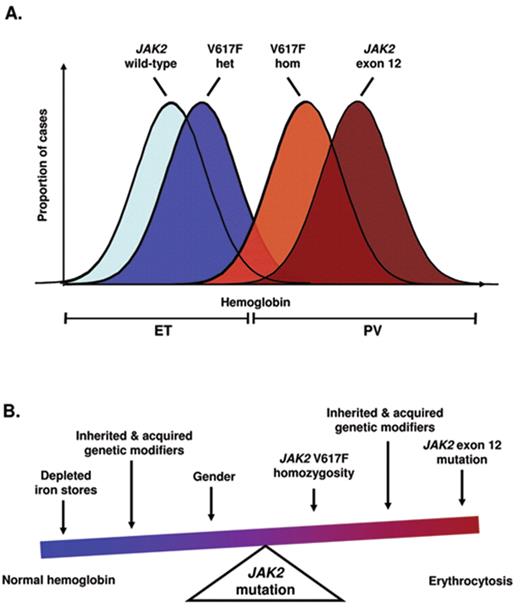
The same JAK2 V617F mutation is present in the vast majority of patients with PV and around half of those with ET or PMF, raising questions as to how a single mutation is commonly associated with apparently distinct clinical phenotypes. Compared to V617F–negative ET, patients with V617F–positive ET were found to have multiple features resembling PV, including higher hemoglobin levels and white cell counts, lower erythropoietin levels, increased bone marrow erythroid and granulocytic activity and higher rates of venous thrombosis.7 These findings suggest a phenotypic overlap between ET and PV, with V617F–positive ET representing a forme fruste of PV. According to this concept, the precise phenotype manifested following acquisition of a JAK2 mutation will depend on additional constitutional and/or acquired modifiers (Figure 1 ). Evidence that a constitutional genetic background modulates disease phenotype comes from mouse models, in which expression of JAK2 V617F produces different phenotypes depending on the genetic strain of the recipient animal.8 One acquired modifier is likely to be the development of homozygosity for the JAK2 mutation. The majority of patients with PV harbor a JAK2 V617F–homozygous clone, whereas such clones are rare in ET,9 suggesting that the ratio of mutant to wild-type JAK2 may be important in determining disease phenotype. Consistent with this notion is the observation that mutations of JAK2 exon 12 are associated with stronger downstream signaling compared with JAK2 V617F and are found in patients with PV but not in those with ET.10 Further support comes from mouse models, in which low levels of mutant JAK2 tend to be associated with thrombocytosis whereas higher levels give rise to erythrocytosis.11,12 Lastly, activation of STAT5 in human CD34-positive cells favors erythroid differentiation, whereas reduced levels favor a megakaryocyte fate.13 Taken together, these findings suggest that the level of JAK2-STAT5 signaling provides a rheostat that determines whether the disease phenotype is predominantly erythroid or megakaryocytic.
Although ET and PMF have traditionally been considered as separate entities, several lines of evidence suggest a blurring of the distinction between these disorders. A proportion of patients diagnosed with ET (see Table 1 for criteria) harbor increased levels of bone marrow reticulin in the absence of other features suggesting a diagnosis of PMF14 (eg, splenomegaly, anemia and/or a leukoerythroblastic blood film). Moreover, in a proportion of ET patients their disease evolves into a clinical syndrome indistinguishable from PMF. It therefore seems likely that the variable degree of reticulin accumulation reflects the combined effects of genetic background, disease duration, therapy, clonal burden and the acquisition of additional genetic lesions. The natural accumulation of reticulin over time in ET should be clearly distinguished from the clinical syndrome of myelofibrotic transformation, which represents accelerated phase disease and presumably reflects the accumulation of additional genetic events. Patients labeled as having PMF may in fact be presenting in accelerated phase of a pre-existing but undiagnosed MPN1; consistent with this notion, some patients with PMF have thrombocytosis many years prior to diagnosis.
