Abstract
In recent years, our understanding of the pathophysiology of chronic lymphocytic leukemia (CLL) has advanced significantly. It is now clear that CLL is a relatively proliferative disorder that requires the help of its microenvironment to be maintained and to progress. The stimulation of the CLL cell occurs in most, if not all, patients through antigen stimulation via the BCR. In addition, there is now a clearer appreciation of the role of the p53 pathway leading to chemoresistance. These insights are allowing a more targeted approach with the use of p53-independent drugs such as mAbs and high-dose steroids to overcome genetically poor-risk CLL. The elucidation of the molecular and intracellular signaling mechanisms of disease is just beginning to facilitate the development of several targeted small molecules that promise to revolutionize the treatment of CLL. The measurement of the level of minimal residual disease (MRD) in CLL is becoming more available, facilitating approaches in which the aim of therapy is the eradication of detectable MRD. This also promises to improve personalization of therapy to the individual. Recently, the addition of rituximab to fludarabine plus cyclophosphamide (FCR) has improved overall survival in CLL for the first time, and it appears that this will only be the first small step on the path to much more effective therapies and, hopefully, less toxic targeted therapies.
Introduction
Our understanding of chronic lymphocytic leukemia (CLL) has changed dramatically over the last decade. The notion that the disease was characterized by the accumulation of quiescent terminally differentiated “memory” lymphocytes has now been shattered. It is now clear that the leukemic cells found in the peripheral blood of patients with CLL are only part of these cells' “life cycle.” The CLL cells rapidly divide, undergo apoptosis, and accumulate depending on the balance between the two. In addition, they are constantly being stimulated through their BCRs and their interaction with the microenvironment.1 In some cases, the tumor has the propensity to develop resistance to therapy, whereas in others, the major consequences of the disease (and its therapy) are immune dysregulation. All of these features probably explain why CLL has been incurable with conventional chemotherapeutic approaches, uncover potential novel therapeutic targets that are now being exploited, and define new therapeutic end points that promise to facilitate more effective treatment strategies.
Advances in our understanding of CLL
Pathophysiology: CLL cellular life cycle
The peripheral blood CLL cells are only part of the life cycle of the CLL cell. From the peripheral blood, the cells migrate into the tissue compartment (BM, lymph nodes, and spleen), where they receive a proliferative stimulus. It appears that this is at least in part driven by antigen that is presumably presented to the CLL cells by components of the microenvironment and is recognized by the BCR on the surface of the CLL cell.2 It also seems likely that the CLL cells recruit the cells of the microenvironment (macrophages, follicular dendritic cells, T cells, nurse-like cells, etc), forming the proliferation centers (previously defined as pseudofollicles; Figure 1). In these proliferative areas, the CLL cells form a synapse or “signalosome,”3 which appears to center on the BCR and other costimulatory molecules such as CD40/CD40L, CD38/CD31, CXCR5/CXCL13 (BCA-1), CXCR4/stromal cell-derived factor-1 (SDF-1), and possibly other molecules, leading to downstream signaling in the CLL cell and a proliferative burst. The cell then migrates from the proliferating centers back to the peripheral blood, where the cell stops dividing. The CLL cell then either undergoes apoptosis or continues to cycle back into the proliferation centers so that it is continuously cycling between a proliferative state in the tissue compartment and a quiescent state in the periphery.
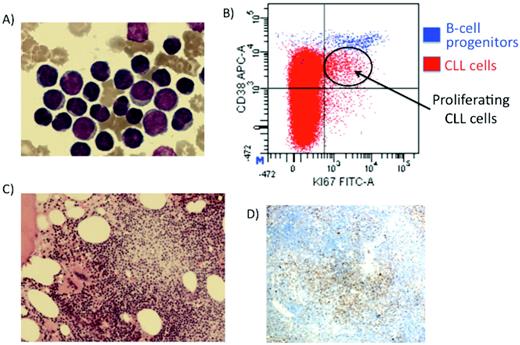
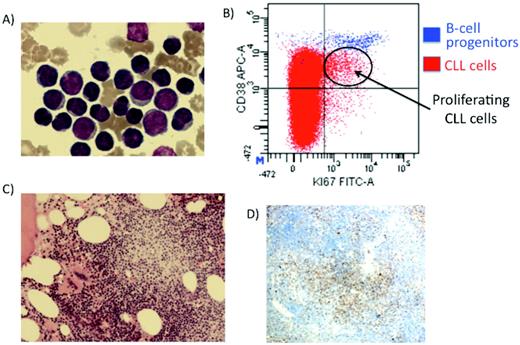
Proliferating cells in CLL. (A) Mature peripheral blood cells with occasional larger cells. (B) Flow cytometry identifying the proliferative compartment in CLL (Ki-67 and CD38 + cells). (C) BM trephine-containing proliferation centers. (D) Ki-67 staining demonstrating CLL cells proliferating in the proliferation centers.
