Abstract
Advances in nanotechnology research have led to the creation of new generation of contrast agents, therapeutics, and delivery systems. These applications are expected to significantly improve the diagnosis and treatment of a variety of diseases. Two nanotechnologies—semiconductor and metallic nanostructures—are the most advanced in this young field and have been extensively investigated for clinical use. These nanostructures are currently the “model” for the developments of many novel nanostructures. This review describes their chemical design, tunable properties, and utility in medicine. Furthermore, we will describe the current understanding of their toxicity, which could be barriers to their use for human.
Advancements in the synthesis, characterization, and surface modifications of nanoscale structures have provided a foundation for their utility in medical applications.1–4 The unique aspect of these nanostructures is their tunable optical, electronic, magnetic, and biological properties. Due to these properties, the incorporation of nanostructures in detection schemes or the use the nanostructures as contrast agents could improve the specificity and accuracy of current diagnostics.5–9 Furthermore, nanostructures can improve the effectiveness of drugs in the treatment of a variety of diseases when they are used as delivery vehicles because of the increase in therapeutic payload.10–12 In the last fifteen years, researchers have characterized the tunable properties by altering the nanostructure size, shape, and chemical composition and have developed reproducible strategies to make nanostructures of desired properties.13–15 Further, researchers have also developed strategies to render nanostructures biocompatible and capable of being coated with biological molecules.16–19 For example, semiconductor nanocrystals synthesized via organometallic methods contain the hydrophobic ligand tri-n-octylphosphine oxide (TOPO). These nanocrystals can subsequently be made water-soluble by covering the TOPO with the amphiphillic polymer polyacrylic acid grafted with octylamine.20,21 The hydrophylic carboxylic acid groups of the polymer coat render the nanocrystal stable in an aqueous environment. Furthermore, these groups can be used for conjugation to polymers and/or biological molecules such as antibodies and oligonucleotides via the formation of peptide bonds.22,23 See Figure 1 for some surface designs of nanostructures for biomedical applications. Although a select few have advanced to clinical use, many are being examined as in vitro and in vivo diagnostic contrast agents, drug delivery vehicles, and therapeutics. Here, we review two of the oldest and most established nanostructures for biomedical applications: semiconductor quantum dots and gold nanoparticles. We also provide a brief description of nanotoxicity, which could be a potential limitation for their use in biological systems.
Semiconductor Nanocrystals
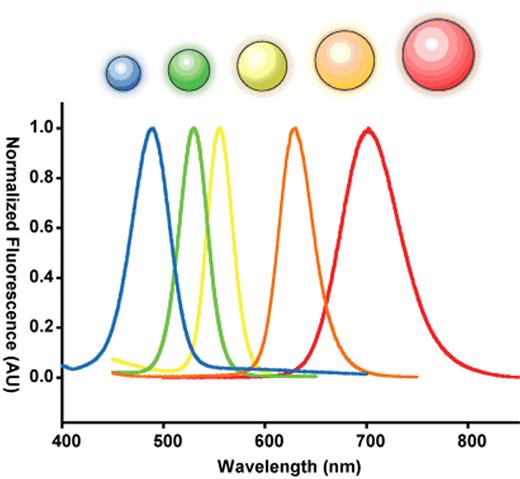
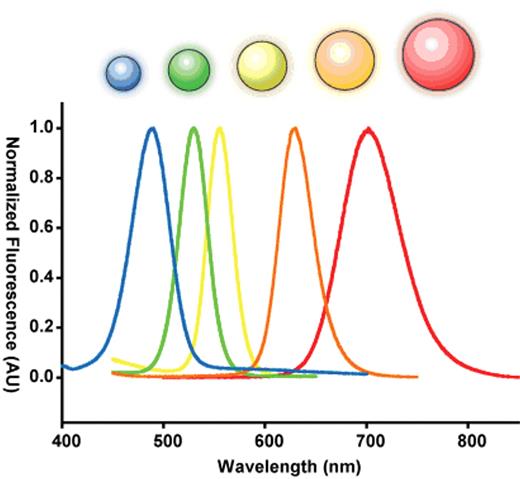
Semiconductor nanocrystals are commonly used as probes for labeling cells and tissues owing to their intense, tunable fluorescence.24–28 The origin of this unique physical property is quite interesting. A semiconductor crystal’s band-gap energy becomes strongly size-dependent as its physical dimensions approach the exciton Bohr radius (the natural radius of an electron-hole pair in bulk). This dependence is a result of a phenomenon known as “quantum confinement,” in which the allowed electronic states of the exciton are broadened when its position is restricted by the dimensions of its parent crystal, in a manner analogous to the pedagogical “particle in a box.”29–31 In this size range, semiconductor nanocrystals are considered electronically zero-dimensional and are referred to as quantum dots. The dimension at which quantum confinement becomes significant depends on the exciton Bohr radius in bulk and thus the particular material in question. For the well-studied II–VI direct band-gap semiconducting material cadmium-selenide (CdSe), strong confinement occurs in the range of 1–10 nm.32–34 This results in a shift in fluorescent band-edge emission wavelength from approximately 700 nm down to 450 nm as the crystal size is reduced (Figure 2 ).32,35
The bright, tunable fluorescence emission of quantum dots has encouraged their use as labels in biomedical applications demanding high sensitivity, spectral resolution, and prolonged imaging. Examples include quantitative in vitro immunoassays5,36–39 and the labeling of fixed cell samples40,41 and tissue specimens.42 Labeling of the membrane receptors of live cells at the single-molecule level has also been achieved.43 This is significant as it allows tracking of membrane receptor diffusion and internalization. In one example, QDs conjugated with epidermal growth factor (EGF) were used to label erB1 receptors to monitor endocytosis and transport along filopodia.44 The erb/HER family of membrane tyrosine kinases are overexpressed in many types of invasive cancer and thought to play a pivotal role in proliferation and differentiation. Other plasma membrane structures have been labeled, including glycine receptors45 and membrane lipids.46 Cytoplasmic structures may also be labeled with QDs for tracking movement, proliferation, and survival in transplanted tissue47 or during embryonic development.48 QDs have also found use as contrast agents in vascular or lymphatic circulation to identify angiogenesis and lymph nodes with a metastatic invasion.49 Finally, QDs have been found to extravasate preferentially in certain tumor types over healthy tissue, with potential significance as an early diagnosis tool and as a guide during surgery.50