Familial Predisposition to ET and Other Myeloproliferative Neoplasms
An inherited predisposition to ET and other MPNs was initially recognized in families containing multiple affected members. Such individuals often harbor acquired mutations in JAK2 and appear clinically indistinguishable from those with sporadic disease.15 This familial predisposition was confirmed by analysis of the comprehensive Swedish Cancer Registry, which identified a relative risk of 7.4 for developing ET in those with an affected first-degree relative.16 Three studies published in early 2009 account for approximately half of this inherited predisposition.17–19 These studies all demonstrated that acquisition of a JAK2 V617F mutation is strongly associated with inheritance of a constitutional haplotype that includes the JAK2 gene. Importantly, in heterozygotes for this haplotype the JAK2 mutation was observed much more frequently in cis to the predisposition allele. There are two main models to explain these findings. The hypermutability model proposes that the predisposition allele is particularly prone to acquiring mutations as a consequence of an unknown mechanism. In the second “fertile soil” model, JAK2 mutations are equally likely to occur on either allele, but are more likely to result in a clonal expansion with manifest disease if they are acquired in cis to the predisposition haplotype, perhaps due to a difference in the expression or function of the JAK2 protein encoded by this allele.
Are Mutations in JAK2 Disease-initiating Events?
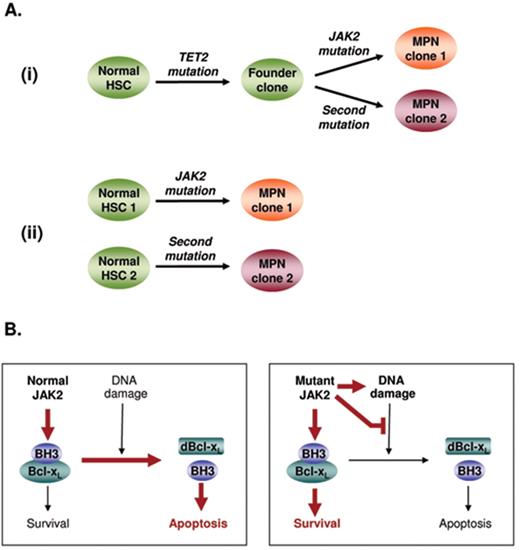
Several lines of evidence suggest that JAK2 mutations play a central role in the pathogenesis of their associated MPN. Expression of mutant JAK2 confers cytokine-independent growth to various cytokine-dependent cell lines, is associated with ligand-independent activation of downstream signaling pathways and is sufficient to produce an MPN-like disease in vivo when expressed in murine bone marrow cells.1,2 However, several lines of circumstantial evidence have accumulated in support of the concept of a pre-JAK2 phase. Such studies include (1) familial tendency to develop MPN,15,16 leading to the suggestion that JAK2 mutations may be insufficient alone to cause a clinical phenotype; (2) patients in whom granulocyte clonality measured by X-chromosome inactivation pattern or quantitation of a cytogenetic abnormality exceeds clonality measured by JAK2 V617F mutant allele burden20; (3) progression to JAK2 wild-type leukemia following a JAK2 V617F–positive MPN;21 and (4) patients with a JAK2 V617F–positive MPN who produce erythropoietin-independent erythroid colonies that are negative for the JAK2 mutation.22,23 Although these studies provide only indirect evidence for the existence of a pre-JAK2 phase, more recently patients have been reported in whom acquisition of a JAK2 mutation was preceded by either a deletion of chromosome 20q24 or a mutation in TET2.6 Thus, direct evidence now exists demonstrating that JAK2 mutations are not the disease-initiating event in some patients, although the frequency of this scenario remains unclear. The situation has been further complicated by the identification of biclonal disease in a proportion of patients with chronic-phase MPN, in whom two genetic events (such as mutations in JAK2 or MPL or deletions of chromosome 20q) can be detected in separate clonal expansions.23,25 Analysis of such patients has demonstrated two pathways leading to the development of biclonal disease, with mutation-bearing clones representing either the related progeny of a shared TET2-mutant founder clone26 or independent proliferations arising from distinct stem cells25 (Figure 2A ). Only a limited number of mutations have so far been identified in ET, and so it seems likely that current studies underestimate the prevalence of biclonal disease.
Progression to Acute Myeloid Leukemia
Progression to acute myeloid leukemia (AML) occurs in a small minority of ET patients and involves the accrual of further genetic events. Recent work has illuminated the mechanisms underlying disease progression. Studies of cell lines and human progenitors suggest expression of JAK2 V617F is associated with increased DNA damage and aberrant DNA repair.27 Moreover JAK2 V617F–positive cells from patients with PV or PMF fail to undergo apoptosis in response to DNA damage,28 thus providing a previously unrecognized mechanism for the accumulation of genetic lesions (Figure 2B ).