Proliferating cells in CLL. (A) Mature peripheral blood cells with occasional larger cells. (B) Flow cytometry identifying the proliferative compartment in CLL (Ki-67 and CD38 + cells). (C) BM trephine-containing proliferation centers. (D) Ki-67 staining demonstrating CLL cells proliferating in the proliferation centers.
Evidence for CLL cell life cycle
Stereotyped BCR.
The BCR of CLL cells is not random; different patients have so-called stereotyped receptors, meaning that there is a highly selective use of a small number of immunoglobulin genes. In some cases, identical immunoglobulin receptors are observed in different patients with CLL and this could not happen by chance.4 Therefore, these receptors are preferentially used in CLL and must have been positively selected for due to their specificity to a particular antigen. Therefore, if there are a limited number of antigens responsible for the development of CLL, then by “chance” different clones might be selected for due to their antigen specificity. This receptor/antigen interaction almost certainly drives the survival and/or proliferation of the CLL cell. Up to 30% of patients have stereotyped receptors, and the antigen is probably an auto-antigen such as the myosin heavy chain or a common alloantigen.
Proliferation centers.
There are structures in the lymph nodes and/or BM of virtually all patients with CLL with a component of rapidly dividing CLL cells. These structures are known as proliferating centers and contain CLL cells with a proliferative morphology and immunophenotype.5,6 In the proliferating centers, the CLL cells are large cells that express proliferation markers such as Ki-67, CD24, CD43, and CD38 (Figure 1).
Dynamic cellular kinetics in CLL.
Heavy-water experiments by Messmer et al demonstrated that, rather than CLL cells being exhausted end-stage lymphocytes, they are in fact frequently highly proliferative.7 These experiments demonstrated that the “birth rate” (the proportion of CLL cells produced each day) in CLL is very high, with up to 2% of CLL cells being “born” each day in some patients. The immunophenotype suggesting proliferation of CLL cells is uncommonly expressed by the CLL cells in the peripheral blood, and it seems that the small number of CLL cells in the peripheral blood with a proliferative phenotype probably represents the cells exiting the tissue compartment before they become quiescent again. The CLL cell then remains in a resting state before it presumably either apoptoses or recirculates through the tissue microenvironment again.
Telomere length.
The telomeres of CLL cells, particularly in unmutated CLL, are short, indicating that they have undergone a large number of cell divisions.8 In fact, recent studies have revealed that the telomeres in CLL are some of the shortest reported in primary human tissues. These findings have been used to suggest that the genomic instability associated with severe telomere shortening might contribute to the progression of CLL.9
Chemoresistance in CLL
Response rates in CLL have improved significantly with the use of combination immunochemotherapy. The combination of fludarabine with cyclophosphamide and rituximab (FCR) is associated with higher complete remission rates (between 44% and 72%), with approximately 90% of patients achieving a response and improved overall survival (OS) compared with fludarabine plus cyclophosphamide (FC).10 However, even with FCR given as the initial therapy, ∼ 10% of patients fail to respond and therefore have “primary” resistance.
P53 pathway (chromosome 17p) abnormalities.
