Abstract
A major breakthrough in the treatment of acute lymphoblastic leukemia (ALL) was the availability of targeted therapies targeting either specific transcripts, such as bcr-abl fusion protein by tyrosine kinase inhibitors (TKIs), or specific antigens by mAbs. ALL blast cells express a variety of specific antigens (eg, CD19, CD20, CD22, CD33, and CD52) that serve as targets for mAbs. To date, the most data are available for anti-CD20 (rituximab), which has been combined with chemotherapy for the treatment of mature B-ALL/Burkitt lymphoma. Studies with rituximab have also been completed in B-precursor ALL. Another antigen, CD19, is of great interest due to a very high rate of expression in ALL. It can be targeted by a bispecific mAb, blinatumomab, directed against CD19 and CD3. Smaller studies or case reports are also available for the anti-CD52 (alemtuzumab), anti-CD22 (epratuzumab), and anti-CD33 (gemtuzumab) mAbs. Available data demonstrate that mAb therapy in ALL is a highly promising treatment approach. However, several details for an optimal treatment approach, such as the required level of antigen expression, timing, schedule, dosage, and stage of disease, still need to be defined.
Introduction
Treatment outcome of adult acute lymphoblastic leukemia (ALL) has been substantially improved in the last decade mainly by the intensification and optimization of chemotherapy, the risk-adapted use of stem-cell transplantation, and improved supportive care.1,2,3 Individualized treatment strategies based on prognostic factors, including the evaluation and monitoring of minimal residual disease (MRD), have contributed to improved outcomes. However, results in adult patients are still considerably inferior to those in pediatric ALL patients, and treatment-related toxicity is a barrier to further intensification of standard chemotherapy, particularly in older patients. Recently, significant progress has been achieved by individualized and targeted therapy, particularly treatment with mAbs. Therapy with mAbs is not only targeted, but is also subtype-specific and compared with chemotherapy has different mechanisms of action and side effects. mAbs can be administered (1) in an unconjugated form (eg, rituximab); (2) conjugated to immunotoxins or chemotherapeutic agents, which are delivered to the target cell by the antibody (eg, gemtuzumab); (3) conjugated to radioactive molecules, which deliver radiation selectively to malignant cells; or (4) as bispecific antibodies, which are directed to 2 target antigens or recruit immunologically active cells to the leukemia blasts. Further, the synergistic effect of combined antibody and chemotherapy can also be used. This article reviews mAb therapy in depth based on the available clinical experience in ALL.
Expression of surface antigens in ALL
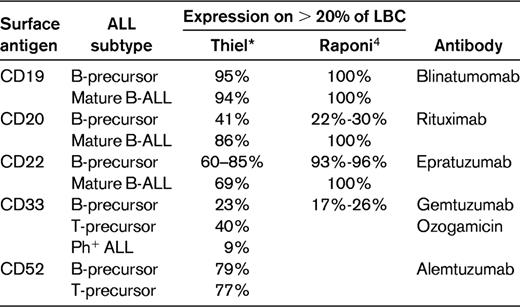
ALL blast cells express a variety of lineage-specific antigens, and combinations of antigens are used for establishing the diagnosis and defining immunological subtypes. A prerequisite for mAb therapy was generally the presence of the target antigen on at least 20% of the leukemic blasts, but this cutoff point is not used by all investigators. Table 1 depicts the surface antigen expression with a cutoff of > 20% positive leukemia blast cells according to ALL subtypes. The results are from 2 large adult ALL series, including more than 500 patients each for the most relevant surface antigens and the mAbs directed against them.
It is presumed that the response to a mAb is correlated with the percentage of ALL cells expressing a specific antigen. There is some evidence that the activity of antibodies depends on the amount of antigen expressed on the cell surface. Antigen expression on individual cells is of interest, although little data are available so far.2,4 The degree of antigen expression measured by mean fluorescence intensity (MFI) and/or the antibody capacity (ABC) very recently analyzed by Raponi et al4 might influence the treatment outcome. Additionally B- or T-lineage ALL subtypes during different maturation stages have an individual antigen expression pattern and thus respond differentially to mAb therapy.
Because target antigens are not expressed exclusively on malignant cells, but also on the surface of normal hematopoietic cells, the cytotoxic effects are less selective and lead to mAb-specific side effects such as profound B- or T-cell lymphopenia, with related clinical consequences, particularly infections.
