Abstract
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of maternal mortality during pregnancy. DVT and PE are commonly suspected due to many mimicking signs and symptoms that are normal in pregnancy. However, validated diagnostic approaches are lacking, and a fear of teratogenic/oncogenic exposure from imaging procedures affects the acceptability of diagnostic approaches used for VTE during pregnancy. DVT and PE treatment in pregnancy is also challenging due to this lack of validated diagnostic approaches, changes in maternal physiology, and the need for intact hemostasis at the time of delivery/epidural analgesia. Prevention requires an optimal balancing of absolute increased bleeding risk from pharmacologic thromboprophylaxis and the absolute benefit of reduced DVT and PE, which, while serious, are relatively uncommon.
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), remains a leading cause of maternal death in the developed world, causing 1.2 to 4.7 deaths per 100,000 pregnancies.1–3 Symptomatic VTE is estimated to occur antepartum (from conception to delivery, or ∼40 weeks) in 5 to 12 per 10,000 pregnancies, and postpartum (6 weeks) in 3 to 7 per 10,000 deliveries.4 Compared with age-matched, nonpregnant controls, this translates into a per-day risk that is increased 7- to 10-fold for antepartum VTE and 15- to 35-fold for postpartum VTE.5,6 The heightened clinical risk of VTE rapidly diminishes after delivery,5 returning to the antepartum level of risk by 3 weeks postpartum, and then returning to the nonpregnant level after 6 weeks.7,8
Management of VTE in Pregnancy
Prevention, diagnostic, and therapeutic management of PE in pregnancy are all complicated by the lack of validated approaches in this unique population, which make firm, evidence-based recommendations difficult. Therefore, practice guidelines are developed by extending the evidence from nonpregnant patients, considering the limited data that are available in pregnant patients, and interpreting this evidence with the unique circumstances that surround pregnancy. These include concerns about teratogenicity and oncogenicity to the unborn child from radiation exposure, the need for reversal of anticoagulation at the time of delivery, and the need for dosing alterations given the increased volume of distribution and renal clearance of heparins in pregnancy. These factors lead to the need for individualized treatment recommendations that should be made in conjunction with a physician specializing in the care of VTE. This review provides a conservative but pragmatic approach to the prevention, diagnosis, and treatment of VTE in pregnancy.
Prevention: Pathophysiology/Etiology/Risk Factors
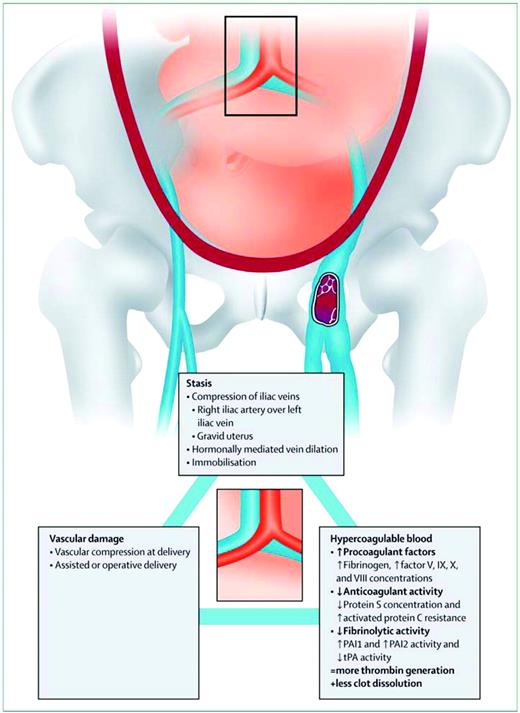
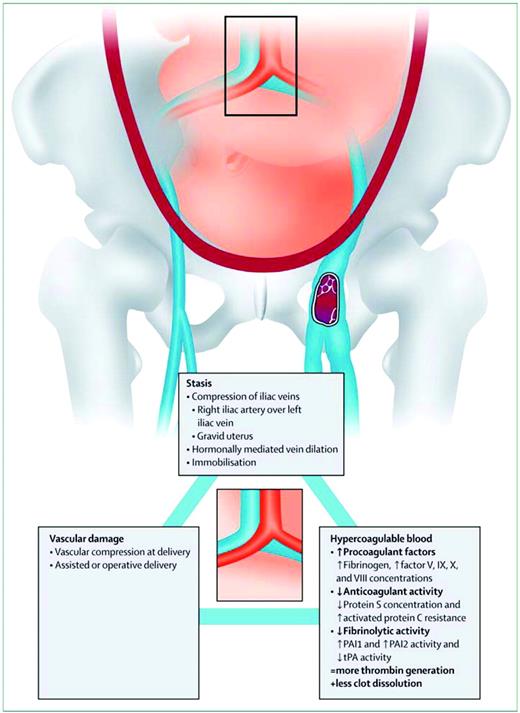
The causal link between pregnancy and VTE is best explained by Virchow's triad, a framework that categorizes elements of the pathophysiology of VTE into three broad categories: venous stasis, vascular damage, and hypercoagulability (Figure 1). Venous stasis, which begins in the first trimester and peaks at 36 weeks,9 is thought to be caused by progesterone-induced venodilation, pelvic venous compression by the gravid uterus, and pulsatile compression of the left iliac vein by the right iliac artery.9 The latter may lead to the marked propensity for left-leg DVT in pregnancy (over 80%).10 DVT in pregnancy appears to more commonly arise from proximal veins (iliac and femoral) rather than calf veins, the usual location in nonpregnant patients, leading to a higher propensity for isolated iliac vein thrombosis and ileofemoral thrombosis in pregnant patients with DVT.11 As a consequence of the latter, pregnancy-associated DVT is more likely to be associated with long term post-phlebitic syndrome.12 Vascular damage to the pelvic vessels can occur during pregnancy due to venous distension, as well as after normal vaginal, assisted vaginal, or cesarean deliveries. Hypercoagulability occurs as the hemostatic system is progressively activated to prepare the pregnant women for the hemostatic challenges of delivery. This hemostatic challenge is illustrated by the fact that peripartum hemorrhage remains the leading cause of maternal mortality in the developing world, and has likely been the leading cause of maternal mortality throughout human evolution.13
