Abstract
Allogeneic hematopoietic stem cell transplantation (allogeneic HSCT) remains a curative treatment for hematological malignancies resistant to other treatment approaches through the unique GVL effect. However, relapse remains a major cause of treatment failure after allogeneic HSCT for patients with high-risk hematological malignancies. Further improvements in exploiting the GVL effect to prevent relapse in high-risk leukemias while minimizing toxicity have focused on the use of targeted antileukemic immunotherapy. These strategies include methods to boost the GVL effect with leukemia vaccines or the adoptive transfer of leukemia-specific lymphocytes. Vaccines can be classified as those against defined antigens such as minor histocompatibility antigens (mHags) or leukemia-associated antigens (PR1, WT1, and BCR-ABL) and those that have broad “antileukemic” activity such as engineered irradiated leukemia cells or leukemia-derived dendritic cells (DCs). The unique posttransplantation milieu, which is characterized by lymphopenia, regulatory T-cell depletion, and the release of growth factors, provides a unique opportunity for effective antitumor immunotherapy and augmenting specific GVL responses. This review focuses on approaches to enhancimg the GVL response by combining allogeneic HSCT with vaccination.
Introduction
Although important advances have been made in the treatment of hematological malignancies using chemotherapy, and more recently with targeted therapies such as tyrosine kinase inhibitors, curative treatments often require allogeneic hematopoietic stem cell transplantation (allogeneic HSCT). Unfortunately, even this intensive treatment fails to prevent relapse in 10%-60% of cases, depending on whether the disease was treated early or was refractory at the time of transplantation. The effectiveness of allogeneic HSCT for hematological malignancies is due primarily to immunologic recognition and elimination of recipient leukemia cells by donor T cells, the so-called GVL effect.1
Some of the antigens that drive this GVL response have been characterized and can be categorized broadly into 3 classes: (1) ubiquitously expressed alloantigens, also known as minor histocompatibility antigens (mHags), widely expressed by normal tissues in the recipient as well as by leukemia cells, and capable of initiating both GVHD and GVL responses2 ; (2) alloantigens expressed uniquely by cells of the hematopoietic system (tissue-restricted mHags) such as HA-1 and HA-22 ; and (3) leukemia antigens including leukemia-specific antigens such as BCR-ABL in Philadelphia-chromosome–positive leukemia,3 and over- or aberrantly expressed leukemia-associated antigens (LAAs) such as proteinase 3 (PR3),3 Wilms tumor 1 (WT1),4,5 the preferentially expressed antigen of melanoma (PRAME),6,7 and BMI-1.8 Several studies have shown a temporal inverse relationship between circulating T cells directed against mHags or LAAs and minimal residual disease in patients with acute and chronic leukemia after allogeneic HSCT.9–14
The increasing array of both alloantigens and leukemia antigens that can elicit antileukemia T-cell responses provides the basis for development of vaccines for leukemia both inside and outside of the context of allogeneic HSCT.
Vaccines under clinical development for leukemia
Vaccines can be classified as those against defined antigens and those with broad antileukemic activity that are not directed against a specific tumor antigen. Examples of vaccines against defined antigens include peptides15 and DNA or RNA encoding the entire sequence or major antigenic elements of the tumor protein.16 Vaccines with broad antileukemic activity include leukemic dendritic cells (DCs), DC fusions with leukemia cells,17 cell lysates,18 and tumor cells engineered to secrete GM-CSF.19 The advantage of the latter approach is that a prior knowledge of tumor antigens is not required and multiple antigens can be targeted simultaneously.
