Abstract
Survival rates for children with acute lymphoblastic leukemia (ALL) have increased dramatically over the past 4 decades, with 5-year survival rates of > 90% in recent trials. With the increasing number of children and adolescents cured of ALL, identifying and characterizing the occurrence of long-term adverse late effects has become increasingly important. In this young population, successful treatment of ALL is associated with increased risk of adverse outcomes such as late mortality, second neoplasms, chronic health conditions, endocrine dysfunction, and psychological function. Research efforts conducted through large survivor cohorts, such as the Childhood Cancer Survivor Study, are providing new and important insights into the very long-term consequences of ALL therapy, while providing direction for screening recommendations and intervention-based approaches for reducing late morbidity and mortality.
Introduction
Annually in the United States there are an estimated 14 382 newly diagnosed cases of cancer among the population under the age of 20 years. Approximately 2970 (21%) of these cases are acute lymphoblastic leukemia (ALL). The annual US incidence rate for ALL under the age of 20 years is 35.0 per million population, with males having a higher incidence than females.1 There is a marked difference in the occurrence among white and black children, with white children having an almost 2-fold greater incidence. The age-specific incidence for childhood ALL is characterized by a peak between the ages of 2 and 5 years. Internationally, there is considerable variation in the in the incidence of childhood and adolescent ALL, with annual rates ranging from 9-47 per million for males and from 7-43 per million for females.2
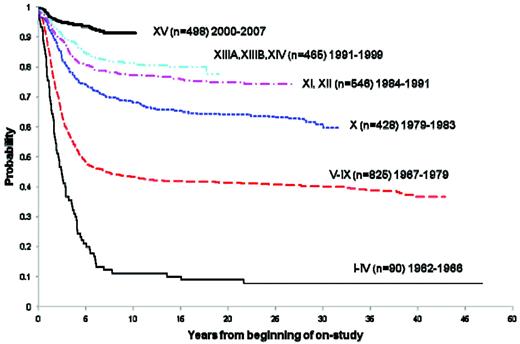
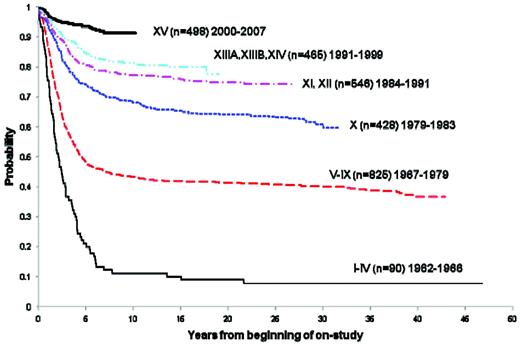
Over the past 4 decades, there has been a striking improvement in the 5-year survival rates among childhood cancer patients.3 Childhood ALL reflects one of the diagnoses for which the most impressive improvements have been realized. At St Jude Children's Research Hospital, the long-term survival has increased from approximately 10% in the early- to mid-1960s to more than 90% today (Figure 1). Estimates derived from 2005 from the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute placed the number of survivors of childhood ALL in the United States at 49 271.4
Overall survival of childhood ALL patients treated at St. Jude Children's Research Hospital trials between 1962 and 2007.
Overall survival of childhood ALL patients treated at St. Jude Children's Research Hospital trials between 1962 and 2007.