Virchow's triad in pregnancy. (From Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375(9713):500–512. Used with permission.)
Virchow's triad in pregnancy. (From Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375(9713):500–512. Used with permission.)
The modern side effect of this teleological maternal hypercoagulable state is an increased risk of VTE. The biological mechanisms driving this state are the following changes to the hemostatic system: 1) the anticoagulant activity of protein S is decreased and protein C resistance is increased14 ; 2) procoagulant activity is increased through higher levels of fibrinogen and factor V, IX, X, and VIII levels, leading to increased thrombin production14 as measured by increased thrombin/anti-thrombin complexes, increased soluble fibrin, and F 1.2 levels15 ; and 3) thrombus dissolution is reduced through decreased fibrinolysis from increased plasminogen activator inhibitor type 1 and 2 (PAI-1 and 2) activity and decreased tissue plasminogen activator (t-PA) activity.15 During the postpartum period, defined as the 6-week interval following delivery, the pro-coagulant maternal hemostatic system gradually returns to the nonpregnant state, as evidenced by progressive normalization of markers of coagulation activation to prepregnancy levels.16,17
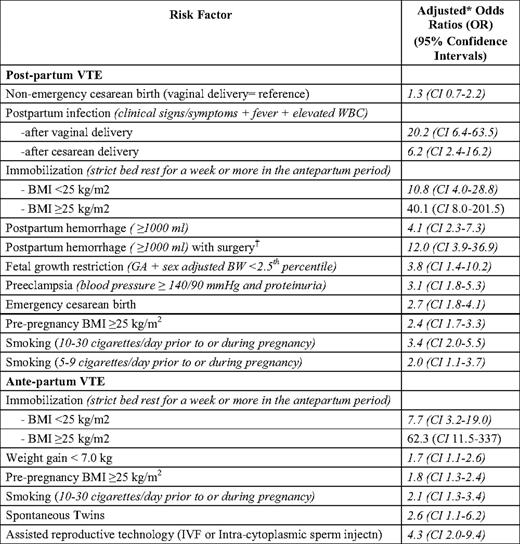
To date, epidemiologic research assessing potential VTE risk factors in the pregnant population has been methodologically limited by being predominantly conducted using large, administrative databases and underpowered, case-control studies4,5,18–20 .2,18 These studies have not contained rich enough potential confounder/risk factor data to permit an adequate exploration of risk factors, which may confound “known” associations (e.g., cesarean section and postpartum VTE) and/or identify new important risk groups. This research has also largely lumped antenatal and postnatal VTE together, despite their having different levels of risk and different risk factors. A large, well-conducted case-control study from Norway avoided these limitations and offers promise in identifying pregnant women at high risk of VTE.7 Jacobsen et al. compared 559 women with pregnancy-associated VTE (268 with antenatal VTE and 291 with postnatal VTE) with 1229 control women from a single hospital who gave birth at the same time, and examined a rich dataset of potential risk factors. Postnatal VTE risk factors and antenatal VTE risk factors differed (Table 1). While prior research has suggested that VTE rates are higher following cesarean delivery, these studies could not control for many independent risk factors for postpartum VTE identified by Jacobsen. Indeed, the association between cesarean delivery and postnatal VTE was confounded by many independent VTE risk factors associated with cesarean delivery, including preeclampsia, fetal growth restriction, and antepartum immobilization.2,18 In Jacobsen's study, uncomplicated cesarean delivery was not associated with an increased risk of pregnancy-associated VTE. Of particular interest were the interactions identified between risk factors, in particular the multiplicative effect of certain risk factors such as antepartum immobilization and body mass index (BMI) > 25 (adjusted odds ratio > 40) (Table 1). Jacobsen et al. also reported that 50% of postpartum women had two or more risk factors for postpartum VTE and 50% had no or one risk factor.
