Abstract
Clinical scenario: An otherwise healthy 25-year-old woman returns to your office for management of chronic primary immune thrombocytopenia. She was diagnosed 6 months earlier and continues to require prednisone 15 mg daily and periodic infusions of intravenous immunoglobulin to maintain a hemostatic platelet count. You discuss second-line treatment options, including splenectomy. The patient asks if there are any means by which to predict likelihood of response to splenectomy. You have heard about the use of indium-labeled autologous platelet scanning for this purpose and wonder what the evidence shows.
Immune thrombocytopenia (ITP) comprises a heterogeneous group of disorders characterized by autoimmune-mediated platelet destruction by tissue macrophages in the spleen and elsewhere, variable impairment of thrombopoiesis, and an elevated risk of bleeding. Splenectomy remains a highly effective and well-tolerated treatment for patients refractory to or intolerant of first-line therapy with long-term complete response rates of 66%.1–3 Nevertheless, splenectomy is not without its drawbacks, including complications related to surgery and anesthesia in the short-term and lifelong risks of serious infection and possibly thromboembolism.2
Indium-labeled autologous platelet scanning (ILAPS) is a nuclear imaging modality in which autologous platelets are reinfused into the patient after ex vivo labeling with 111In. Subsequent scintigraphy demonstrates the site(s) of platelet sequestration and clearance. It has been proposed that patients with purely or predominantly splenic sequestration determined by ILAPS may be more likely to respond to splenectomy than those exhibiting hepatic, mixed, or diffuse patterns.4 To evaluate the potential of ILAPS to predict response to splenectomy, we performed a comprehensive literature review of all studies in which ILAPS was performed prior to splenectomy in patients with ITP.
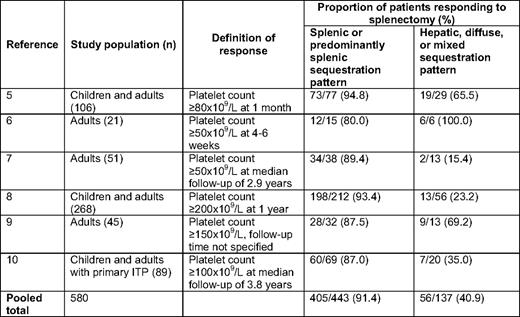
A search of the PubMed database limited to English language references was conducted by combining the MeSH (Medical Subject Heading) term “indium” with the keywords “ITP,” “immune thrombocytopenic purpura,” “idiopathic thrombocytopenic purpura,” and “autoimmune thrombocytopenia” between 1950 and June 30, 2010. This strategy yielded 51 citations. Excluded were 32 studies in which either ILAPS or splenectomy was not performed, 6 case reports, and 3 reviews. Of the 10 remaining references, 1 did not include data on response to splenectomy, 1 did not provide information on the sequestration pattern observed with ILAPS, and 3 were preliminary reports of ongoing case series for which updated iterations have been published, leaving 5 eligible studies.5–9 A search of abstracts from the American Society of Hematology, the International Society on Thrombosis and Haemostasis, and the European Haematology Association since 2003 identified a sixth eligible study (n = 580 for all eligible studies). A manuscript describing this study was in press at the time of our literature search and was graciously provided by the authors for inclusion in the present analysis.10
In a pooled analysis of the six eligible studies (Table 1), patients with a splenic or predominantly splenic pattern of platelet sequestration by ILAPS demonstrated a higher response rate following splenectomy than those with a hepatic, diffuse, or mixed pattern (0.914 vs 0.409; odds ratio 15.4; 95% CI 9.6–24.8). The validity of this analysis is limited by differences among the six studies with respect to the study population, definition of response, timing of assessment for response, and ILAPS methodology and interpretation. Nonetheless, we conclude that ILAPS holds promise as a tool for predicting response to splenectomy in patients with chronic ITP. Additional prospective studies utilizing well-defined patient populations, recommended definitions of response,11 and standardized methods for ILAPS12 are needed to confirm the value of this procedure. Until higher level evidence is available, we do not recommend the routine use of ILAPS for predicting response to splenectomy in centers without extensive experience with this modality (grade 2C).13
Disclosures
Conflict-of-interest disclosure: D.B.C. has consulted for Glaxo-Smith-Kline and Amgen. A.C. declares no competing financial interests.
Off-label drug use: None disclosed.
Correspondence
Adam Cuker, MD, MS, Penn Comprehensive Hemophilia and Thrombosis Program, Hospital of the University of Pennsylvania, 3 Dulles, 3400 Spruce St., Philadelphia, PA 19104; Phone: (215) 615-6555; Fax: (215) 615-6599; e-mail adam.cuker@uphs.upenn.edu