At the time of initial treatment, ∼ 7% of previously untreated patients with CLL requiring therapy have a deletion involving the short arm of chromosome 17 detected by FISH that covers the locus of the p53 gene. In addition, a small number of patients have mutations of the p53 gene without a 17p deletion. Both 17p deletion and the p53 mutation render patients resistant to chemotherapy because the p53 pathway is critical in the cellular response to DNA damage, either by facilitating the repair of the damaged DNA or, if the damage is too great, leading to cell-cycle arrest and/or apoptosis.11 In addition to FISH analysis for 17p deletion, there are increasing data on other methods of assessing p53 abnormalities, such as single nucleotide polymorphism microarray and sequencing of the p53 gene, which carry the same implications as 17p deletion (Figure 2). Therefore, patients with progressive disease in whom the CLL cells have deleted 17p would be expected to have primary resistance to therapies that depend on DNA damage or interference with its repair.12 In trials of chemotherapy and chemoimmunotherapy, the response rates for 17p-deleted CLL are universally very poor. For example, in the German CLL Study Group CLL8 trial, the complete remission rate for patients with 17p-deleted CLL treated with FCR was ∼ 5% compared with 50% for those patients who did not have this genetic abnormality.10 In addition, the median progression-free survival (PFS) for patients with 17p deletion was only 11.2 months, compared with 51.8 months for FCR generally and with only 38.1% surviving 36 months after frontline FCR therapy in patients with 17p deletion. In addition to the patients with deletions of chromosome 17, there is a smaller group of patients with inactivating mutations of the p53 gene without abnormalities detected by FISH, and these patients also appear to have a poor response to conventional therapy and inferior survival.13
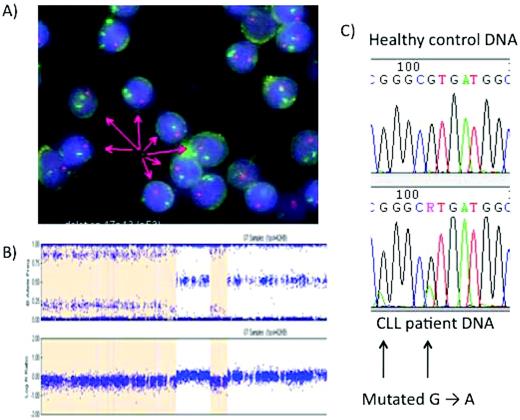
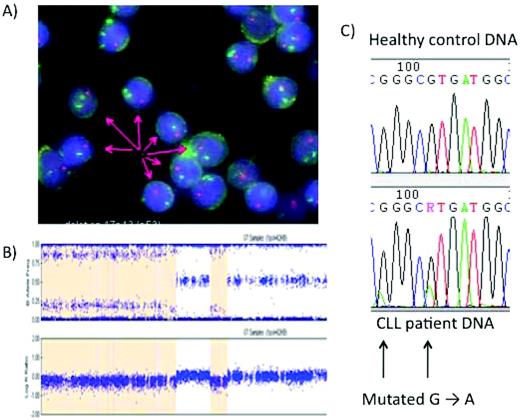
Analysis of the p53 pathway in CLL. (A) FISH with a green fluorescent chromosome 17 centromeric probe and a red 17p probe demonstrating the deletion of 17p in most, but not all, CLL cells. (B) Single-nucleotide polymorphism microarrays in CLL. The patient has loss of heterozygosity in part of the genome covered by the p53, suggesting that the CLL clone may have no active p53. The orange shaded area in the figure indicates a deletion of 17p. Direct sequencing is then possible. (C) Direct sequencing allows the identification of single p53 mutations. These mutations are often found when the other allele is deleted, although this is not the case here.
Analysis of the p53 pathway in CLL. (A) FISH with a green fluorescent chromosome 17 centromeric probe and a red 17p probe demonstrating the deletion of 17p in most, but not all, CLL cells. (B) Single-nucleotide polymorphism microarrays in CLL. The patient has loss of heterozygosity in part of the genome covered by the p53, suggesting that the CLL clone may have no active p53. The orange shaded area in the figure indicates a deletion of 17p. Direct sequencing is then possible. (C) Direct sequencing allows the identification of single p53 mutations. These mutations are often found when the other allele is deleted, although this is not the case here.
FISH chromosome 11q deletion.