Prophylaxis
Prevention requires an optimal balancing of absolute increased bleeding risk from pharmacologic thromboprophylaxis and the absolute benefit of reduced VTE, which while serious, are relatively uncommon. The focus must remain on known high-risk groups with the understanding that recommendations for prophylaxis, even in high-risk groups, are based on a limited dataset. A recently updated Cochrane review addressed the effectiveness and safety of prophylaxis for VTE in pregnancy and the early postpartum period.24 The reviewers concluded that “there is insufficient evidence on which to base recommendations for thromboprophylaxis during pregnancy and the early postnatal period. Large scale randomized trials of currently-used interventions should be conducted.” The relative absence of evidence in this area is highlighted by the fact that over 65,000 patients have been randomized in published studies of postoperative thromboprophylaxis after major orthopedic surgery25 (most were industry sponsored), but only 236 participants have been randomized in postpartum prophylaxis studies (none of which were industry sponsored).
Unselected Pregnant Women
Implementing universal low-molecular-weight heparin (LMWH) thromboprophylaxis after delivery is not feasible given the costs and potential side effects of prophylaxis and the low absolute event rate of postpartum VTE if all women (i.e., low- and high-risk) are targeted (recall that the absolute risk of postpartum VTE is 3–7 per 10,000 deliveries). Post-delivery LMWH prophylaxis is not without risk; these risks include bleeding (>1.5% risk of any bleed, major and minor), wound complications following cesarean delivery, allergic reactions (skin reactions in 1.8% and, rarely, anaphylaxis), and, very rarely, heparin-induced thrombocytopenia (∼1:5000).
This review focuses on selected high-risk groups in which pharmacologic prevention should at least be strongly considered. All women at high risk of pregnancy-associated VTE should be counseled about the signs and symptoms of DVT and/or PE, and a plan developed should these symptoms arise.
Following Cesarean Section
While more recent data, as discussed above, suggest that C-section may not be an independent risk factor for VTE, and despite the absence of randomized, controlled trials providing evidence that thromboprophylaxis following C-section is of net clinical benefit, many authorities advocate risk assessment in women following C-section and the use of thromboprophylaxis in women with at least one additional VTE risk factor.26 Pharmacologic thromboprophylaxis or mechanical thromboprophylaxis (intermittent pneumatic compression or graduated compression hose) are recommended in those with a single additional risk. In those with multiple additional risk factors,26 combined pharmacologic and mechanical thromboprophylaxis is suggested. Finally, in those at very high risk, extended prophylaxis (4–6 weeks after delivery) is suggested.26
Prior VTE (but now off anticoagulants)
Antepartum.
In a study of 125 women with a single previous VTE, Brill-Edwards demonstrated that the lowest risk of recurrent VTE was in a subgroup of patients with a previous VTE that was secondary to a temporary risk factor (i.e,. a provoked DVT) who did not have an identifiable thrombophilia (0 of 44 women had recurrences; 95% confidence interval [CI] 0%–8%).23 Although the authors concluded that it was safe to withhold antepartum prophylaxis from this group, given the small sample size and the 95% CI of 0% to 8%, some providers still offer prophylaxis in this group. There is consensus that antepartum prophylaxis should be recommended in women with a prior unprovoked VTE and identifiable thrombophilia with laboratory testing (1 of 10, or 10%, of women had antepartum recurrences; 95% CI 0.3%–44%) and should be considered in all women with a prior VTE and an identifiable thrombophilia with laboratory testing (2 of 21, or 10%, of women had antepartum recurrences; 95% CI 1%–30%). Identifiable thrombophilias include anti-phospholipid antibodies; factor V Leiden; prothrombin gene mutations; and deficiencies of protein C, protein S, or antithrombin.