The use of combination chemotherapy was responsible for the increasing number of long-term survivors beginning in the 1960s. With the recognition that CNS relapse was common among children in bone marrow remission, presymptomatic CNS radiation and intrathecal chemotherapy were introduced into the treatment of childhood ALL.5 During the 1970s and early 1980s, additional treatment strategies were adopted to continue this improvement in survival, including the addition of other agents such as anthracyclines and alkylating agents, along with incorporation of a delayed intensification phase of chemotherapy. Subsequently, with the emergence of adverse late effects, clinical investigations addressed the potential reduction in intensity and duration of therapy, including approaches that produced sustained CNS remissions without the use of cranial radiation.6–9
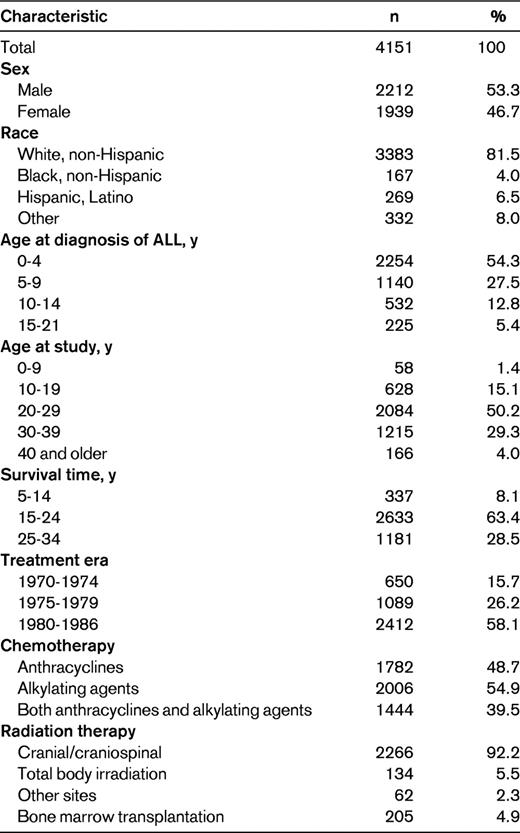
With the success in treating childhood ALL came the responsibility to investigate the long-term morbidity and mortality associated with the treatments responsible for this increase in survival. Early efforts to describe the late effects of pediatric and adolescent ALL and the associated treatments were largely conducted through single-institution and limited consortia studies, and occasionally within the cooperative clinical trials groups. Single-institution investigations provided many of the initial observations on those sequelae with relatively high frequency or those associated with severe morbidity. However, by the mid-1980s, it became increasingly clear that these approaches had inherent limitations, including small sample size, convenience sampling, incompletely characterized populations, and limited length of follow-up. Most importantly, deficiencies in follow-up may obscure the true frequency and nature of the late effects of therapy. To overcome these limitations and to augment ongoing and future research efforts, the Childhood Cancer Survivor Study (CCSS) was initiated to establish and follow a large cohort of 5-year survivors of childhood cancer, including a large population of survivors of childhood ALL.10,11 In 2008, Mody et al published the results of a 25-year follow-up of ALL survivors in the CCSS cohort.12 This chapter summarizes expanded findings from the CCSS regarding a broad spectrum of long-term outcomes among 5-year survivors of ALL diagnosed and treated from 1970-1986 (Table 1).
Late mortality
Among the 5-year survivors of ALL eligible for the cohort (n = 5760), there were 730 confirmed deaths, representing a standardized mortality ratio of 9.6 (95% confidence interval [95% CI]: 9.4-10.7).13 The overall survival rate at 25 years from initial diagnosis was 86.8% (95% CI: 85.9-97.8).12 Recurrent ALL accounted for 66% of deaths, whereas 12% died of second neoplasms and 2% from cardiac causes. Although recurrence was the most common cause of death among 5-year survivors of all forms of childhood and adolescent cancer, the mortality rate from recurrence among the ALL survivors markedly declined with extended follow-up: 1.32% at 5-9 years from initial diagnosis, 0.35% at 10-14 years, 0.11% at 15-19 years, and 0.04% at 20-24 years. Among 5-year ALL survivors, the cumulative rate of recurrence observed between 10 and 15 years from initial diagnosis was negligible at 0.8%. Therefore, ALL patients who survive 10 or more years from initial diagnosis have a remarkably low risk of recurrence and death due to their initial ALL, and with the rare exception, are cured.