CD20 antigen
CD20 is a 33- to 37-kDa, nonglycosylated B lymphocyte–specific integral membrane phosphoprotein. The function of CD20 has not been clarified in detail, but it seems involved in the regulation of transmembrane calcium conductance.5 CD20 is only very slowly internalized.
CD20 is expressed on normal and malignant B lymphocytes, but not on normal stem cells. Rituximab is a chimeric human/mouse mAb to CD20. From in vitro studies, various mechanisms of action have been postulated, including complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis.6 The rationale for testing the addition of rituximab to chemotherapy for ALL was an increase in overall survival (OS) by ≥ 20% in studies of high-grade nonHodgkin lymphoma (NHL).7 CD20 is found on ∼ 30%-40% of B-precursor ALL blasts, and its expression is more common in older adults with B-precursor ALL (40%-50%). CD20 is expressed in the majority of mature B-ALL blast cells (80%-100%).
Prognostic impact of CD20 expression
The prognostic impact of CD20 expression in B-precursor ALL is controversial (Table 2). In the largest series of 253 adult B-lineage ALL patients from the M.D. Anderson Cancer Center,8 which included 52 Philadelphia chromosome–positive (Ph+) ALL cases, the complete remission duration (CRD) and the OS at 3 years were inferior for CD20+ ALL at 20% and 27%, respectively, compared with a CRD of 55% and an OS of 40% for the CD20− ALL group. The outcome was particularly favorable for the CD20− younger age group. In contrast, another recent series of 143 patients 15-60 years of age showed no significant difference between the 97 CD20− ALL patients, with a complete remission (CR) rate, event-free survival (EFS), and OS of 92%, 54%, and 59%, respectively, compared with the 46 CD20+ ALL cases at 89%, 47%, and 55%.9 However, there was a negative prognostic value of CD20 expression within the high-risk (HR) patients (WBC ≥ 30 × 109/L) due to a higher cumulative incidence of relapse (Table 2). Similarly, in a study with 169 children,10 CD20 expression was not associated with inferior outcome; EFS was 84% in CD20+ compared with 78% for CD20− ALL.
These data indicate that the prognostic impact of CD20 expression in B-precursor ALL may be age related, with no impact in children due to a lower percentage of HR patients, but with an adverse influence with increasing age. The inferior outcome of elderly (50-60 years of age) CD20+ ALL patients receiving rituximab supports this idea (discussed in “Rituximab in older adults with CD20+ B-precursor ALL”).
In addition, like other prognostic factors, a potential adverse factor such as CD20 expression could lose its impact with better treatment outcome, particularly in children, as shown by earlier reports of inferior EFS for CD20+ ALL. This assumption is supported by a recent study that allogeneic hematopoietic stem cell transplantation overcomes the prognostic impact of CD20 expression in children and in adults with ALL.11 In this cohort of 125 patients, there was no association among the factors of age, Ph+, WBC count at diagnosis, and CD20+.
Up-regulation of CD20 expression
A new observation may lead to an extended application of rituximab in ALL. Dworzak12 found CD20 up-regulation in children with B-cell-precursor ALL during induction treatment, most likely due to corticosteroids. This observation was confirmed in another childhood ALL study.13 Dworzak et al confirmed that drug-induced modulation rather that clonal selection causes this phenomenon, and that up-regulation of CD20 is more persistent than that for other antigen expressions (eg, for CD45).14
Whether this modulation of antigen expression in B-cell-precursor leukemias (common and pre-B-ALL) will provide a benefit for patients with < 20% CD20 expression at diagnosis has to be evaluated in ongoing studies.
Rituximab in mature B-ALL and Burkitt NHL
The outcome of mature B-ALL and Burkitt NHL has been improved substantially with short, intensive chemotherapy regimens derived from pediatric studies. The OS achieved with a variety of protocols was 50%-70%. In adult patients, further treatment intensifications by increased dose intensity (eg, of methotrexate) were limited. Therefore, several study groups combined rituximab with chemotherapy (Table 3). The GMALL (German Multicentre Study Group for Adult ALL) study group initiated protocol B-ALL/NHL-2002, which uses rituximab in combination with chemotherapy. The regimen includes 6 5-day chemotherapy cycles based on previous GMALL studies.15 The major new feature was that rituximab was administered at a dose of 375 mg/m2 on day −1 before each chemotherapy cycle and thereafter twice for consolidation in monthly intervals for a total of 8 applications.
