Abstract
What is the most sensible way of organizing the disorderly spectrum of acquired marrow failure states collectively known as the myelodysplastic syndromes (MDS)? While the 2008 version of the World Health Organization classification is the current standard, the recent proliferation of MDS prognostic tools illustrates the usefulness of supplemental information for clinical purposes. Many cases of acquired bone marrow failure do not fit cleanly into established MDS categories, yet an alternative diagnosis is not apparent. The term “idiopathic cytopenias of undetermined significance” (ICUS) has been proposed to describe these cases, but there is a paucity of information about the natural history of ICUS. New data on the natural history of MDS associated with a broad range of cytogenetic abnormalities that were not included in the International Prognostic Scoring System (IPSS), as well as the emerging picture of karyotypically occult DNA changes, promise to inform future classifications.
Classifications and taxonomies are, by nature, artificial: simple mental constructs imposed upon an ambiguous and complex physical reality, and therefore intrinsically imperfect.1,2 Despite their inherent limitations, classifications of human disease are both necessary and useful. Standardized nomenclature facilitates communication among clinicians and researchers, and also fulfills the desire of many patients to put a name on their ailment, so that they can begin to understand that illness and guess how it might evolve.
What makes a disease classification successful? To begin with, high-quality nosologies are comprehensive, leaving few cases unclassifiable, yet are comprised of categories that are mutually exclusive, so that a single case can not fit in more than one place at the same time.1,3 Good diagnostic taxonomies are also modifiable, so that new information is quickly accommodated—yet they resist succumbing to fashionable trends, making them sufficiently stable to avoid changing multiple times during the course of a clinical trial or laboratory project. The best disease classifications are clinically relevant, offering accurate prognostic information and guiding treatment. And as tools of communication, first-rate schemata are straightforward (yet avoid oversimplification); serve to homogenize terminology the way Noah Webster’s 19th-century dictionaries standardized American English orthography; are reproducible with little inter-observer variance; require only investigative tools that are widely available (that is, not esoteric technology or assays requiring highly subjective interpretation) and are evidence-based, and therefore accepted by the majority of users in the field.
With respect to classification of the myelodysplastic syndromes (MDS), some of the criteria for a satisfactory classification have been met, while others have proven elusive. On the positive side, over the last 3 decades, for the most part only one MDS classification system has prevailed at any given time. The 1982 French-American-British Cooperative Group (FAB) classification was dominant until about 2000, and continues to be used for some purposes in the 21st century, such as for clinical trial eligibility and response criteria (for example, the 2006 International Working Group criteria, where evolution to a more advanced FAB subtype is one criterion for disease progression), coding and billing, and drug indication language approved by regulatory agencies. Approximately 10 years ago, the first of the World Health Organization (WHO) classifications of MDS succeeded the FAB system, upon which it was based (Figure 1 ).3–5 As a result of this natural succession, the complexities that historically troubled areas of medicine where multiple classification systems were used simultaneously (such as lymphoproliferative disorders prior to the 1994 REAL synthesis of the International Lymphoma Study Group) have been largely avoided in MDS.
Neither the FAB nor WHO systems are able to classify all putative MDS cases, and pathologists must still regularly resort to being descriptive in reports as they struggle to assign certain difficult cases neatly to a category. Still, these classifications share merits: they use only widely available diagnostic tools, are relatively simple, and have enjoyed wide acceptance. Some investigators did express concerns about the first WHO classification at its debut, but this may have been in part because a draft of the classification appeared well before the reasoning behind it was fully outlined.6–8
The proliferation of proposed prognostic tools for MDS (Table 1 ) following the 1982 publication of the FAB classification highlights one important limitation of both past and present classification systems: wide variation in patient outcome within diagnostic categories. Accurate prognostication in MDS continues to be challenging, and critical problems also remain with respect to defining minimal diagnostic criteria for MDS, as well as distinguishing MDS from other conditions with similar features (for example, aplastic anemia or myeloproliferative neoplasms). Unfortunately, our limited pathobiological understanding of MDS means that most diagnoses are still based almost exclusively on light microscopy—always somewhat hazardous, because morphological changes can be non-specific. Valuable supplemental information may be available from karyotyping, but cytogenetic results are normal, and therefore unhelpful, in approximately one half of de novo disease and in 10% to 20% of cases of treatment-related, secondary MDS.