Postpartum.
All women with prior VTE should receive postpartum prophylaxis; in the Brill-Edwards study, the risk of postpartum recurrence was 3 of 125 women (2.4%; 95% CI 0.5%–7.0%) despite all study participants being recommended postpartum prophylaxis. Two of the three events occurred after hospital discharge, arguing in favor of longer postpartum prophylaxis (6 weeks).
Recent VTE (and on anticoagulants)
Where possible, vitamin K antagonists should be avoided in pregnancy because they are associated with congenital malformations (especially with exposure from 6–12 weeks) and with fetal and neonatal hemorrhage.27 Women on vitamin K antagonists with a current or recent VTE should be advised to discontinue oral anticoagulants as soon as they become pregnant (missed menses and /or positive urine pregnancy test). In women who become pregnant and have had a recent VTE, the urgency and aggressiveness of ongoing treatment should be dictated by the timing of the VTE. Full-dose therapeutic LMWH should be initiated immediately if the previous VTE occurred in the last month; aggressive prophylaxis in the form of a 3/4-treatment dose of LMWH in the next 24 h if the VTE occurred in the previous 12 months; the full prophylactic dose should be considered if the VTE occurred more than 12 months previously.
Identifiable Thrombophilia with Laboratory Testing but No Prior VTE
Antepartum.
Although thrombophilic women are at greater relative risk of developing VTE in the antepartum period, the current standard of care is to observe most of these women without prophylaxis. Several large, prospective cohort studies of pregnant women with factor V Leiden or prothrombin gene mutation but no prior VTE have shown a very low absolute risk of antepartum VTE without prophylaxis.28–31 However, in the rare patient with potent thrombophilia, such as compound heterozygotes and type I antithrombin deficiency, antepartum VTE prophylaxis is likely warranted.
Postpartum.
VTE prophylaxis should be considered at the very least while women remain in the hospital.
Diagnosis
Radiation Exposure
Of significant concern to clinicians and patients is the radiation exposure to the unborn child and mother associated with diagnostic imaging used in the management of suspected VTE in pregnant women. The amount of radiation exposure to the fetus from imaging for DVT and PE is well below the 5 cGy recommended by the National Commission on Radiation Protection as the maximum allowable exposure for the entire pregnancy. Ginsberg et al. concluded that radiation exposure in utero of up to 5 cGy may increase the risk of childhood malignancy by up to 2-fold, and may increase the risk of congenital eye abnormalities slightly.32 On the other hand, there appears to be no increased risk of fetal growth restriction, miscarriage, stillbirth, or infant death.32 The estimated radiation exposure to the unborn child is up to 0.58 cGy with ventilation/perfusion lung (V/Q) scanning and up to 0.066 cGy with computed tomography (CT) scanning.33 Maximizing efforts to limit radiation exposure using low-dose perfusion scanning (omitting ventilation scanning for negative perfusion studies) (<0.012 cGy) results in very low radiation exposures to the unborn child. Despite the potentially small increased risks of radiation exposure to the unborn child, clinicians and patients can be comforted that more good than harm can be achieved by the appropriate management of suspected VTE in pregnancy when one considers the known high mortality of untreated VTE and of major bleeding with therapeutic anticoagulation in women without VTE.
Deep Vein Thrombosis
Suspected DVT is common in pregnancy, given that leg swelling is a frequent complaint or finding. In addition, due to the fact that isolated iliac vein thrombosis occurs with increased frequency during pregnancy, patients may manifest with unusual presentations such as isolated buttock, groin, flank, or abdominal pain.11
Diagnostic Tools: Clinical Assessment
While clinical assessment using clinical decision rules has been demonstrated to be very useful in assigning pretest probability outside of pregnancy,34 the studies deriving and validating this model did not include pregnant patients. In a recent prospective cohort study, Chan et al. reported on 194 women with suspected DVT.35 Expert clinicians collected clinical information and synthesized this information in a subjective pretest probability assessment; 17 of 194 women had DVT on an initial ultrasound, 182 women had a normal initial ultrasound, and 152 then underwent serial leg compression ultrasounds (d 3 and/or d 7). If the ultrasound was negative, participants were followed for 3 months for further clinical events, and one additional patient developed DVT (total DVT = 18). The subjective opinion of the expert clinicians managing these patients demonstrated that the high negative predictive value was 98.5% (95% CI 94.6%–99.6%). The “LEFt” clinical decision rule was derived, which considers three variables in pregnant women with suspected DVT: 1) left leg presentation, 2) ≥ 2 cm calf circumference difference, and 3) first trimester presentation. If none of the LEFt variables is present, then the negative predictive value is 100% (95% CI 95.8%–100%). Clinical decision rules often perform worse in validation studies, so this rule should not be used in clinical practice until it is validated. However, it appears to be very promising.