In a pilot study in 2003, 82 patients from 39 centers entered the protocol. Of 53 evaluable patients, the CR rate in mature B-ALL was 91% and in Burkitt NHL 96%; the OS was 70% and 80%, respectively. Rituximab administration showed no excessive toxicity. Since that time, more than 227 patients have been included. The OS was 88% for Burkitt NHL and 70% for mature B-ALL, which is a considerable improvement over the previous trial.16
In a study by Cancer and Leukemia Group B (CALGB 9251), adult patients with Burkitt or Burkitt-like leukemia/lymphoma received a similar high-intensity chemotherapy and rituximab, intensified in cycle 2 and given for a total of 8 times.17 Of 105 enrolled patients, the 7 planned courses of therapy could be completed in 75; 82% achieved a CR and 87% of those remained in CR. There was a clear difference in outcome based on the International Prognostic Index score, with a 2-year EFS and OS for low-risk patients of 98% and 92%, respectively, compared with 55% and 55%, respectively, for HR patients.
In the M.D. Anderson Cancer Center study, rituximab was added to the hyper-CVAD regimen (cyclophosphamide/vincristine/Adriamycin/dexamethasone HDMTX/ HDAraC).18 Rituximab was given at the beginning and end of the first 4 chemotherapy cycles for a total of 8 doses. In 31 patients with newly diagnosed Burkitt NHL or mature B-ALL, 86% CRs were observed and the 3-year OS was 89%. The investigators observed a significant reduction in relapse rate and an improvement in outcome, particularly in elderly patients. There was apparently no additional toxicity compared with the previous protocol with chemotherapy only. In the above 2 studies, the improvement in OS was approximately 30%: 88%/89% compared with 50%/53%.
Rituximab was also added to the CODOX-M/IVAC regimen for a total of 4 doses.9 In the 40 patients in the rituximab cohort (R+), the CR rate was 90% compared with 81% in the 47 R− patients. The PFS and OS in the R+ arm was 70% and73%, respectively, compared with 61% and 68% in the R− arm. The outcome for the R+ patients was not statistically significant superior to the R− patients, and it was discussed whether more frequent dosing of rituximab might provide the optimal benefit. When the GMALL immunochemotherapy approach was applied in HIV+ Burkitt NHL and combined with antiretroviral HAART therapy, the OS improved to 77%.20 Overall, despite the one negative study (in which the addition of rituximab did not result in inferior results), it appears that rituximab added to a short, intensive chemotherapy regimen has substantially increased the survival rate of adult patients with mature ALL/Burkitt NHL, as well as in HIV+ patients. The feasibility of rituximab was also explored recently in newly diagnosed pediatric patients with mature B-cell NHL and Burkitt leukemia.23
Rituximab in younger patients with CD20+ B-precursor ALL
The effect of rituximab with a chemotherapy induction and consolidation therapy was studied in CD20+ Ph/Bcr-abl− B-precursor ALL in the GMALL Study 07/2003.24 Adult standard-risk (SR) ALL patients (age, 15-55 years) with CD20+ B-precursor ALL (41% antigen expression of > 20%) received 375 mg/m2 rituximab at day −1 before each induction course (phase I and II) and before each of the 6 consolidation courses, for a total of 8 doses. The aim was to reduce MRD load and thereby the relapse rate. HR patients were candidates for a stem cell transplantation (SCT) in CR 1 and received rituximab 3 times (on day −1 of induction) in an attempt to reduce tumor load before SCT. SR was defined in this setting as WBC count < 30 000/μL, achievement of CR for ≤ 4 weeks, and the absence of the cytogenetic aberrations t(4;11), t(9;22). HR patients were those having one or more of these factors.25 t(9;22) patients were treated differently.
A total of 185 CD20+ ALL patients were included in this study; 133 were SR and 52 HR patients. 117 received rituximab (R+ arm) and were compared with 70 patients recruited earlier without rituximab (R− arm), but who had identical chemotherapy and supportive therapy. In SR patients, there was no difference in the CR rate of 94% and 93%, respectively, in the R+ compared with the R− arm, or in early death rates of 5% and 4% or failure/PR 1% and 2%, respectively. However, the MRD course differed substantially. The decrease in MRD load in the R+ compared with the R− arm was faster, with a molecular CR (MRD < 10−4) at day 21 of 60% versus 19% and at week 16 of 89% versus 57%. The probability for continuous CR at 3 years was correspondingly higher, at P = .64 versus P = .48, for R+ compared with R− patients (P = .009) and for OS P = .75 versus P = .54. For HR patients, the probability for OS at 3 years was P = .54 versus P = .32 in the R+ versus the R− group. In the 66% HR patients receiving a SCT in CR 1, the OS was superior for the R+ vs. R− arm at P = .75 versus P = .40 (nonsignificant) due to fewer relapses. There was no excess toxicity in R+ compared with the R− patients; death in CR 1 was 4% in R+ versus 3% in the R− arm.