Late mortality among ALL survivors differed significantly for those treated with radiation (87.3%) compared with those who did not receive radiation as part of their treatment for ALL (96.1%). The CCSS results are similar to those reported from the Nordic countries,14 but are in contrast to those more recently reported by the British CCSS for ALL patients diagnosed and treated between 1940 and 1991, in whom a standardized mortality ratio of 21.5 (95% CI: 20.0-23.0) was found for this older cohort of childhood ALL survivors.15
Second neoplasms
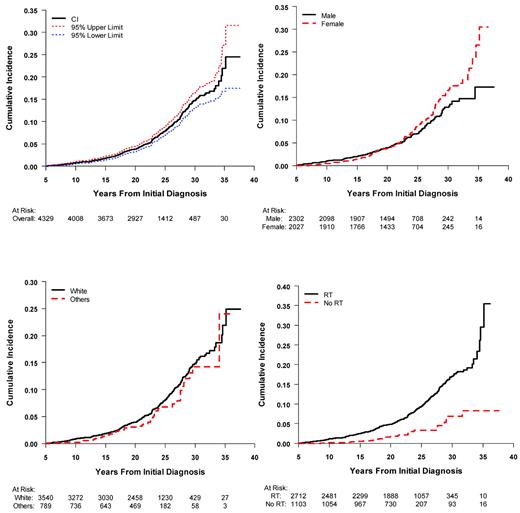
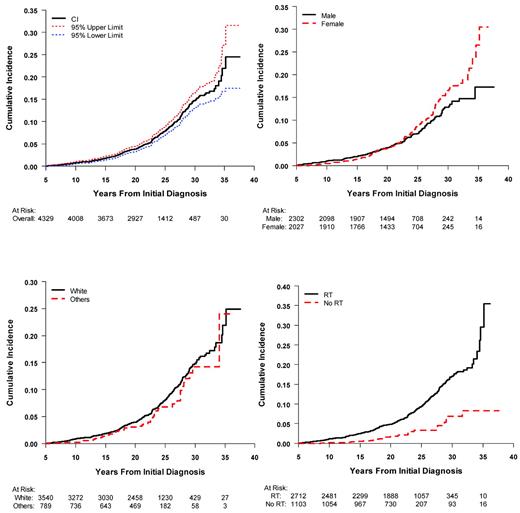
For more than 4 decades, it has been well-recognized that survivors of pediatric and adolescent ALL are at increased risk of developing subsequent neoplasms.16–19 Second and subsequent neoplasms are predominantly in the skin (43%) and CNS (31%). The majority of secondary skin cancers are basal cell carcinoma, whereas 70% of the CNS neoplasms consist of meningioma. Beyond skin and CNS, there are an increasing number of solid tumors consisting of breast, bone, soft tissue, and thyroid malignancies. Results from cohorts with long-term follow-up are now providing a more detailed description of the incidence and risk factors for second and subsequent malignancies.12,20–22 Provided in Figure 2 are recent data from the CCSS cohort of ALL survivors for the cumulative incidence of second neoplasms, in which the estimated cumulative incidence at 30 years is 14.6% (95% CI: 12.9-16.4). Whereas the risk of second neoplasms in ALL survivors is strongly associated with radiation therapy, they are also observed among nonirradiated survivors (Figure 2). With the elimination of cranial radiation as standard therapy for ALL, it is anticipated that the occurrence of second neoplasms will markedly decline in future cohorts of ALL survivors. Because of these risks, recommendations are made for enhanced screening for breast and skin cancer among specific exposed populations (www.childrensoncologygroup.org/disc/le/), which may include ALL survivors.
Cumulative incidence of second neoplasms among 5-year survivors of childhood ALL in the CCSS cohort. Shown are the overall incidence and 95% confidence limits (top left panel); by sex (top right panel); by race (bottom left panel); and by radiation treatment (bottom right panel).
Cumulative incidence of second neoplasms among 5-year survivors of childhood ALL in the CCSS cohort. Shown are the overall incidence and 95% confidence limits (top left panel); by sex (top right panel); by race (bottom left panel); and by radiation treatment (bottom right panel).