Diagnostic Tools: D-Dimer
D-dimer levels increase throughout normal pregnancy.16 Near term and in the postpartum period, most normal pregnant women will have abnormal D-dimer levels. D-dimer assays are, in general and even more so in pregnancy, sensitive but nonspecific markers for VTE. Chan et al. recently examined altering the D-dimer cutoff in pregnancy, which improved specificity without altering sensitivity.36 A recent small study demonstrated promising diagnostic performance for a whole-blood agglutination D-dimer in pregnant women. This D-dimer had a sensitivity of 100% (13 of 13 patients; CI 77%–100%), a specificity of 60% (CI 52%–68%), and a negative predictive value of 100% (81 of 81 patients; CI 95%–100%). The D-dimer was positive in 0% (CI 0%–60%), 24% (CI 14%–37%), and 51% (CI 40%–61%) of women in the first, second, and third trimesters, respectively.37 While D-dimer appears to be a promising tool in diagnosing suspected DVT in pregnancy, given the limited evidence base, it requires further validation prior to being routinely used to exclude DVT without diagnostic imaging.
Diagnostic Tools: Compression Ultrasound Leg Vein Imaging
Compression ultrasound leg vein imaging has become the test of choice in patients with suspected DVT. In clinical management studies, serial venous ultrasound imaging has been found to safely exclude DVT in unselected patients, and in accuracy studies to have high sensitivity and specificity for proximal DVT (i.e., DVT in the popliteal vein or above).38 It is less sensitive and specific for distal DVT (i.e., DVT isolated to the paired calf veins: the peroneal, anterior tibial, and posterior tibial veins) and for DVT in the pelvic vasculature, which is more common in pregnancy. Nonetheless, compression leg vein ultrasound is the diagnostic imaging procedure of choice for suspected DVT in pregnancy. Venous ultrasound imaging is noninvasive and there is no radiation exposure to unborn child. While further validation of serial ultrasound imaging for DVT in pregnancy is warranted, the results of Chan et al. suggest that a serial compression ultrasound approach (at d 0, d 3, and d 7) in pregnancy is valid given a high negative predictive value of 99.5% (181 of 182 women; 95% CI 97%–99%).
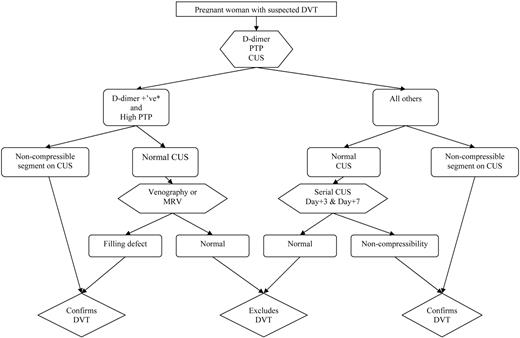
Putting the Diagnostic Tools into Practice
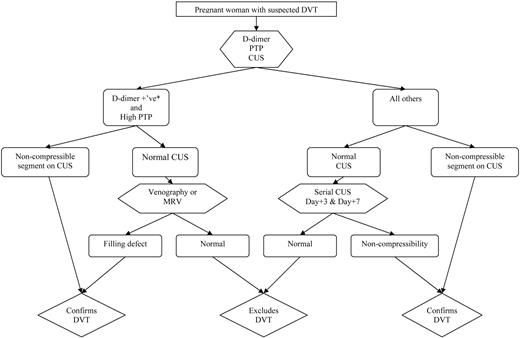
While ongoing studies will help to clarify the validity of non-imaging-based diagnostic approaches (i.e., clinical decision rules and D-dimer) for suspected DVT in pregnancy, we suggest that all women with suspected DVT should be assigned a pretest probability, have D-dimer testing, and then undergo venous ultrasound imaging (Figure 2). If a proximal DVT is detected, then treatment should be continued. In women with high pretest probability and positive D-dimer and a normal initial ultrasound, magnetic resonance venography (MRV) should be considered to rule out isolated pelvic DVT. If MRV is unavailable, limited venography with pelvic shielding that does not obscure visualization of the iliac vein is an acceptable alternative. All other women (i.e., those with normal D-dimer or non-high pretest probability) should undergo serial compression ultrasound imaging 3 d and 1 week later, with anticoagulation withheld.35 Leg venous ultrasound imaging is insensitive to calf DVT and iliac DVT, which may propagate in pregnancy, and this is the reason for the recommendation for serial compression ultrasound imaging. If no DVT is detected by d 7, then DVT can be considered safely excluded.