It was concluded that intensive chemotherapy plus immunotherapy with rituximab is feasible in adult SR and HR B-precursor ALL patients. There was a faster and higher molecular CR rate in the rituximab arm with an improvement of continuous CR and OS. Therefore, rituximab plus chemotherapy can improve the outcome of younger adults with CD20+ B-precursor ALL.
The M.D. Anderson Cancer Center evaluated a modified hyper-CVAD regimen combined with rituximab in de novo Ph− B-precursor ALL when CD20 expression was ≥ 20%.26 In this study, younger (< 60 years) and older (> 60 years) patients were included. The CR rate was 95% in the younger (age < 60 years) CD20+ subset, the 3-year CRD was 70% in the modified R+ arm compared with 38% (P < .001) in the R− arm, and the OS was 75% compared with 47% (P = .003). Because CRD and OS for the CD20− counterparts were unchanged with the different hyper-CVAD modifications, it seems likely that rituximab was responsible for the CRD and OS improvement. In addition, in the CD20+ group, the percentage of patients with MRD− (measured by multiparameter flow cytometry) was higher at 81% compared with 58% without rituximab. The absence of MRD was also associated with significantly better 3-year CRD (82% R+ vs 24% R−, P = .002), but not OS (70% vs 27%, P = nonsignificant), which was in part related to deaths in CR for the MRD− group. The conclusion was that younger CD20+ B-precursor ALL patients benefit from chemoimmunotherapy with rituximab.
Rituximab in older adults with CD20+ B-precursor ALL
The outcome of older adults with ALL (differentially defined as > 50 years or > 60 years depending on the study) is still poor, with survival rates of approximately 10% in recent trials. Approximately 40%-50% of the patients with B-precursor ALL (common or pre-B-ALL) are CD20+, with an expression level > 20%. Because the options for chemotherapy intensification in older adults are limited, these patients might benefit from the addition of a noncytotoxic approach such as rituximab. In a GMALL study, CD20+ elderly ALL patients received rituximab before each cycle of a dose-reduced chemotherapy for a total of 8 applications. For an interim analysis, 26 patients were evaluable. The CR rate in CD20+ ALL patients was 63% and the OS after 1 year was 54%.16 The early mortality and relapse rate in this study was still considerably higher than in younger adults in both CD20+ and CD20− ALL patients. The risk of infections during induction therapy and in remission remains a major problem, although there was no clear evidence that this problem was aggravated by rituximab therapy. Therefore, in elderly patients with B-precursor ALL, the combination of chemotherapy and rituximab is feasible, but long-term results are awaited.
In the M.D. Anderson Cancer Center study,25 the older age group with 58 patients had a CR rate of 88% with a low early death rate; the 3-year CRD was 53% and the OS was 29%. Although the toxicity profile for CD20− and CD20+ ALL patients was similar, the number of deaths in CR in the CD20+ subset was higher and was predominantly related to infections with multidrug-resistant organisms in the older group during consolidation chemotherapy. Other causes of death in this subgroup included complications related to secondary myelodysplastic syndrome, cardiovascular events, or seizure-related anoxic encephalopathy. It was concluded that older patients did not benefit from rituximab-based chemoimmunotherapy.
Other study groups, such as the French GRAALL (Group for Research on Adult Acute Lymphoblastic Leukemia) or the British NCRI (United Kingdom National Cancer Research Institute) have randomized trials ongoing between rituximab and non-rituximab-continuing regimens in induction.