Patients with deleted chromosome 11q also have a poorer survival than those without this abnormality (although not as poor as patients with 17p deletion).14 Chromosome 11q is the locus of the ataxia telangiectasia mutated (ATM) gene that lies on the p53 pathway but is not as pivotal in that pathway as the p53 gene itself.15,16 The addition of rituximab to fludarabine plus cyclophosphamide appears to overcome the poor prognostic impact of the chromosome 11q deletion,17 bringing these patients closer to standard-risk patients in terms of response rates and their early PFS. However, there is however mounting evidence that 11q deletion still confers a poor prognosis, because patients appear to relapse more frequently in the medium term (3-5 years) and inferior OS becoming evident in longer-term follow-up.18
Immunoglobulin gene mutation analysis.
The long-term PFS and OS are worse for patients with unmutated immunoglobulin genes compared with patients with mutated immunoglobulin genes. This appears to be independent of the depth of remission, because similar numbers of patients achieve complete remission in both groups.19 The explanation for this seems to be that mutated CLL is genetically more stable than unmutated CLL. Therefore, when patients with unmutated CLL relapse, they are far more likely to have developed deletion of chromosome 17p (or presumably mutation of the p53 gene) and therefore are more likely to be resistant to salvage chemotherapy. This concept leads to the natural conclusion that the approach to treatment for these 2 major subsets of CLL should probably be different. A less intense, and therefore less toxic, approach may be appropriate for mutated CLL, in which patients can be relatively easily salvaged with second-line therapy. Conversely, more effective initial therapy but with the possibility of increased toxicity might be considered for unmutated CLL. For example, in patients with poorer-risk CLL, several approaches are currently being studied, including: (1) intensifying the chemotherapy backbone in FCR by the addition of mitoxantrone (FCM-R)20,21 ; (2) the use of consolidation after FCR with mAbs, in particular alemtuzumab, which clearly has some toxicity but also appears to be effective22 ; and (3) maintenance therapy after FCR with lenalidomide (or other immunomodulatory drugs). However, an alternative approach might be that unmutated CLL is genetically unstable and that exposing such patients to therapy that damages DNA might be detrimental and should be avoided if possible. It might therefore be worth considering approaches that do not use the p53 pathway, such as the combination of mAbs (alemtuzumab or rituximab) and steroids, the use of alternative therapies such as lenalidomide, or novel therapies such as the emerging BCR pathway inhibitors. It is clear that well-designed clinical trials are needed to address all of these questions. However, it should be emphasized that these approaches currently have very little evidence to support their use in patients with non-17p-deleted CLL and chemoimmunotherapy therefore remains the standard approach outside of clinical trials.
Response assessment
One of the most important predictors of both PFS and OS is the depth of remission after therapy.23 This is true regardless of the exact therapy used or the line of therapy. In fact, there is now emerging evidence that improving the depth of remission in an individual patient will improve outcome. For example, in the German CLLSG CLL8 trial, the PFS was the same for patients who achieved remission with no detectable minimal residual disease (MRD−) after either FC alone or FCR.24 However, almost twice as many patients were MRD− after FCR compared with FC, indicating that approximately half of the patients who were MRD− after FCR would not have been with FC; the survival of these patients appears to be similar to other MRD− patients (ie, extremely good).25 In addition, the conversion of patients from MRD+ to MRD− with the use of alemtuzumab consolidation is associated with improvement in PFS for these patients. One of the key questions to be answered is whether we can identify a therapy that is relatively safe when used in consolidation, in which case we may consider this approach in all patients, or at least those who are MRD+ after initial therapy; until that time, another question is which patients (if any) should have consolidation with alemtuzumab. It is certainly possible to identify patients who will almost certainly relapse within 1 or 2 years of completing therapy, such as patients who had a short initial remission and are now in a second remission or those who are MRD+ after therapy, particularly those who do not have good-risk disease. It appears that using alemtuzumab consolidation in patients with unmutated immunoglobulin genes who are MRD+ at the end of therapy may be appropriate, because these patients will relapse early and may have evolved a p53 pathway abnormality.