Algorithm for suspected DVT in pregnant women. *Using nonpregnant D-dimer cutoff (i.e., usual D-dimer cutoff in local practice); CUS, venous compression ultrasound imaging; PTP, pretest probability
Algorithm for suspected DVT in pregnant women. *Using nonpregnant D-dimer cutoff (i.e., usual D-dimer cutoff in local practice); CUS, venous compression ultrasound imaging; PTP, pretest probability
Pulmonary Embolism
Diagnostic Tools: Clinical Assessment
Dyspnea, chest pain, and unexplained tachycardia are common in pregnant women, so the diagnosis of PE is frequently considered. Bedside tests to exclude PE without diagnostic imaging have been developed and validated and have improved patient management in nonpregnant patients,39 but pregnant women were excluded from the studies that developed these non-imaging-based approaches. Further investigation will be required to either validate these tools in pregnant women or develop specific clinical tools for pregnant women.
The prevalence of PE in pregnant patients with suspected PE appears to be even lower than in nonpregnant patients, who are generally older and have other comorbidities. In one small, retrospective cohort study examining V/Q scanning for suspected PE in pregnant women, only 1.8% had high-probability V/Q scans, and less than 6% were treated for VTE after the completion of diagnostic imaging.40
Diagnostic Tools: D-Dimer
See above section on D-dimer, but note that it has never been prospectively studied for suspected PE in pregnancy.
Diagnostic Tools: V/Q Scanning
For decades, V/Q scanning has been used as the imaging procedure of choice for the evaluation of patients with suspected PE.41 A limitation of V/Q scanning in the nonpregnant population is that most lung scans fit into the non-diagnostic category (neither normal nor high probability), in which the incidence of PE varies from 10% to 30%. In pregnant women with suspected PE, fewer patients will have non-diagnostic scans (25%) than in unselected patients likely due to less concomitant respiratory disease and hyperdynamic pulmonary circulation than in other patient populations with suspected PE.40 Further testing is required to exclude the diagnosis of PE in patients with non-diagnostic scans.
Diagnostic Tools: CT Pulmonary Angiography
Management studies have shown that it is safe to withhold treatment in nonpregnant patients with suspected PE in whom the CT pulmonary angiography (CTPA) result is negative.42 Several features make CTPA more attractive than V/Q scanning in nonpregnant patients: 1) the specificity of CTPA is higher than V/Q (>90% vs. 10%), 2) CTPA may identify alternative causes for a patient's presentation (V/Q cannot), and 3) CTPA is more widely accessible and more often available after-hours.43 For the time being, we would consider CTPA in pregnant women with suspected PE as a second-line choice. While the literature provides reassuring estimates of fetal radiation exposure from CTPA for suspected PE in a pregnant woman, CTPA imaging has not been validated in pregnant women with suspected PE. Furthermore, a recent randomized, controlled trial showed that CTPA results in a higher rate of PE diagnosis than V/Q imaging in patients with suspected PE44 without a corresponding increase in recurrent VTE in the V/Q group, calling into question the clinical significance of these additional PEs. A false-positive diagnosis of PE has serious consequences both during the pregnancy (anticoagulant treatment complicating the pregnancy, delivery, and postnatal period) and for the rest of the woman's life (anticoagulation in subsequent pregnancies, lifelong anticoagulation with next VTE, limited contraceptive options, etc.). Also, it is estimated that CTPA is associated with a radiation exposure to the woman's breast of 2.0 to 3.5 cGy,45 increasing her lifetime risk of breast cancer. Finally, the most attractive characteristic of CTPA for suspected PE relative to V/Q and outside of pregnancy is the low proportion of non-diagnostic tests. A recent retrospective study suggested that in pregnant women with normal chest X-ray, CTPA in pregnancy is more likely to be non-diagnostic than V/Q.46