CD19 antigen
In nearly all B-precursor ALL patients, the CD19 antigen is expressed because it appears during the early stages of B-cell maturation and development. Therefore, CD19 appears to be an attractive target antigen. Several mAbs targeting the CD19 antigen have been developed, most of them conjugated to immunotoxins. These antibodies are being tested in early-phase clinical trials and some have demonstrated activity.1
In a CALGB study of 82 patients 17-82 years of age, 46 received a consolidation course with anti-B4–blocked ricin. It was generally well tolerated, but molecular monitoring before and after the experimental course of intensification did not show a consistent change in the number of leukemia cells remaining, and the immediate posttreatment PCR studies did not correlate with remission duration. It was concluded that anti-B4–blocked ricin is feasible, although with little evidence of an additional clinical benefit.27
Anti-CD19 (Blinatumomab)
Blinatumomab is a new bispecific, single-chain antibody construct specific for CD19 and CD3.28 It is designed to target CD19-expressing cells and to recruit CD3 cytotoxic T cells to lyse CD19-expressing B cells. Therefore, it combines 2 antigen-binding sites, one specific to T cells and the other one to the CD19+ leukemia blast cells, and it allows T cells to kill both resting and proliferating tumor cells.
Blinatumomab in relapsed B-cell NHLs
Blinatumomab was first explored in advanced refractory B-cell NHLs.28 The clinical activity and safety of increasing doses of this bispecific antibody was studied in 38 patients, who received blinatumomab at doses from 0.0005-0.06 mg/m2/d. A total of 11 major responses (4 CRs and 7 partial regressions) were observed. All occurred at doses of 0.015 mg/m2/d and higher, indicating a dose-response relationship. All 7 patients treated at the latest completed dose level of 0.06 mg/m2/d showed objective responses occurring within 4 weeks of treatment. Adverse events of blinatumomab treatment included pyrexia, lympho- and leukopenia, chills, increase of C-reactive protein, and CNS symptoms including disorientation, confusion, speech disorders, tremor, and convulsions, all of which were fully reversible.28
Blinatumomab in MRD+ adult ALL
Blinatumomab was first investigated in adult B-precursor ALL patients in hematological CR who had detectable MRD using clone-specific PCR methods to monitor the malignant clones (MRD+). This study included patients who had persistence of MRD despite clinical remission or patients who had molecular relapse after ≥ 3 cycles of chemotherapy.29 Blinatumomab was given at a dose of 50 μg/m2/d as a continuous infusion for 4 weeks, with a 2-week break between cycles. Patients achieving molecular CR received 3 consolidation cycles. Molecularly stable patients may have received 6 additional cycles in an attempt to achieve molecular CR. The primary aim of this study was to convert MRD+ by blinatumomab into a MRD− state using a quantitative PCR with a sensitivity of ≥ 1 × 10−4. Secondary end points were time to hematologic relapse and incidence of severity of adverse events (Goekbuget N, Brueggemann M, Arnold R, et al, unpublished data).
In this GMALL study, 21 patients were evaluable30 (Table 4). Of 14 BCR-abl−, MRD+ ALL patients, 12 became MRD−. This included some very high-risk patients; of 2 t(4;11) patients, 1 became MRD− and of 5 BCR-abl+ patients, 3 became MRD− (including 1 patient with a t315I abl kinase mutation). This accounts for an overall MRD response rate of 80%. Most patients showed a rapid response occurring within the first cycle of treatment. The probability for relapse-free survival is 78%, with a median follow-up of 405 days. Nonhematological toxicities such as infections and nervous system disorders (1 syncope/convulsion, 1 seizure, 1 headache, 1 somnolence) were rare,30 most likely due to the lower dose of blinatumomab.
Blinatumomab in refractory/relapsed ALL
Blinatumomab was also evaluated in 3 children with far-advanced B-precursor ALL and recurrence after allogeneic matched unrelated donor SCT. All children achieved a molecular remission after blinatumomab with tolerable toxicity.31 In an interim analysis of an ongoing study, 7 adult patients 18-77 years of age with relapsed/refractory B-precursor ALL received blinatumomab at 2 different dose levels, 15 or 5 μg/m2/d, for the first 7 days, followed by 15 μg/m2/d for week 2 in both groups. Four of 5 evaluable patients had a CR, 3 of whom were MRD−. Side effects, including cytokine release syndrome (1 patient) and a CNS event (1 patient), were manageable and reversible.32
Possible future strategies for blinatumomab include its use as monotherapy during consolidation or maintenance cycles, monotherapy for elderly frail patients, or in combination with chemotherapy.
CD52 antigen
CD52 is a cell-surface glycoprotein on most lymphoid cells of both T- and B-cell lineage, which may have a higher expression in more mature subtypes of B- or T-lineage ALL. CD52 antibodies have been first used for ex vivo T-cell depletion of allogeneic bone-marrow grafts to prevent GVHD without further GVHD prophylaxis, because CD52 is not expressed on hematopoietic stem cells.