Chronic medical conditions
With longer follow-up from ALL diagnosis, survivors are at increasing risk for the development of a wide range of chronic medical problems, including neurologic, neurosensory, endocrine, pulmonary, cardiac, musculoskeletal, neoplasia, gastrointestinal, and genitourinary conditions.23 These chronic health conditions can, to varying degrees, be associated with increased morbidity and mortality. Applying the Common Terminology Criteria for Adverse Events (version 3) from the National Cancer Institute, it was determined that 50% of the ALL survivors followed in the CCSS cohort reported one or more chronic medical conditions.12 This incidence is statistically significantly higher than the rate of 38% reported by siblings. Compared with siblings, ALL survivors were 3.7 times more likely to report a severe or life-threatening (grade 3 or 4) chronic health condition (95% CI: 3.0-4.5) and 2.8 times (95% CI: 2.4-3.2) more likely to report multiple chronic health conditions. ALL survivors were at highest risk for developing musculoskeletal, cardiac and neurological conditions, with relative risks of 7.7, 6.9, and 5.3, respectively. The 25-year cumulative incidence of any chronic health condition was 64.9%. When considering chronic health conditions graded as severe or life-threatening, the cumulative incidence at 25 years was 21.3% (95% CI: 18.2-24.4). Radiation therapy, for the treatment or prevention of CNS leukemia was significantly associated with a 4.1-fold increased risk of an endocrinologic chronic health condition and a 1.7-fold increased risk for having a grade 3 or 4 (severe or life-threatening) chronic health condition. Moreover, when considering self-reported health status, exposure to radiation therapy was associated with a 2.2-fold increased risk of severe functional impairment. For survivors of all forms of childhood and adolescent cancers, long-term risk of serious chronic health conditions was significantly associated with radiation therapy, surgery, and chemotherapy, particularly exposure to alkylating agents and anthracyclines. Although combinations of these therapeutic exposures were not assessed specifically within the ALL survivor population, it is anticipated that comparable levels of risks would apply.
Treatment of ALL, particularly glucocorticoid therapy and radiation, is known to be associated with an increased occurrence of osteonecrosis. Among long-term survivors of childhood and adolescent ALL, the cumulative incidence of osteonecrosis at 20 years has been found to be low (0.43%), but significantly higher than expected (6.2-fold higher than that observed in siblings). The risk of osteonecrosis is highest in long-term survivors of ALL who received a stem-cell transplant.24
Obesity
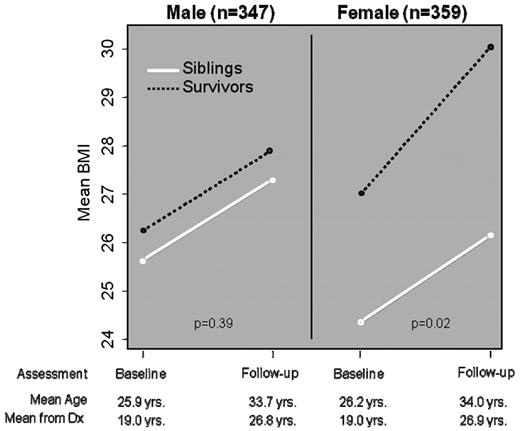
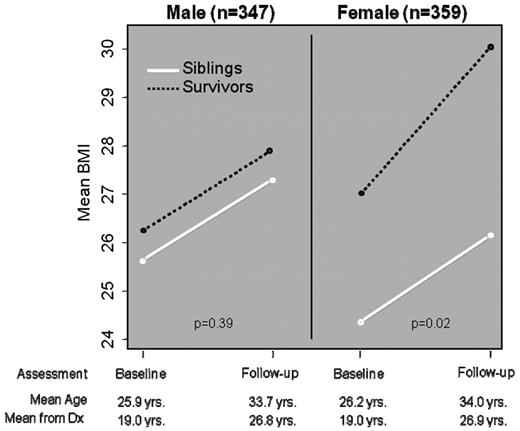
Increased adiposity is a significant public health problem in the general population. Survivors of childhood ALL treated with cranial radiation have been found to be at substantially increased risk of obesity.25 In the original report from the CCSS cohort, it was found that ALL survivors treated with > 20 Gy cranial radiation were significantly more likely to be obese (BMI > 30) compared with siblings. Whereas this was true for both male and female ALL survivors, it was found that the risk was higher among females (odds ratio of 2.59, P < .001) than in males (odds ratio of 1.86, P < .001). Beyond the risk associated with cranial radiation dose, it was also found that younger age at cranial radiation was a significant risk factor, with female ALL survivors treated with > 20 Gy cranial radiation before the age of 4 years having a 3.8-fold increased risk (95% CI: 2.3-6.0) of obesity as an adult. Subsequent follow-up of the ALL survivors demonstrated the increased risk of obesity continued for both males and females, but the proportion of obese female survivors increased at a greater pace (Figure 3). Among those females treated with > 20 Gy cranial radiation, the mean BMI was 29.3 (SD 7.3), with 40% being obese as defined by a BMI of > 30.26 To further define the risk profile for obesity among childhood ALL survivors, CCSS investigators assessed the potential role of the leptin receptor gene (Gln223Arg). Female ALL survivors who were overweight or obese were significantly more likely to be homozygous for the arginine allele, and a statistically significant interaction was found between genotype and radiation exposure.27