Selection of therapy according to biology
These new insights into the pathophysiology of CLL have changed the way CLL is treated and promise to completely revolutionize our approach to the treatment of CLL in the near future. Tailoring of CLL therapy according to the biology of an individual patient and individual disease is already upon us. For example, the combination of FCR is now the standard therapy for CLL, showing an improvement in OS compared with FC, which was the previous “gold standard.” However, there are already patients for whom FCR may not be the most appropriate therapy. Patients with abnormalities of the p53 pathway, usually identified by deletion of chromosome 17p by FISH, have a very poor response to FCR (5% complete remissions in 17p-deleted CLL compared with nearly 50% for those patients without 17p deletion). In contrast, when therapies are used that do not require an intact p53 pathway, such as mAbs or high-dose steroids, then better responses are seem. For example, alemtuzumab is the single agent with the highest efficacy in 17p-deleted CLL, and in a recent trial, the small cohort of patients with 17p deletion had a 24% complete remission rate to alemtuzumab monotherapy.26 Recently, the combination of alemtuzumab with high-dose steroids has been reported to be even more effective in 17p-deleted CLL. The combination of alemtuzumab with high-dose methyl prednisolone (CamPred)27 resulted in all 17 previously untreated patients with 17p deletion responding and 65% of them achieving a complete remission. In contrast, the complete remission rate in previously treated patients with 17p deletion was only 14%, indicating that patients should receive targeted therapy as their first treatment rather than after they have failed conventional chemotherapy-based treatments. A second recent trial combined alemtuzumab with oral dexamethasone (CamDex),28 which showed promising activity but not quite as good as that for CamPred, although the patient groups were slightly different. Although FCR might be considered effective cytoreductive therapy in patients with 17p-deleted CLL before allogeneic stem cell transplantation because 68% of patients will respond, transplantation is only appropriate for a minority of younger, fitter patients. The combination of mAbs with corticosteroids appears to be more effective cytoreductive therapy and should be considered for patients with deletion of chromosome 17p, particularly because only a few patients are likely to be candidates for transplantation. It is therefore extremely important that all patients are tested by FISH for 17p deletion before they receive any line of therapy, because such a finding will alter the treatment offered.
Identifying the “achilles' heel” of CLL
Some of the major advances in the therapy of malignancy, and in particular hematological malignancy, have arisen after the identification of key disease mechanisms specific for the underlying malignancy and not present in normal cells. The classic example, of course, is the development of specific tyrosine kinase inhibitors such as imatinib in chronic myeloid leukemia. The recent progress in our understanding of CLL, particularly in the central importance of a proliferative signal through the BCR and of the interaction between the CLL cell and its microenvironment promises to open the door on a series of potential targets, both the microenvironment, its interaction with CLL cells, and the downstream signaling from the BCR.
Microenvironment interactions
The interaction with the microenvironment is critical to maintaining the CLL cell's cycle and, therefore, interfering with this interaction would be a rational therapeutic approach. It is likely that at least some of the activity of lenalidomide is due to directly modifying this interaction. In addition, there are ongoing studies examining the role of the CXCR4 chemokine receptor inhibitor plerixafor in interfering with the interaction between the CLL cell and its microenvironment.
BCR downstream signaling
There are multiple receptors and their ligands that participate in the proliferative signal generated by the interaction between the CLL cell and the microenvironment. It appears that the role of the BCR is central to this interaction. Therefore, targeting the BCR and its downstream signaling is an attractive therapeutic avenue. There are several molecules that are important in this pathway, including Syk, Akt, Btk, PI3Kδ, and ZAP-70. The initial reports targeted Syk with, for example, fostamatinib, which resulted in responses in over half of relapsed, refractory patients with CLL.29 The effect of inhibitors of the BCR pathway on these targets appears dramatic and similar; the most promising at present, CAL-101 (a PI3Kδ inhibitor)30,31 and PCI-32765 (a Btk inhibitor),32 have both recently been described to have effects as oral agents in phase 1 trials in chemorefractory CLL. In both cases, there is a rapid and often dramatic reduction or even resolution of lymphadenopathy within the first few months of therapy but coinciding with an increase in lymphocytosis. After continuous treatment for 6 or more months, the lymphocytosis usually resolves. This pattern of response suggests that the proliferative compartment in the tissues has been disrupted, as would be expected knowing the mode of action of these agents, and that the CLL cells have been pushed into the periphery. Over the next few months, because the proliferative fraction appears to have been disrupted, the lymphocytosis improves. These studies were in relapsed, refractory CLL, and it might be anticipated that these agents would be more effective earlier in the disease course or in combination with other active agents, such as mAbs.