Anti-CD52 (Alemtuzumab; Campath-1H)
Campath-1H is a genetically engineered, humanized, IgG1κ mAb that is specific for the 21- to 28-kDa lymphocyte surface glycoprotein CD52. The humanized antibody Campath-1H showed antitumor activity in CLL, T-PLL, and other T-NHLs. In a few cases, clinical effects were observed in patients with single-drug treatment in relapsed adult ALL.1
In CALGB 10102, 24 CR 1 patients received single-agent alemtuzumab33 as their fourth treatment module during a phase 1 portion of the study. The median age was 37 years (range, 18-77 years); 80% had precursor B-cell ALL; 19% had precursor T-cell ALL. Nonhematologic toxicities were mild. Grade 3-4 myelosuppression was reported during 4 weeks of alemtuzumab treatment in 4 patients; 2 had grade 3-4 lymphopenia. During subsequent postremission therapy, 8 patients developed CMV viremia, 2 had herpes simplex infections, and 3 had herpes zoster reactivation. Serial assessment of MRD using quantitative clone-specific PCR was possible in 11 of 24 cases. There was a median 1-log decrease in MRD during alemtuzumab therapy. The median follow-up time for the 14 surviving patients was 51 months (49-54 months). The median disease-free survival (DFS) was 53 months and the median OS was 55 months. Pharmacokinetic analysis revealed increasing alemtuzumab serum levels during treatment in all dose cohorts, and levels were still detectable in some patients 10 weeks after completing alemtuzumab. There was, however, no significant correlation between serum alemtuzumab level and change in MRD. The investigators concluded that because the DFS results were encouraging, they will be confirmed in an additional 70 patients in a phase 2 portion of this study.
In 2006 the M.D. Anderson Cancer Center reported using alemtuzumab to treat 6 patients with advanced ALL.34 Patients were included if they had 20% CD52+ leukemic blast cells and alemtuzumab was given 30 mg IV 3 times a week for a total of 4-12 weeks. Of the 6 ALL patients, including 3 with recurrent Ph+ disease, 4 were withdrawn early from treatment because they had progressive disease. Alemtuzumab was found to be myelosuppressive in all patients and infectious complications occurred in most of them. It was concluded that the combination of alemtuzumab with other active agents needs further investigation.
From these studies, it appears that alemtuzumab may have activity in both B- and T-cell precursor ALL. The optimal dose seems to be 30 mg IV 3 times per week for 4 weeks or, even better, up to 3 months. Although DFS in small selected cohorts might be encouraging, this has to be weighed against a high rate of infectious complications, despite adequate antiviral and antibacterial supportive therapy. Close follow-up of CMV viremia seems to be required. The definitive role of alemtuzumab in the treatment of adult ALL, including factors such as combinations, the setting, consolidation, and maintenance, is, however, as yet undefined.
CD22 antigen
CD22 antigen, a 135-kDa type I transmembrane sialoglycoprotein, is expressed specifically on B cells and is observed during B-cell development at low levels in the cytoplasm of pro- and pre-B cells and on the cell surface of mature cells with IgM and IgD positivity.35 It is not expressed on hematopoietic stem cells. The function of CD22 is not entirely clear, but it appears to be involved in the regulation of B-cell function and survival. CD22 is expressed in > 90% of cases of B-precursor ALL (Table 1).
Anti-CD22 (Epratuzumab)
Epratuzumab is a humanized anti-CD22 mAb that binds to the third extracellular domain of CD22. After binding, the receptor/antigen complex is internalized. Epratuzumab appears to modulate B-cell activation and signaling. Mechanisms of action include antibody-dependent cellular cytotoxicity, CD22 phosphorylation, and proliferation inhibition with cross-linking.36
Epratuzumab has been explored in one small study in pediatric ALL. In a Children's Oncology Group pilot study, a chemoimmunotherapy reinduction with epratuzumab was investigated in 15 children with relapsed ALL. Patients received 4 doses of epratuzumab, 360 mg/m2/dose IV twice weekly during the 14-day reinduction phase, followed by 4 weekly doses, 360 mg/m2/dose, administered with chemotherapy.36 Two patients died as a result of infections and 1 was removed from the protocol. Nine patients achieved a CR and 7 of these achieving a morphologic CR had also no detectable MRD. Of the 12 patients fully assessable for toxicity, acceptable grade 1 or 2 infusion reactions characterized by rigors, fever, and nausea were noted. Two patients experienced dose-limiting toxicity: one had a grade 4 seizure and the other experienced a grade 3 alanine transaminase elevation that resolved.