The 2008 World Health Organization (WHO) Classification of MDS
In 2008, the WHO International Agency for Research on Cancer (IARC) published the 4th edition of Classification of Tumours of Haematopoietic and Lymphoid Tissues (Table 2 ), which included numerous changes from the 3rd edition that had in turn been proposed in 1999 and formally published by the IARC in 2001.3,5,9 (The 1967 1st edition and 1981 2nd edition of the WHO cancer classification, then titled International Histological Classification of Tumours, did not attempt to classify MDS, as these syndromes were poorly characterized prior to the 1980s and not recognized as neoplastic.) The rationale for the specific changes to the WHO myeloid disease classification between the 3rd and 4th editions were recently published by Vardiman and colleagues in Blood.10
Refractory Cytopenia with Unilineage Dysplasia
The first notable new feature of the 2008 MDS classification is the term “refractory cytopenia with unilineage dysplasia (RCUD).” Most patients with MDS who have unilineage-dominant dysplasia are anemic with erythroid-restricted morphologic abnormalities, and are therefore classifiable as refractory anemia (RA) or as RA with ring sideroblasts (RARS). However, a small number of patients present with neutropenia and dysplasia limited to neutrophils and their precursors, or with thrombocytopenia and isolated megakaryocyte/platelet dysplasia.11,12 The new WHO classification groups RA (but not RARS) together with two new entities created to describe these latter patients, who would have been unclassifiable in the FAB and previous WHO schemata: refractory neutropenia (RN) and refractory thrombocytopenia (RT). Special caution is indicated in making these latter diagnoses, however, since isolated neutropenia or thrombocytopenia due to medications, immune dysregulation, or other causes appear to be much more common than clonal unilineage MDS restricted to either of those cell lines.
Cytogenetic Abnormalities with Diagnostically Insufficient Morphology
The second key change between the 3rd and 4th editions of the WHO classification is that the WHO structure now allows for patients with refractory cytopenias and certain MDS-associated clonal cytogenetic abnormalities (Table 3 ) to be classified presumptively as MDS, even in the absence of diagnostic morphologic features.13 This important change foreshadows a day when molecular profiling will be an essential complement to traditional morphology for diagnosing MDS, as is already the case for some other conditions (for example, chronic myeloid leukemia, diagnosis of which requires demonstration of BCR-ABL fusion by some laboratory method).
Some might question the particular karyotype choices in Table 3 . While the most common MDS-associated abnormalities are listed, and three common but less disease-specific abnormalities are purposefully excluded [del(20q), trisomy 8, and loss of the Y chromosome], several rare translocations were also chosen, such as t(2;11)(p21;q23), even as other uncommon, but recurrent, MDS-associated karyotypes were not included (for example, trisomy 19 or translocations involving NUP98 at 11p15). We can expect the list of disease-defining cytogenetic and molecular abnormalities to be plastic and to expand in the future.
In contrast to the increased importance attached to an abnormal karyotype, the WHO revision committee took a wait-and-see approach with respect to flow cytometry in MDS diagnosis. Although numerous immunophenotypic abnormalities are associated with MDS and have prognostic value, their diagnostic specificity is still being established.14 Recent work of the European Leukemia Network to standardize flow cytometry scores may help establish the role of flow cytometry in evaluation of suspected MDS cases more firmly.
Sometimes Ring Sideroblasts Don’t Matter
While the 2001 WHO classification distinguished cases with refractory cytopenias and multilineage dysplasia (RCMD) without ring sideroblasts from RCMD cases with at least 15% ring sideroblasts (RCMD-RS), these two entities are merged in the 2008 WHO classification. Just as ring sideroblasts do not portend an indolent disease course if excess myeloblasts are also present, the negative prognostic impact of multilineage dysplasia trumps any ameliorating influence from ring sideroblasts.15 RCMD had been separated from RCMD-RS in the 3rd edition of the WHO classification without much of a supporting evidence base; this is a reminder that although sometimes provisional classification choices must be made to prompt further research, even widely used classification systems should be viewed with a critical eye.