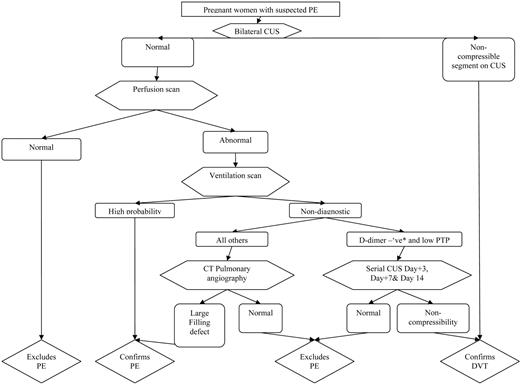
Putting the Tools into Practice
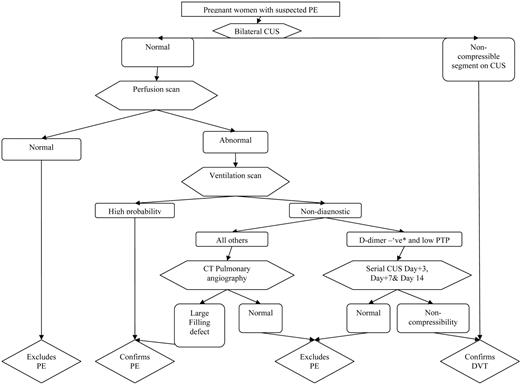
The V/Q-based algorithm outlined in Figure 3 is suggested for the diagnosis of PE in pregnant women. This approach favors a conservative diagnostic algorithm that unfortunately leads to a lot of diagnostic imaging until less-imaging-intensive tools and approaches are validated in pregnancy. It recommends that leg vein imaging be conducted in all pregnant women with suspected PE, given that venous ultrasound imaging is noninvasive and therefore there is no radiation exposure to the unborn child. If positive, leg vein imaging answers the therapeutic question. If V/Q is unavailable, CTPA can be used instead of V/Q; however, if an isolated sub-segmental filling defect is identified in patients with non-high-pretest clinical probability, additional testing is suggested due to a high risk of false-positive PE diagnoses with this CTPA result.
Algorithm for suspected PE in pregnant women. *Using nonpregnant D-dimer cutoff (i.e., usual D-dimer cutoff in local practice); CUS, venous compression ultrasound imaging; PTP, pretest probability
Algorithm for suspected PE in pregnant women. *Using nonpregnant D-dimer cutoff (i.e., usual D-dimer cutoff in local practice); CUS, venous compression ultrasound imaging; PTP, pretest probability
Treatment
If thromboembolism is suspected, diagnosis and treatment should be immediate and effective. Treatment of PE can be considered in four phases: acute (first 24 h), subacute (d 1–week 3 or 4), medium-term (after week 3 or 4), and peripartum.
Acute Period
It is estimated that 10% of cases of PE lead to rapid death prior to diagnosis.47 Thrombolytic therapy is considered in patients who are hemodynamically unstable and who have refractory hypoxemia.48 Fewer than 200 pregnant patients who have received thrombolytic therapy have been reported. The maternal mortality rate from thrombolytic therapy has been reported to be 1.2%, the bleeding rate 8.1%, and the fetal loss rate 5.8%.49 Hemorrhagic complications are most often seen peripartum. Tissue plasminogen activator is not teratogenic, and appears to be the safest fibrinolytic drug in pregnancy. Confirmed VTE in pregnancy requires immediate anticoagulation with intravenous unfractionated heparin or therapeutic-dose LMWH. LMWH has become the drug of choice for the treatment of VTE in pregnant patients due to ease of use and less heparin-induced osteoporosis.50 A recent systematic review of 64 reports documenting 2777 pregnancies concluded that LMWH is safe during pregnancy.51 In this review, there were no deaths reported and serious side effects were rare. Heparin-induced thrombocytopenia is rare but can occur.52,53 In the systematic review by Greer, allergic skin reactions to LMWH were reported in 50 women (1.80%), some of which may have been associated with heparin-induced thrombocytopenia antibodies.54 Osteoporotic fracture was reported in one patient (0.04%) in that review. Significant bleeding was more common (1.98% overall; 55 events), with 12 (22%) cases of significant antenatal bleeding, 26 (47%) cases of postpartum hemorrhage, and 17 (31%) wound hematomas. LMWHs do not cross the placental barrier, making them safe for the fetus when administered during pregnancy. Greer's review had limited data on fetal outcomes; of 2215 pregnancies treated with LMWH, 94.7% were reported to have successful outcomes, defined as live birth.51 LMWH is only minimally secreted in the breast milk.55 The pharmacokinetics of LMWH in pregnancy are poorly understood, but studies clearly show that drug clearance is dependent on gestational age.56,57 Therefore, treatment with full-dose LMWH in pregnancy may best be accomplished with monitoring of drug effect with target anti-Xa levels of 0.5 to 1.1 at 3 to 6 h post-dose. Graduated compression stockings providing 30 to 40 mmHg should be considered in patients with pregnancy-associated DVT to help reduce the risk of long-term post-phlebitic syndrome.