Metallic Nanoparticles
Quasi-spherical nanoscale metallic particles also exhibit interesting optical properties. For instance, light of a wavelength much larger than the diameter of the particle can produce coherent oscillations in the metal’s electron cloud relative to its stationary core. This occurs via the coupling of light in a resonant fashion to free electrons within the metal.51–53 These oscillations are called “surface plasmons,” and lend their name to the effect, which is known as “localized surface plasmon resonance” (LSPR). LSPR results in absorption and scattering of incident radiation at the resonant wavelength, with the strength of the interaction depending on the material. For noble metals, the plasmon resonance condition is satisfied in the visible to near-IR portion of the spectrum, and the coupling is extremely efficient, resulting in a strong extinction. The LSPR wavelength depends as well on the dielectric constant of the surrounding environment and on the presence of inter-particle interactions.54,55 For well-separated gold particles of around 20 nm in an aqueous environment, the resonant condition is satisfied at approximately 520 nm, giving a solution of these particles a distinctive red color (see Figure 3 for details).
Gold nanoparticles are the most commonly studied metallic nanoparticles because of both their ease of synthesis and efficient resonance coupling. The strong dependence of their surface plasmon resonance position and efficiency on the dielectric properties of the surrounding environment offers the potential to create a sensor for adsorption events at the surface. This is a colloidal analogue to the commercialized surface plasmon resonance (SPR) sensor and can be used to monitor antigen-antibody binding kinetics and stoichiometry.54,56–58
The peak SPR wavelength of gold nanoparticles shifts when they are aggregated due to plasmonic coupling. Single monodisperse gold nanoparticle solutions appear as a red solution but change to blue when they are aggregated. Mirkin and co-workers used this principle to create simple diagnostic systems for detecting proteins and genes.59–62 In their system, an oligonucleotide strand is coated onto the surface of the gold nanoparticle via thiol chemistry. When the gold nanoparticle solution is exposed to a target gene, it hybridizes with the oligonucleotides on the surface of the gold nanoparticles and aggregates them, resulting in a change of solution color from red to blue. The aggregated nanoparticles can be subsequently de-aggregated upon heating. This simple color test for detecting genes (or proteins) can be made in a high-throughput format for the rapid analysis of complex biological samples.63 This principle has also been applied during cell labeling where a 9 nm red-shift in the peak was observed with microabsorption spectroscopy upon gold nanoparticle binding to the EGF receptor in cultured human oral squamous carcinoma cell lines.64 Because of the intense scattering at the LSPR wavelength, gold nanoparticles may be used as labels under confocal reflectance microscopy. This technique was employed, under laser illumination, to image epithelial growth factor receptors (EGFR) in a cervical cancer cell line.65
One of the most promising areas of application for gold nanoparticles is as an agent for photothermal therapy, or the thermal ablation of tumorigenic cells. Conventional strategies use organic molecules for localized heating, but the absorption cross-section of gold nanoparticles is five orders of magnitude greater than that of the popular agent indocyanine green, making them far more efficient thermal centers. Recently, Hauck et al studied the effect of thermal ablation in several carcinomas using gold nanorods. In addition to observing cell death under laser light illumination, they also showed a strongly synergistic effect when combined with chemotherapeutic agents.66
Nanotoxicity