With Respect to MDS, Children Are Little Adults—But Only Sometimes
Childhood MDS are now formally recognized in the WHO classification, including a provisional entity, refractory cytopenia of childhood (RCC), which is characterized by cytopenias and by fewer than 5% blasts in a (usually) hypocellular marrow.16 Childhood MDS are distinct from adult MDS in several important ways. Hypocellularity and pancytopenia are much more common in childhood than in adult MDS, and RARS or MDS with isolated del(5q) are rare in the pediatric age group. Down syndrome–associated MDS are categorized separately by the WHO as “myeloid proliferations related to Down syndrome.” MDS in children are rare—fewer than 5% of pediatric hematological malignancies—and often associated with congenital disorders of DNA damage surveillance and repair pathways. As a result, many cases are idiosyncratic, and therefore difficult to classify.16 Likewise, RCC itself can be quite difficult to distinguish from inherited marrow failure states such as Fanconi anemia. The WHO Clinical Advisory Committee believed that children with 2% to 19% blasts in the peripheral blood or 5% to 19% marrow blasts could be classified using the same MDS categories defined for adults.10
Therapy-Related Myeloid Neoplasms: Does Etiology Matter?
A fifth revision between the 2001 and 2008 WHO classifications of MDS is that therapy-related myeloid neoplasms (t-MDS/t-AML) due to exposure to alkylating agents or radiation are no longer considered separately from t-MDS/t-AML due to topoisomerase II inhibitor exposure. Many patients developing t-MDS/t-AML have received combination chemotherapy or chemoradiotherapy with both classes of drugs; the specific agent predisposing to MDS in such cases may be difficult to ascertain with certainty and is usually irrelevant with respect to the clinical course.
Notably, some patients without a known exposure to an MDS-inciting drug or ionizing radiation have molecular changes similar to those with t-MDS/t-AML, suggesting an unrecognized environmental exposure in a subset of cases.17 In the 2001 WHO classification, t-MDS was combined with t-AML because the prognosis is dismal for both groups of patients, and the blast proportion and other morphological features have only modest predictive value with respect to outcome.18
Some Changes That Weren’t Made: Hypocellular MDS and MDS with Fibrosis
The WHO classification committee did not define separate categories for MDS with marrow fibrosis or marrow hypocellularity, because of lack of consensus on the precise definition or the importance of these findings (recent data from Della Porta et al19 indicate that 2+ or greater reticulin fibrosis, as defined by the European consensus system, is an independent negative prognostic marker in MDS). However, the diagnostic challenges that such cases present are discussed frankly in the WHO manual, with a recommendation that hematopathologists specifically comment upon hypocellularity or extensive fibrosis in interpretative reports. This issue raises the knotty question of when a particular disease feature is distinctive enough that a separate diagnostic category is indicated. In general, until there is evidence that a feature is associated with distinct clinical behavior or requires a specific therapy, it seems best to err on the side of “lumping” rather than “splitting.” Such considerations apply not only to morphological and immunological features, but also to molecular findings.