Mean BMI among 706 long-term survivors of childhood ALL treated with ≥ 20 Gy cranial radiation. (Adapted from Garmey et al.26 )
Mean BMI among 706 long-term survivors of childhood ALL treated with ≥ 20 Gy cranial radiation. (Adapted from Garmey et al.26 )
Social and psychological outcomes
The diagnosis and treatment of ALL during childhood has the potential to disrupt the social development, emotional health, and academic progress of survivors. Investigations of survivors of childhood ALL have documented that diagnosis and treatment can increase the risk for adverse psychological outcomes including lower cognitive functioning, executive function, depression and somatic distress.28–30 CNS-directed therapy, including radiation and/or chemotherapy, for the prevention or treatment of CNS leukemia is well-recognized to be associated with decreased cognitive function, and particularly vulnerable are the youngest patients (eg, those of preschool ages).31 In the CCSS cohort, treatment-related impairment resulted in decreased educational attainment and greater utilization of special education services.32 It was found that those ALL survivors who were provided with special educational services had comparable educational attainment to siblings, whereas those not reporting use of special education had lower educational attainment. With extended follow-up, ALL survivors have been found to have a higher frequency of impairment in task efficiency (P < .001), memory (P = .004), and emotional regulation (P < .001), as well as lower executive functioning.30 Survivors of childhood ALL have also been extensively evaluated relative to marriage, employment, education, and access to health insurance.12,29,33–35 Within the CCSS cohort, leukemia survivors were significantly more likely to report being unemployed compared with siblings (relative risk 1.26, 95% CI: 1.11-1.41). Moreover, analyses stratified for sex, rates of college graduation, marriage, and having health insurance were significantly lower among ALL survivors than their sibling counterparts. Lower rates of marriage, educational attainment, and having health insurance are associated with history of cranial radiation.
Summary
With > 90% of childhood ALL patients achieving survival beyond 5 years, efforts need to be focused not only on innovative approaches for treating and curing those who continue to have a poor prognosis, but also on further refinement of ALL treatments with the objective of maintaining a high cure rate but minimizing long-term adverse effects. The population of more than 50 000 survivors of childhood ALL in the United States will continue to grow and age. Long-term surveillance of this population is important to better understand the incidence of late-occurring events and defining high-risk features. Moreover, long-term follow-up efforts should integrate intervention strategies for early detection and prevention. The Children's Oncology Group has developed comprehensive guidelines for clinical follow-up on survivors of cancer diagnosed and treated during the pediatric and adolescent period.36 The guidelines (www.childrensoncologygroup.org/disc/le/pdf/) use the available literature to formulate exposure-based recommendations that are the clinical consensus of experts in the field. With continued research, it is anticipated that a larger proportion of recommendations will transition to evidence-based recommendations.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
L. L. Robison, Department of Epidemiology and Cancer Control, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; phone: (901) 595-5817; fax: (901) 595-5845; e-mail: les.robison@stjude.org.