Unexpectedly, leukemia developing in the context of a preceding JAK2 V617F–positive MPN is negative for the JAK2 mutation in around a half of cases.21,29 In theory, this phenomenon might reflect leukemic transformation arising in the wild-type daughter cell resulting from mitotic recombination in a JAK2 V617F–heterozygous cell, but this mechanism has been excluded in all 12 cases examined to date.21,26,29 Two alternative models exist to explain the development of JAK2 wild-type AML following a JAK2-mutant MPN: (i) the two phases of disease are phylogenetically related, having arisen from a shared (pre-JAK2) founder clone, or (ii) the MPN and AML are clonally unrelated and reflect transformation of independent stem cells. Some insight has been gained from detailed studies of two patients in whom TET2 mutations were detected at the time of transformation to JAK2 wild-type leukemia. In both cases, the TET2 mutation was absent from JAK2-mutant erythroid colonies, demonstrating that mutations in JAK2 and TET2 were present in separate clonal expansions26 but leaving open the possibility of a shared founder clone preceding both JAK2 and TET2 mutations. At the time of writing there is no direct evidence to distinguish the two models described above, and different mechanisms may well operate in different patients.
Diagnosis and Management
Diagnostic Criteria
ET has traditionally been a diagnosis of exclusion, requiring the absence of reactive conditions and other myeloproliferative and myelodysplastic syndromes that may present with an isolated thrombocytosis. The identification of mutations in JAK2 and MPL now allows for the positive identification of this disorder in over a half of all cases.4 Testing for the JAK2 V617F mutation is generally considered a first-line investigation for suspected ET, with screening for mutations in MPL recommended in JAK2 V617F–negative cases. Mutations in JAK2 exon 12 are not thought to occur in patients with ET.10 The combination of an isolated thrombocytosis with a pathogenetic mutation, in the absence of iron deficiency (which may mask PV) or features of PMF (splenomegaly, anemia or a leukoerythroblastic blood film), is usually sufficient to make a diagnosis of ET. In patients with suspected ET who lack JAK2 and MPL mutations, exclusion of reactive causes is particularly important. In this context a careful history, assessment of inflammatory markers and bone marrow histology are all recommended in order to confirm the diagnosis. Occasional patients with chronic myeloid leukemia present with an isolated thrombocytosis in the absence of leukocytosis. Given the major therapeutic implications, testing for BCR-ABL1 should be considered in all patients who lack mutations in JAK2 and MPL.
Suggested diagnostic criteria for ET are presented in Table 1 . These criteria are similar to the WHO 2008 classification but differ in three important respects. First, bone marrow trephine biopsy is not always required in the presence of a pathogenetic mutation (eg, JAK2 or MPL), on the basis that PV can usually be excluded by normal iron studies and in some patients PMF can be excluded by the absence of splenomegaly, anemia and a leukoerythroblastic blood film. Second, bone marrow histology is not used to subdivide ET into “true-ET” and “prefibrotic myelofibrosis” as the existence of the latter proposed entity remains controversial and the underlying histological criteria are difficult to apply reproducibly, even by experienced hematopathologists.30 Third, this classification includes patients with bone marrow reticulin of greater than grade 2 (on a 0–4 scale) without other features of PMF or myelofibrotic transformation. It is clear that there is histological heterogeneity within ET, and we favor the view that patients with ET (or PV) gradually accumulate reticulin fibrosis as an inherent part of their disease, with the degree of fibrosis reflecting interplay between factors such as disease duration and inherited or acquired genetic modifiers.