Lord of the Rings, Not Lord of the Ringeds
Perhaps the least weighty change between the 2001 and 2008 WHO classifications is that it is no longer proper to speak of “ringed” sideroblasts. Instead, the term “ring” sideroblasts is preferred for historical reasons (although neither term was used in Sven-Erik Björkman’s seminal 1956 report20 of 4 cases of idiopathic sideroblastic anemia). A consensus definition of a pathological ring sideroblast—an erythroid precursor with 5 or more iron granules encircling at least one third of the nucleus—was recently ratified by an International Working Group on morphology of MDS.21
Classification Versus Prognostication
Although both the FAB and WHO classification systems for MDS offer general prognostic guidance, the wide variety of outcomes within FAB or WHO categories, as well as reliance on morphology to classify cases when other information of prognostic value is available (for example, cytogenetics), resulted in numerous attempts to derive more accurate MDS prognostic tools, beginning with the Bournemouth score22 in 1985 (Table 4 ). These efforts culminated in the landmark 1997 International Prognostic Scoring System (IPSS), which continues to be the most widely used prognostic tool in MDS for stratification of patients enrolling in clinical trials.23
Despite its ubiquity and utility, the IPSS has several important limitations. The IPSS was only validated in previously untreated patients with de novo MDS, because prior to 1997 most patients with MDS were treated only with supportive care. In addition, the 2001 version of the WHO classification decreased the acute leukemia-defining marrow blast threshold from 30% to 20%, thereby making the IPSS appear dated, since the IPSS blast scoring system used the older threshold. Another substantive criticism leveled at the IPSS is that while the scoring system accounts for the prognostic value of numbers of cytopenias, it does not consider cytopenia severity (for example, the IPSS scores a patient with a platelet count of 90 × 109/L the same as one who is platelet transfusion-dependent, even though the natural history for such patients is distinct.) Furthermore, the IPSS includes only a limited number of cytogenetic abnormalities, and it may overemphasize the significance of marrow blast proportion while underemphasizing high-risk karyotypic findings.
As a result of these concerns, over the last few years investigators have again begun to propose a variety of new prognostic systems. Recent proposals include the WHO-based Prognostic Scoring System (WPSS) derived by Malcovati and Cazzola in Pavia and their colleagues,24 two prognostic tools for MDS developed by the Department of Leukemia at the MD Anderson Cancer Center,25,26 and a modified version of the WPSS that accounts for marrow fibrosis.19 The introduction of several new prognostic systems in rapid succession since 2007 is reminiscent of the frenzied period just prior to the development of the IPSS. It remains to be seen whether one of these newer systems will eventually supersede the 1997 IPSS, or if instead the revised version of the IPSS that is currently in development will come to fruition and be widely adopted.
The Influence of Cytogenetics and Molecular Genetics in Classification
Unlike the 1976 and revised 1985 FAB leukemia classifications, which were based solely on morphologic and immunologic findings, the 2001 WHO classification of acute leukemia formally incorporated recurrent genetic abnormalities into the diagnosis of AML, recognizing the dramatic influence of cytogenetic and molecular genetic findings on prognosis and, in some cases, treatment of these diseases. In contrast, the only specific chromosomal or genetic abnormality that was part of the 3rd edition WHO MDS classification was del(5q). Although cytogenetic aberrations are just as important in MDS as they are AML, a key difference between AML and MDS is that the former is frequently characterized by balanced translocations where rearrangements of specific genes can be confirmed by fluorescence in situ hybridization (FISH) and other assays. In MDS, in contrast, large deletions with varied breakpoints and numerical chromosomal abnormalities predominate, and in most instances it is not yet clear which specific genes are critical and responsible for the phenotype.
A More Detailed Picture of the Prognostic Value of Cytogenetics in MDS Is Emerging
Hundreds of recurrent cytogenetic abnormalities have been described in MDS and are reviewed elsewhere.27,28 While the 1997 IPSS included just five of the common MDS-associated cytogenetic findings—del(20q), del(5q), abnormalities of chromosome 7, loss of the Y chromosome, and a complex karyotype (≥ 3 abnormalities)—in 2007 a German-Austrian consortium published data from 2072 MDS patients with 684 different karyotypes, allowing better characterization of outcomes associated with 13 rarer abnormalities.29 The German-Austrian results were subsequently validated in more than 1700 patients treated at the MD Anderson Cancer Center30 and have most recently been combined with a Spanish cohort and the IPSS-IMRAW cohort to create a four-tier cytogenetic prognostic grouping for MDS (Table 4 ).31