Before engineered nanomaterials can be applied in a clinical setting, their potential adverse impact on human health must be evaluated. Much of the initial nanomaterial research overlooked the health risks, in favor of exploring the unique physicochemical properties that enable their use as diagnostic and therapeutic agents. The major toxicological concerns of nanomaterials are (1) the materials is composed of heavy metals with known toxicity and (2) nanomaterials may elicit unique and unpredictable biological responses because of their tunable properties. Further, because of their size, they are accessible to vital cells and organs.49 Within a physiological compartment, many nanomaterials interact with the resident cell populations. This results in either adherence to the surface or internalization by a diverse set of pathways including translocation through the plasma membrane, receptor-mediated endocytosis, and pinocytosis.67–72 Once internalized, particles can remain in endosomes and accumulate within the cell, escape to the cytosol to interact with biomolecules and subcellular organelles, or be recycled to the cell surface.67,68 The eventual localization of the nanomaterial, along with its subsequent cellular interactions, dictates its toxicological effect. For example, a number of studies have shown that nanomaterials are cytotoxic in cultured cell models. For example, CdSe quantum dots were found to be toxic to primary rat hepatocytes73; carbon nanotubes, nanofibers, and nanoparticles were toxic to lung tumor cells74; and iron-containing nanoparticles were toxic to nerve cells.75 However, at the same time, other studies have concluded that certain formulations are non-toxic to cells in culture.48,69
Recently, researchers have focused on evaluating nanomaterials in animal models, which are more physiologically relevant. In one study, mice injected with long multi-walled carbon nanotubes showed asbestos-like pathogenic effects.76 However, acute and chronic injections of single-walled carbon nanotubes into mice yielded no apparent indication of toxicity, despite localizing in the intestine.77,78 It is worth noting that particles less than 5 nm in size have been observed to be eliminated through renal-filtration, raising the possibility of total-body clearance of nanomaterials and eliminating a great deal of the concern for chronic exposure.79 Similar to the cell data, the experiments from the animals are not conclusive.
As illustrated above, many of the results of these studies are contradictory, with some confirming the toxicity of engineered nanomaterials, while others find no indication of toxicity. This lack of uniformity can be largely attributed to the enormous variety of nanomaterials under study. For example, it is entirely feasible that certain particle formulations are toxic to a particular cell type, while being benign to another. At the same time, a different nanomaterial of a different composition, size, or shape may be toxic to a different set of cells or under a different set of exposure conditions.11 Alternatively, a number of studies have also determined that the reagents used in synthesizing nanoparticles may be the culprits in inducing toxicity, and as a result the nanoparticles could be toxic, not because of the particle itself, but because of the synthetic reagents or chemical by-products.80 This highlights the need for more systematic evaluation of nanoparticle formulations under study as the presence of impurities, adsorbates, and reaction byproducts as well as the stability of a particle system are all important factors. To complicate matters further, there is a lack of standardization in model systems and test assays. An assay may be performed to measure apoptosis, but fail to recognize an arrest in cell proliferation, or a high level of DNA damage or mutation suggesting genotoxicity, leading a researcher to erroneously conclude that the particle is non-toxic.
Conclusion
Nanotechnology is a young research field and enabling technology that still requires development. Despite its youth, nanotechnology research has already demonstrated some interesting applications, and in the last 5 years nanomaterials have begun entering clinical trials (eg, gold nanoshells, gold nanoparticles). The major limitation of this field is the unknown toxicology of nanomaterials, but this is a growing sub-discipline of nanotechnology and many questions will be answered in the next few years.
An example of the different surface chemistry design of nanoparticles for biomedical applications. The base particles are usually designed with organic functional groups (eg, amines and carboxylic acids) that allow the particles to be conjugated to targeting agents (eg, antibodies, aptamers), polymers (eg, polyethylene glycol [PEG]), cell-penetrating peptides (eg, TAT peptides), or imaging agents (eg, fluorophores, radiolabels). In other nanoparticle designs, the nanoparticles has a porous interior that can store therapeutic agents.
An example of the different surface chemistry design of nanoparticles for biomedical applications. The base particles are usually designed with organic functional groups (eg, amines and carboxylic acids) that allow the particles to be conjugated to targeting agents (eg, antibodies, aptamers), polymers (eg, polyethylene glycol [PEG]), cell-penetrating peptides (eg, TAT peptides), or imaging agents (eg, fluorophores, radiolabels). In other nanoparticle designs, the nanoparticles has a porous interior that can store therapeutic agents.