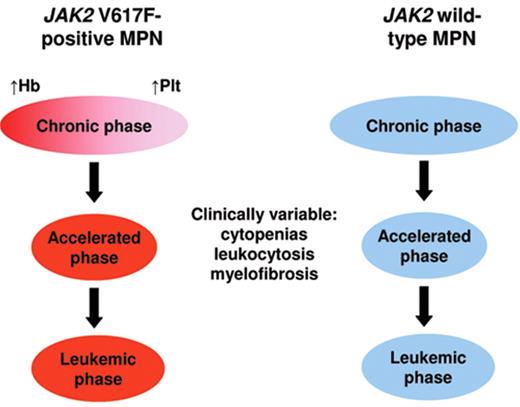
Recent insights have initiated a move towards a molecular classification of the myeloproliferative neoplasms (Figure 3 ). JAK2 V617F–positive ET resembles a forme fruste of PV, with the two disorders sharing several laboratory and clinical features.7JAK2 V617F–negative ET presents with a more isolated thrombocytosis and is heterogeneous; 10% of patients harbor mutations in MPL,4,5 but the molecular lesion(s) responsible for the remainder are currently unknown. In a proportion of patients the accumulation of genetic or epigenetic changes results in acceleration of their disease, which may manifest, inter alia, as myelofibrotic transformation. Given that myelofibrotic transformation of ET or PV is indistinguishable from PMF, it seems likely that at least some, and possibly most, patients labeled as PMF represent individuals presenting in accelerated phase of a pre-existing MPN.1
Prognosis
There is a lack of good quality prospective data concerning long-term survival in ET. From retrospective studies, mortality rates are similar to population controls in the first decade after diagnosis but appear to increase thereafter.31 This excess mortality results from disease complications such as thrombosis and transformation to myelofibrosis or AML. However management paradigms have changed significantly over the last 10 to 20 years, and so the relevance of these data for current patients is unclear.
Age over 60 years or history of prior thrombosis remain the best predictors of thrombotic complications in patients with ET. Risk factors associated with atherosclerotic disease in general, such as diabetes, hypertension, hypercholesterolemia or tobacco use, may also predict for thrombosis in patients with ET, although definitive data are lacking. The predictive utility of the platelet count remains unclear, and an association between the degree of thrombocytosis and risk of thrombosis or hemorrhage is not well established. More recently leukocyte count at diagnosis has been reported as an independent predictor of thrombosis in both ET and PV,32 but it is unclear whether the relationship is causal. Patients with JAK2 V617F–positive ET are reported to exhibit higher rates of venous and overall thrombosis compared with those without the mutation,7 although a direct association between JAK2 V617F–positive ET and arterial events is not well established. The presence of an MPL mutation predicted for higher rates of arterial thrombosis compared with JAK2/MPL-negative patients in the Italian5 but not the PT-14 cohort. More recently follow-up from the PT-1 trials has identified increased bone marrow reticulin fibrosis at diagnosis as an independent predictor of subsequent thrombotic and hemorrhagic complications.14 Although correlated with both increased white cell and platelet count, bone marrow reticulin at diagnosis remained a predictor for thrombosis and hemorrhage in multivariate analysis, implying that reticulin fibrosis serves as a marker for the aggressiveness or clonal dominance of the MPN.
Therapy
There is a general consensus that cardiovascular risk factors such as hypertension, diabetes, smoking, hypercholesterolemia and obesity should be identified and treated accordingly. Unless contraindicated, low-dose aspirin is generally recommended for all patients. Although data in ET patients are lacking, a large randomized trial in PV demonstrated a reduction in thrombotic events in those taking aspirin without a concomitant increase in the risk of hemorrhage.33
Cytoreductive therapy in ET is aimed primarily at reducing the frequency of thrombotic complications, with treatment decisions based on the stratification of patients according to known risk factors. At the present time, age over 60 years or history of previous thrombosis is generally considered to mandate cytoreductive therapy. Although the degree of thrombocytosis is not a reliable indicator of thrombotic or hemorrhagic risk, many physicians consider cytoreductive therapy in patients with a very high platelet count (for example, greater than 1500 × 109/L). The predictive utility of other risk factors such as leukocytosis, mutation status and bone marrow fibrosis await further validation before they can be used in treatment stratification. In the absence of high risk features, patients can be divided by age into low risk (age less than 40 years) and intermediate risk (age 40–60 years). Cytoreductive therapy is unlikely to offer a significant benefit for those with low-risk disease, in whom the a priori risk of thrombosis is small. There is currently little evidence available to guide treatment decisions in the intermediate risk group. The on-going PT-1 trials (http://www.ctsu.ox.ac.uk/projects/leuk/pt1/), comprising a randomized trial of hydroxyurea and aspirin versus aspirin alone for intermediate-risk patients and an observational study of low-risk patients treated with aspirin alone, will provide prospective data to help clarify therapeutic decisions for these patients.