Beyond Cytogenetics: DNA Array Approaches
For patients with a normal karyotype, emerging data from array-based technologies for assessing DNA copy number variation and loss of heterozygosity/uniparental disomy (UPD) in MDS reveal provocative complexity and new recurrently abnormal chromosomal regions. Heterogeneous, karyotypically occult chromosomal changes in MDS are present in at least half of patients who have normal cytogenetic results. A few of these changes are recurrent; for instance, in one series, 25% of patients with RARS, 17% of those with RAEB, and 12% with RCMD had UPD on chromosome 4q, the location of the TET2 gene.32 Such changes may also have prognostic value.32–35 Furthermore, it seems likely that MDS cells will soon be subject to high-throughput “next-generation” DNA sequencing, following the example of the high-profile 2008 report of a normal-karyotype AML M1 patient whose blast cells and germline control cells were sequenced to greater than 90% diploid coverage, thereby identifying 63,277 tumor cell-restricted single nucleotide polymorphisms and 10 non-synonymous coding mutations, 8 of them new.36
Point Mutations and Single-Gene Abnormalities in MDS
For now, however, aberrations at the molecular level are still poorly characterized in MDS. The most common point mutations in MDS (such as, RUNX1, TP53, NRAS) are still present in only a minority of patients and are found primarily in patients with t-MDS where the prognosis is already known to be poor.37 Other somatic mutations modify the MDS phenotype without clearly changing the natural history, such as ATRX mutations associated with acquired thalassemia38 or those yet-to-be-characterized abnormalities that underlie acquired erythrocyte enzymopathies or platelet functional defects. Such variants underscore the necessity for newly discovered mutations in MDS to be fully functionally evaluated before pathobiological significance can be attributed to them, since there may be many “bystander” mutations that accumulate during clonal evolution, as recently described for childhood leukemia.39 Recent descriptions of rearrangements or mutations in CBL, IER3, TET2, ASXL1 and other factors newly linked to myeloid disease point out how much is yet to be learned about MDS. It is not clear if common themes will emerge, such as the activating mutations of JAK2 that are so common in myeloproliferative neoplasms, or if MDS will ultimately prove to be as heterogeneous molecularly as these syndromes are morphologically.
Myeloagnosticism: Idiopathic Cytopenia(s) of Undetermined Significance (ICUS)
Commonly, patients present to clinicians with peripheral blood cytopenias and a diagnosis of MDS is suspected, but formal diagnostic criteria for MDS, such as those specified by the WHO, are not met and an alternative diagnosis is not apparent. In the past, this situation has informally been termed “not quite MDS” or “not yet MDS” – the latter expression implying that MDS is likely to evolve eventually, in the same way “preleukemia” carried (inaccurate) connotations of inevitable disease progression to AML. An international group of investigators recently proposed formal criteria for a label expressing diagnostic uncertainty that can be applied to such patients: “Idiopathic Cytopenia(s) of Undetermined Significance” (ICUS) (Table 5 ).40 A key distinction of ICUS from other potential precursor conditions familiar to hematologists—monoclonal gammopathy of undetermined significance (MGUS),41 monoclonal B-cell lymphocytosis (MBL),42 and T cell clonality of undetermined significance (TCCUS)43—is that an ICUS designation does not necessarily imply a clonal disorder. In fact, evidence of restricted clonality of hematopoiesis favors a diagnosis of MDS.
Few data are available about the frequency or natural history of ICUS, but it is clear that there is some risk of evolution to overt MDS.44 A recent Mayo Clinic series45 reviewed 2899 patients who underwent marrow examination for idiopathic cytopenias over a 12-year period: 1716 were diagnosed with MDS, 535 were found to have non-myeloid malignancies or were excluded from further analysis because they were younger than 18 years old, and 69 had normal marrow morphology but an MDS-associated cytogenetic lesion, and therefore could presumptively be diagnosed as MDS using the 2008 WHO classification criteria. This left 579 patients: 397 patients who had cytopenias with minimal dysplastic changes that did not quite meet WHO criteria for MDS, and 182 patients with cytopenias but entirely normal marrow morphology. Presumably, then, for every 3 patients diagnosed with MDS, 1 patient assessed could be called ICUS, because no specific diagnosis can be made at the time of initial clinical and morphological evaluation. The 182 patients with entirely normal morphology were evaluated further: 80 were ultimately suspected to have other causes for cytopenias (such as hypersplenism or autoimmune disease) or the peripheral blood counts normalized spontaneously, and 92 patients had less than 6 months follow-up, leaving just 10 patients with long-term data, 6 of whom ultimately developed overt MDS.