Bcl-2 antagonists
CLL cells almost universally have high expression of the antiapoptotic molecule Bcl-2, although without the classical translocation seen in follicular lymphoma. Therefore, Bcl-2 is a sensible target in CLL, and a variety of agents, such as antisense technology and small molecules, have been developed to inhibit this target. The latest showing some promise is navitoclax (ABT-263).
CDK inhibitors
Flavopiridol showed some promise as one of the cyclin-dependent kinase (CDK) inhibitors. However, there have been difficulties in identifying an acceptable schedule of administration and issues of toxicity that might ultimately limit the use of this agent. There are now second-generation CDK inhibitors in development.
Conclusion
Currently, the identification of p53 pathway abnormalities, principally with the identification of deletions of the p53 locus on the short arm of chromosome 17 using interphase FISH, is being used to modify therapy. Patients with p53 abnormalities have very poor responses to conventional chemotherapy, because these agents largely require an intact p53 response to be effective, and should therefore be avoided. Therapies that do not depend on this pathway appear to be more effective, particularly when used as first-line treatments, and these include mAbs and high doses of corticosteroids. In addition, the use of MRD eradication as an end point of therapy promises to further improve treatment outcomes in CLL.
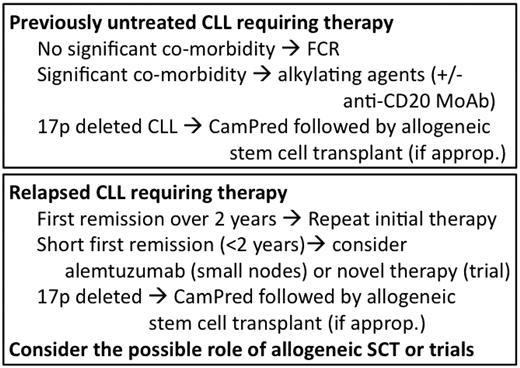
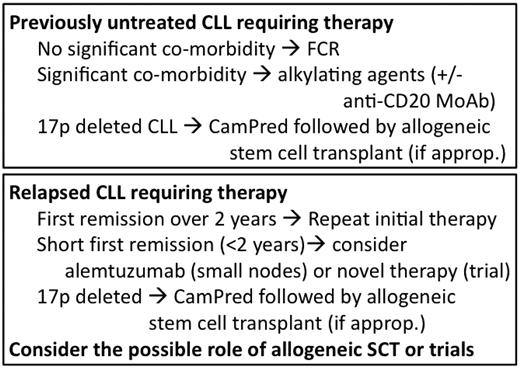
The recent development of inhibitors of B-cell signal transduction, agents that interfere with the CLL microenvironment (some that induce apoptosis), and novel mAbs promise in the relatively near future to revolutionize our therapeutic approach to CLL. It is conceivable that, in the not-to-distant future, many patients may be treated for CLL with relatively nontoxic combinations that do not include cytotoxic chemotherapeutic agents. The era of targeted therapy in CLL is already upon us and promises to lead to a paradigm shift in our approach to treating the disease. My schema for the treatment of CLL is shown in Figure 3.
Broad schema of how I treat CLL (assuming that there are no available trials).
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Roche Pharmaceuticals and Genzyme; honoraria from GlaxoSmithKline, Mundipharma, and Roche Pharmaceuticals; and has been affiliated with the speakers' bureaus for Mundipharma, GlaxoSmithKline, and Roche Pharmaceuticals. Off-label drug use: Novel therapies in trials for CLL such as CAL-101 and btk inhibitors.
Correspondence
Peter Hillmen, Professor of Haematology, St James's Institute of Oncology, Level 3, Bexley Wing, St James's University Hospital, Beckett Street, Leeds, LS9 7TF, United Kingdom; Phone: 44-113-206-8513; Fax: 44-113-206-8177; e-mail: peter.hillmen@nhs.net.