Hydroxyurea is widely regarded as first-line therapy for patients requiring cytoreductive treatment, and is the only cytoreductive agent proven to reduce thrombotic events in a randomized controlled trial.34 Concern exists, however, over a possible increased risk of AML transformation in patients treated with hydroxyurea. Clinical studies attempting to address this issue have given contradictory results, often confounded by factors such as small numbers, the use of multiple therapies, lack of proper controls, retrospective data collection, relatively short follow-up and the fact that AML may occur in the absence of cytoreductive therapy. Moreover hydroxyurea appears neither leukemogenic in the treatment of sickle cell disease35 nor mutagenic in patients with MPN,36 and those receiving single-agent hydroxyurea do not appear at increased risk of leukemic transformation.37
Current evidence suggests that if there is any increased risk of AML with hydroxyurea, it is likely to be small and should be balanced against the expected reduction in thrombotic complications, which remain the leading cause of morbidity and mortality in patients with ET.38
Anagrelide reduces the platelet count by selective inhibition of megakaryocyte differentiation. There is no reason to think that anagrelide might be leukemogenic and it does not produce neutropenia, although a dilutional anemia is common. The PT-1 study demonstrated that anagrelide plus aspirin was inferior to hydroxyurea plus aspirin in high-risk patients with ET. Platelet control was equivalent in the two arms. However, patients who received anagrelide were more intolerant of their treatment and experienced higher rates of arterial thrombosis, major hemorrhage and myelofibrotic transformation, although rates of venous thrombosis were higher in the hydroxyurea arm.38 Anagrelide does appear to provide partial protection from thrombosis and may be considered as second-line therapy for patients in whom hydroxyurea is inadequate or not tolerated. Combined therapy with anagrelide and hydroxyurea has also been used successfully where hydroxyurea alone has failed to control the platelet count. Therapy with anagrelide, but not with hydroxyurea, was also associated with progressive anemia and an increase in bone marrow fibrosis.14 The increased fibrosis was reversible in a small number of patients upon withdrawal of anagrelide,14 and follow-up trephine biopsies are therefore recommended for patients receiving this agent, perhaps every 2 to 3 years. The results of the ANAHYDRET trial (comparing hydroxyurea to anagrelide) have been reported to show that anagrelide is not inferior to hydroxyurea in the treatment of ET.39 However, compared with PT-1, the number of patients enrolled was small, duration of follow-up relatively short (539 patient-years compared with 2653 patient-years in PT-1) and considerably fewer end-point events were recorded (Table 2 ). It seems highly unlikely, therefore, that the ANAHYDRET study had the necessary statistical power to detect the differences observed in the PT-1 study. Moreover the ethical basis for conducting non-inferiority trials has been called into question, not least because the design of such trials carries an intrinsic risk of failing to detect a clinically relevant difference between the treatment groups.40
Both conventional and pegylated recombinant interferon alpha are effective at controlling the platelet count in ET, although there is no randomized evidence of efficacy in prevention of thrombosis. Since interferon is not thought to be leukemogenic or teratogenic, it is often used for younger patients or during pregnancy. In patients with JAK2 V617F–positive PV interferon use has been reported to reduce the mutant allele burden, with occasional patients achieving a molecular remission.41 Larger studies are being planned to confirm these data and to see whether molecular responses are associated with improved clinical outcomes.
The discovery of JAK2 mutations has led to the hope that JAK2 inhibitors may prove effective, particularly in PMF or accelerated-phase disease. Several agents are currently being evaluated in early-phase studies (see the accompanying article by S Verstovsek, beginning on page 636).
Models to explain the relationship of essential thrombocythemia to polycythemia vera. (A) At the population level, different pathogenetic lesions are likely to be associated with overlapping distributions of hemoglobin level. In keeping with this model, a proportion of patients harboring a JAK2 V617F-homozygous clone may not manifest erythrocytosis; conversely, erythrocytosis may be seen in the absence of a V617F-homozygous clone. (B) When considering an individual with JAK2-mutant disease, the presence or absence of erythrocytosis likely reflects interplay between JAK2 mutation type, gene dosage and additional factors such as patient gender, iron homeostasis and inherited or acquired genetic modifiers. Het indicates heterozygous; hom: homozygous.
Models to explain the relationship of essential thrombocythemia to polycythemia vera. (A) At the population level, different pathogenetic lesions are likely to be associated with overlapping distributions of hemoglobin level. In keeping with this model, a proportion of patients harboring a JAK2 V617F-homozygous clone may not manifest erythrocytosis; conversely, erythrocytosis may be seen in the absence of a V617F-homozygous clone. (B) When considering an individual with JAK2-mutant disease, the presence or absence of erythrocytosis likely reflects interplay between JAK2 mutation type, gene dosage and additional factors such as patient gender, iron homeostasis and inherited or acquired genetic modifiers. Het indicates heterozygous; hom: homozygous.