Epratuzumab was selected for this study because of high CD22 expression levels. The favorable rate of MRD− after administration of chemotherapy with epratuzumab suggested that the antibody may enhance response to a cytotoxic chemotherapy.
CD33 antigen
Gemtuzumab ozogamicin is a humanized anti-CD33 antibody that is conjugated to calicheamicin, an antitumor antibiotic. CD33 is mainly expressed in acute myeloid leukemia, but is also observed in ALL, with such cases termed ALL with myeloid markers, biphenotypic, or bilineage ALL. Several earlier case reports found responses to anti-CD33 in pediatric ALL and in a few cases of relapsed adult ALL (reviewed in Gökbuget and Hoelzer3 ). Patients responding to gemtuzumab had very high (> 90%) CD33 expression. It would have been of interest to further investigate this agent in CD33+ ALL, particularly in early subtypes such as pro-B-ALL or early T-ALL, which comprise a considerable proportion of cases with coexpression of myeloid antigens37,38 ; however, this drug is not currently available.
T-cell antibodies
In T-lineage ALL, antibody therapy has been far less intensively explored, which may be because immunotherapy was first developed for treatment of B-lineage NHL as a more frequent disease. In addition to alemtuzumab, immunotoxins directed to CD25, CD7, CD5, and CD3/CD7 have been tested in the treatment of cutaneous T-cell lymphoma, other T-cell lymphomas, and GVHD.39
Several antibodies bind the CD25 antigen (IL-2 receptor), which is often overexpressed on activated and malignant lymphocytes, particularly T lymphocytes. CD25 antibodies were conjugated to different toxins and showed activity in treatment of acute GVHD. One of these antibodies induced CRs in single patients with adult T-cell leukemia/lymphoma (reviewed in Gökbuget and Hoelzer1 ).
Other antibodies that have demonstrated activity in T-cell leukemias, either in vitro or in vivo, include anti-CD7-ricin, anti-CD7-PAP, anti-CD2, OKT3, and a humanized anti-CD3 mAb. Overall experience with mAbs in T-cell ALL—with the exception of anti-CD52 alemtuzumab—is scarce.
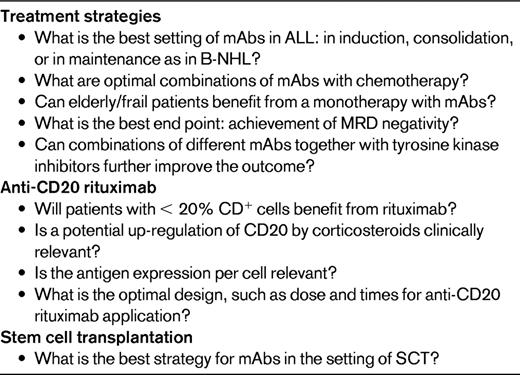
mAbs in adult ALL: challenges for future investigation
mAbs directed against specific surface antigens on ALL blast cells have successfully been explored in recent adult ALL studies; this is particularly true for anti-CD20 rituximab and anti-CD19/CD3 blinatumomab. However, mAb therapy in ALL is far from being evidence based or a routine treatment until ongoing randomized protocols mature (Table 5).
New mAbs
There is an increasing number of new mAbs that have been developed, many directed against CD20. One of these is GA101, an Fc-engineered, type II humanized IgG1 anti-CD20 antibody.40 Ofatumumab binds to a novel epitope of CD20 with greater avidity than rituximab.41 These mAbs and several others that have been developed recently are first being explored in NHL. If they have significant activity in that setting, they will then be tested in the treatment of ALL patients. Given the small numbers overall of adults and children with ALL, investigators will need to carefully select the most promising antibodies for clinical trial testing. Cooperative international trials of carefully defined ALL patient populations will be needed to explore their optimal value and to define their role in improving treatment outcome.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Prof. Dr. Dr. h.c. Dieter Hoelzer, ONKOLOGIKUM, Frankfurt am Museumsufer, Schaubstr. 16, D-60596 Frankfurt, Germany; Phone: +49(0)69-650073-170; Fax: +49(0)69-650073-180; e-mail: hoelzer@em.uni-frankfurt.de.