Size dependent optical effects of semiconductor nanoparticles. Semiconductor nanoparticles contain size dependent electronic and optical properties. A series of five different sized ZnS-capped CdSe semiconductor nanoparticles called quantum dots is used to demonstrate this principle. When these quantum dots are 2 nm, they emit in the blue but at 6 nm, they emit in the red. The size is inversely related the bandgap energy of the nanoparticle. This bandgap energy dictates the fluorescence emission.
Size dependent optical effects of semiconductor nanoparticles. Semiconductor nanoparticles contain size dependent electronic and optical properties. A series of five different sized ZnS-capped CdSe semiconductor nanoparticles called quantum dots is used to demonstrate this principle. When these quantum dots are 2 nm, they emit in the blue but at 6 nm, they emit in the red. The size is inversely related the bandgap energy of the nanoparticle. This bandgap energy dictates the fluorescence emission.
Size-dependent plasmonic properties of metal nanoparticles. Extinction spectra and representative TEM images (inset) for a) 15, b) 30, c) 60, and d) 100 nm colloidal gold in solution. The plasmon resonance peak red-shifts and broadens at larger diameters, while the particle morphology remains quasi-spherical. Of note, the AU refers to arbitrary units.
Size-dependent plasmonic properties of metal nanoparticles. Extinction spectra and representative TEM images (inset) for a) 15, b) 30, c) 60, and d) 100 nm colloidal gold in solution. The plasmon resonance peak red-shifts and broadens at larger diameters, while the particle morphology remains quasi-spherical. Of note, the AU refers to arbitrary units.
Disclosures Conflict-of-interest: The authors declare no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Institute of Biomaterials & Biomedical Engineering, Donnelly Centre for Cellular and Biomolecular Research, Department Materials Science and Engineering, Department of Chemical Engineering, University of Toronto, Toronto, Ontario, Canada

![Figure 1. An example of the different surface chemistry design of nanoparticles for biomedical applications. The base particles are usually designed with organic functional groups (eg, amines and carboxylic acids) that allow the particles to be conjugated to targeting agents (eg, antibodies, aptamers), polymers (eg, polyethylene glycol [PEG]), cell-penetrating peptides (eg, TAT peptides), or imaging agents (eg, fluorophores, radiolabels). In other nanoparticle designs, the nanoparticles has a porous interior that can store therapeutic agents.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2009/1/10.1182_asheducation-2009.1.701/2/m_chan_fig1.jpeg?Expires=1768070158&Signature=Ca2-77Aeyh-wXW~1gydNuhAdPLYYJBFlD9SU~mntl~mp-uXd4ePYwhROhgSL13waAKJF3zptRNQVtDBSG218TYoQ0alDm-sT00aAMTsCpqEh~lyRN7lk7BTfrCBkTHs1cITIJ~5iJik-FuBBZvsWUmgzUQV6W5onNu-DiOAnliOPdzui5TsyIVyxdYfb1L3~n~S~iyhuwar0lB6i6epeaqDdpOdg0zcNjtytWUmtp7WjGLzrpgLzUrwhBngq2nnvcEhlYDbSpi4xGW3c1xQMFCjcQr1pM733aa9dY~WfC6tPiucR811OTCzzC1WyGe~u6D1kgF7YeXHRqBwNctECIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. An example of the different surface chemistry design of nanoparticles for biomedical applications. The base particles are usually designed with organic functional groups (eg, amines and carboxylic acids) that allow the particles to be conjugated to targeting agents (eg, antibodies, aptamers), polymers (eg, polyethylene glycol [PEG]), cell-penetrating peptides (eg, TAT peptides), or imaging agents (eg, fluorophores, radiolabels). In other nanoparticle designs, the nanoparticles has a porous interior that can store therapeutic agents.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2009/1/10.1182_asheducation-2009.1.701/2/m_chan_fig1.jpeg?Expires=1768070159&Signature=FWuYSySle2ZZ4eHbZOD34U2tlMw52EquNZFc~q-Iv93ods1EKq5b4WotfGfkkAYJyNx1LE--BIPhCDSr5fUwHifdKXpos7Vq-T2jfOBhQdpgXp26TGN9sSwxDYj7rWWSgBuL8BbivzFJD5mGyr6I-hsujNcFxZwPnx08V1M6EnG0ADczWBi0JaunkBMeOvbc1ApFcpURyn3aFk7sLbLkEW7MI4gFBpJbuZH4TSibb1yIcDFxyEkjCttyalHkciX67Y09RePPBGY8mb3hR7Z3kJBq~-f2vepVQ5VzjoYSE2CgLAUaNJObxjs1krw7Mjyl07PXoCaWGPSr9DiFjGp~xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)