Models of biclonal disease and the accumulation of genetic damage in the myeloproliferative neoplasms (MPN). (A) Models to explain the presence of biclonal disease in MPN patients, with two clones representing (i) the phylogenetically related progeny of a shared founder clone or (ii) independent clones arising from distinct stem cells. Direct evidence exists for both of these models in patients with chronic phase disease.23,24 (B) Mechanisms of disease progression in the JAK2 V617F–positive myeloproliferative neoplasms. In the presence of normal JAK2 signaling, DNA damage causes a modification (deamidation) in the pro-survival Bcl-XL protein, resulting in release of pro-apoptotic BH3-only proteins and subsequent cell death by apoptosis. Expression of mutant JAK2 not only increases DNA damage but also inhibits the normal Bcl-XL deamidation response; the net result is survival of the MPN clone despite the accumulation of genetic damage. HSC indicates hematopoietic stem cell; dBcl-XL: deamidated Bcl-XL.
Models of biclonal disease and the accumulation of genetic damage in the myeloproliferative neoplasms (MPN). (A) Models to explain the presence of biclonal disease in MPN patients, with two clones representing (i) the phylogenetically related progeny of a shared founder clone or (ii) independent clones arising from distinct stem cells. Direct evidence exists for both of these models in patients with chronic phase disease.23,24 (B) Mechanisms of disease progression in the JAK2 V617F–positive myeloproliferative neoplasms. In the presence of normal JAK2 signaling, DNA damage causes a modification (deamidation) in the pro-survival Bcl-XL protein, resulting in release of pro-apoptotic BH3-only proteins and subsequent cell death by apoptosis. Expression of mutant JAK2 not only increases DNA damage but also inhibits the normal Bcl-XL deamidation response; the net result is survival of the MPN clone despite the accumulation of genetic damage. HSC indicates hematopoietic stem cell; dBcl-XL: deamidated Bcl-XL.
Model to explain the relationship of essential thrombocythemia, polycythemia vera and primary myelofibrosis. Whereas a phenotypic overlap exists between JAK2 V617F–positive polycythemia and thrombocythemia, JAK2 V617F–negative disease is both biologically distinct and heterogeneous, including 10% of patients with MPL mutations and 90% in whom the molecular cause is currently unknown. Accelerated phase disease may follow JAK2 V617F–positive or –negative chronic phase and is clinically variable, including bone marrow fibrosis, increasing or falling white cell count or splenomegaly. A minority of patients undergo disease evolution to acute myeloid leukemia. In this model, patients currently labeled as having primary myelofibrosis may in fact represent those presenting in accelerated phase of a pre-existing myeloproliferative neoplasm (MPN). Hb indicates hemoglobin; Plt, platelet count.
Model to explain the relationship of essential thrombocythemia, polycythemia vera and primary myelofibrosis. Whereas a phenotypic overlap exists between JAK2 V617F–positive polycythemia and thrombocythemia, JAK2 V617F–negative disease is both biologically distinct and heterogeneous, including 10% of patients with MPL mutations and 90% in whom the molecular cause is currently unknown. Accelerated phase disease may follow JAK2 V617F–positive or –negative chronic phase and is clinically variable, including bone marrow fibrosis, increasing or falling white cell count or splenomegaly. A minority of patients undergo disease evolution to acute myeloid leukemia. In this model, patients currently labeled as having primary myelofibrosis may in fact represent those presenting in accelerated phase of a pre-existing myeloproliferative neoplasm (MPN). Hb indicates hemoglobin; Plt, platelet count.
Disclosures Conflict-of-interest disclosure: ARG serves as a consultant for Astex Therapeutics, Shire, Bristol-Myers Squibb, and Incyte; he also receives research funding from Astex Therapeutics. PAB declares no competing financial interests. Off-label drug use: None disclosed.
Acknowledgments
We gratefully acknowledge the contributions of the many researchers whose work we were unable to cite due to space restrictions.
References
Author notes
Department of Haematology and Cambridge Institute for Medical Research, University of Cambridge, Cambridge, United Kingdom
Department of Haematology, Cambridge University and Addenbrooke’s Hospital, Cambridge, United Kingdom