Subacute Period
Therapeutic dose LMWH and weekly peak anti-Xa monitoring in patients should be continued during the subacute period. However, it is important to acknowledge that anti-Xa thresholds for safety and efficacy have not been established and that considerable inter-assay/machine variability of anti-Xa measurements58 are significant potential limitations of this approach.
Medium-Term Period
The dose of LMWH should be reduced to 3/4 of the full treatment dose throughout the remainder of pregnancy and at least throughout the postpartum period. Dose reduction to 3/4 treatment dose after 3 to 4 weeks of full treatment dose appears safe, given evidence that prophylactic-dose LMWH has been shown to have comparable efficacy and safety as oral anticoagulant therapy (international normalized ratio [INR] of 2–3) in the longer-term therapy (secondary prevention) of acute DVT in nonpregnant patients.59 Furthermore, in nonpregnant cancer patients with acute VTE, a randomized trial has shown that LMWH,60 with dose reduction after 3 to 4 weeks, is more effective than warfarin, with a target INR of 2 to 3. Cancer patients are at much higher risk of treatment failure than pregnant women, which provides additional rationale for this approach.61 A higher than prophylactic dose maybe justified given the ongoing hypercoagulability of the pregnant state. A 3/4-treatment dose permits ongoing therapy, likely without the need for laboratory monitoring of drug effect and likely to be associated with a lower bleeding risk. The efficacy and safety of this practice in the context of pregnancy, for which the drug may have different pharmacokinetics, awaits confirmation.
Peripartum Period
In all patients on therapeutic anticoagulants, an induction of labor helps to prevent the risk of labor occurring on full anticoagulation, likely reducing the risk of bleeding and optimizing anesthetic options. Clinicians should keep in mind, however, that even with induction, the onset of labor may be unpredictable and its duration variable.
If the VTE is diagnosed near term (over 37 weeks), then consideration should be given to the placement of an inferior vena cava (IVC) filter (preferably retrievable) and a planned induction performed after reversal of anticoagulation. Reversal of anticoagulation without IVC filter protection is strongly discouraged in the 2-week period after the diagnosis of the VTE, given the mortality of untreated thromboembolism in this period in nonpregnant patients.62 However, it must be noted that complications with IVC filter insertion and retrieval can occur in pregnancy.63 An attempt is usually made to place the filter in the infrarenal vena cava. However, the infrarenal vena cava may be distorted by the gravid uterus, reducing the chances of successful placement. Suprarenal filters may be placed in these cases and are unlikely to be associated with higher complication rates.
Longer un-anti-coagulated periods without IVC filter protection can be considered if the VTE is more remote (>2 weeks), given that the risk of untreated mortality from recurrent VTE diminishes with time from VTE diagnosis. If the VTE was diagnosed 2 to 4 weeks prior to delivery, LMWH should be replaced with intravenous heparin prior to starting induction. Heparin can then be stopped in active labor or reversed with protamine infusion if delivery is precipitous. Postpartum, as soon as hemostasis is achieved after delivery, heparin therapy (full-dose intravenous or full-therapeutic-dose LMWH split every 12 h) can be restarted. If the VTE was diagnosed 1 to 3 months prior to delivery, the last 3/4-dose of LMWH should be given 24 h prior to anticipated delivery and split up every 12 h. The 3/4-dose LMWH should be restarted 6 h postpartum. If the VTE was diagnosed over 3 months prior to delivery, then the last of 3/4-dose LMWH should be given 24 h prior to induction and split up every 12 h. The 3/4-dose LMWH should be restarted 12 to 24 h postpartum.
Disclosures
Conflict-of-interest disclosures: The author has received research funding from the Canadian Institutes of Health Research, Sanofi Aventis, Leo Pharma, Pfizer, and the Heart and Stroke Foundation, and was a member on the board of directors or advisory committees for Sanofi Aventis.
Off-label drug use: LMWH in pregnancy for VTE prevention and treatment.
Correspondence
Marc Rodger, Chief, Division of Hematology, Senior Scientist, Ottawa Health Research Institute, Ottawa Hospital General Campus, 1812-E Box 201, 501 Smyth Road, Ottawa, ON, Canada K1H 8L6; Phone: (613) 737-8899, ext. 74641; Fax: (613) 739-6102; e-mail: mrodger@ohri.ca