Prospects for the Future
MDS diagnosis and classification is currently in a transitional phase from reliance almost entirely on cell morphology supplemented by cytochemistry and G-banded karyotyping, towards a new era in which molecular and perhaps immunophenotypic findings will be fully incorporated. The revised 2008 WHO MDS classification represents a small but valuable step forward, and new prognostic tools, including the more detailed karyotyping stratification in Table 5 , are also useful for clinicians. Although the trend towards greater classification complexity seems likely to increase as additional molecular lesions in MDS are characterized, it is also still possible that additional unifying themes will emerge from the apparent chaos.
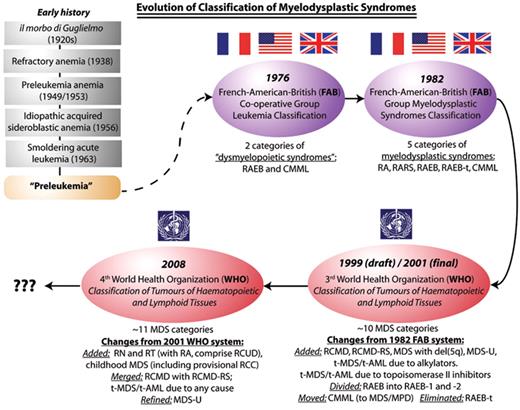
Evolution of classification of the myelodysplastic syndromes, from the era when these syndromes were poorly characterized and collectively known as “preleukemia” (the prevailing term in the 1960s and early 1970s for what is now known as MDS), through the 1976/1982 FAB classifications and, in the last decade, the two WHO systems. FAB indicates French-American-British Co-operative Group; WHO, World Health Organization; MDS, myelodysplastic syndromes; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; CMML, chronic myelomonocytic leukemia; RAEB, refractory anemia with excess blasts; RAEB-t, refractory anemia with excess blasts in transformation; RN, refractory neutropenia; RT, refractory thrombocytopenia; RCMD, refractory cytopenias with multilineage dysplasia; RCMD-RS, refractory cytopenias with multilineage dysplasia and ring sideroblasts; MDS-U, MDS unclassifiable; RCC, refractory cytopenias of childhood; RCUD, refractory cytopenias with unilineage dysplasia; MDS/MPD, myelodysplastic/myeloproliferative diseases.
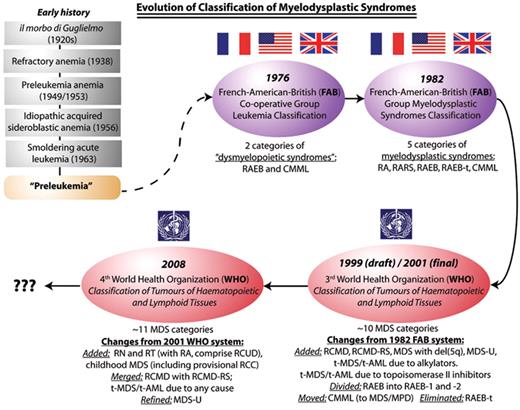
Evolution of classification of the myelodysplastic syndromes, from the era when these syndromes were poorly characterized and collectively known as “preleukemia” (the prevailing term in the 1960s and early 1970s for what is now known as MDS), through the 1976/1982 FAB classifications and, in the last decade, the two WHO systems. FAB indicates French-American-British Co-operative Group; WHO, World Health Organization; MDS, myelodysplastic syndromes; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; CMML, chronic myelomonocytic leukemia; RAEB, refractory anemia with excess blasts; RAEB-t, refractory anemia with excess blasts in transformation; RN, refractory neutropenia; RT, refractory thrombocytopenia; RCMD, refractory cytopenias with multilineage dysplasia; RCMD-RS, refractory cytopenias with multilineage dysplasia and ring sideroblasts; MDS-U, MDS unclassifiable; RCC, refractory cytopenias of childhood; RCUD, refractory cytopenias with unilineage dysplasia; MDS/MPD, myelodysplastic/myeloproliferative diseases.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Dana-Farber Cancer Institute; Harvard Medical School, Boston, MA