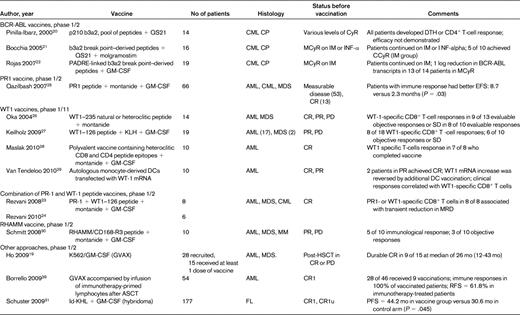
The majority of vaccine trials in patients with leukemia have used peptide vaccines due to their ease of manufacture and relatively low cost (summarized in Table 1). Phase ½ trials on both sides of the Atlantic have investigated the use of BCR-ABL peptide combinations in patients with chronic myeloid leukemia (CML).20–22 In 16 chronic-phase CML patients with various degrees of residual disease on imatinib or IFN-α, Bocchia et al confirmed the immunogenicity of this approach and reported stable and complete cytogenetic remission in a minority of patients.21 In a safety study in 8 patients with myeloid malignancies, a single vaccination with PR1 and WT1 peptides, together with montanide and GM-CSF adjuvants, induced CD8+ T-cell responses to PR1 or WT1 or both vaccines in 8 of 8 patients, with a brief decrease in molecular markers of disease,23 although repeated vaccination failed to induce sustained immune responses against the leukemia antigens.24 A large pilot study of repeated vaccination with PR1, a 9–amino acid human leukocyte antigen A0201 (HLA-A0201)–restricted peptide derived from proteinase 3, in 66 HLA-A0201 patients with acute myeloid leukemia (AML), CML, or myelodysplastic syndrome (MDS) found immune responses in 25 (47%) of patients. Clinical responses ranging from improvements in blood counts to complete cytogenetic remission were observed in 9 of 25 immune responders (36%) compared with 3 of 28 nonresponders (10%). Interestingly, immune response to PR1 was associated with improved event-free survival with a trend toward longer overall survival.25 The group in Osaka were the first to show the safety and immunogenicity of WT1 peptide vaccination in patients with hematological and solid cancer.26 Several investigators have since confirmed immune responses to vaccination with WT1 peptide or WT1 mRNA–electroporated DCs in patients with MDS and AML.27–29 In these studies, clinical responses were observed in 30%-80% of evaluable patients (including stable disease and reduced expression of tumor markers), although correlation with the detection of immunological responses in peripheral blood was variable. A recent pilot study investigated the immunogenicity of a polyvalent WT1 vaccine in patients with AML in complete remission (CR). The vaccine was composed of a mixture of heteroclitic HLA class I peptides designed to induce stronger WT1-specific CD8+ T-cell responses and synthetic longer peptides to induce CD4+ T-cell responses across several HLA types and to provide help for long-lasting immunity. CD8+ and CD4+ T cells directed against WT1 were induced in the majority of patients, with some suggestion of improved survival.28 Another potential leukemia-associated antigen, the receptor for hyaluronic acid mediated motility (RHAMM or CD168), has also been used as a target for vaccine, with reports of clinical and immunologic responses after the administration of the HLA-A0201–restricted RHAMM R3 peptide emulsified with montanide adjuvant and GM-CSF in patients with AML, MDS, and multiple myeloma overexpressing RHAMM/CD168.30 Finally several studies have evaluated the safety and efficacy of other vaccine strategies using heat-shock protein, leukemic DCs (DC), or fusions of DCs with myeloma or lymphoma cells, tumor-derived idiotype protein, and tumor cells engineered to secrete GM-CSF in patients with hematological malignancies, and have shown promising results.17–19,31 A recent phase 3 clinical trial conducted in patients with follicular lymphoma who achieved CR after chemotherapy showed significant improvement in progression-free survival in patients vaccinated with autologous tumor–derived idiotype vaccine conjugated to keyhole limpet hemocyanin plus GM-CSF compared with controls.31
These studies indicate that a variety of leukemia and lymphoma antigen–specific vaccination strategies can induce functional cytotoxic T-cell (CTL) responses that are associated with clinical improvement in some cases, especially in the setting of minimal residual disease, and support the use of such relatively simple approaches to boosting GVL responses posttransplantation.
The posttransplantation immune milieu and antileukemia immune responses
The finding of increased frequencies of BCR-ABL-, PR1-, and WT1-specific CTL and T cells directed against recipient mHags after allogeneic HSCT suggests that GVL could be further enhanced by posttransplantation vaccination.9–14,32,33 The profoundly lymphopenic environment immediately after transplantation provides a favorable milieu for rapid and extensive lymphocyte expansion and facilitates immune responses to weak self-antigens (reviewed in Rezvani and Barrett34 ). TCR Vβ spectratyping reveals that in the first few months after transplantation, the T-cell repertoire is oligoclonal, with skewing of the T cells toward host, leukemia, and viral antigens35 (with the potential to cause GVHD and exert GVL and antiviral activity), despite global immune deficiency. Indeed, massive clonal T-cell expansions have been reported in a patient with severe GVHD in whom the T-cell compartment was nearly completely (> 95%) occupied by one GVHD clone.36
The role of lymphopenia in antitumor immunity in murine models was first reported in the late 1970s.37 More recently, animal studies have shown that lymphoablation enhances the effectiveness of adoptively transferred, tumor-specific CD8+ T cells.38 Several preclinical murine studies have evaluated the role of lymphodepletion combined with vaccination strategies.39 The most direct evidence for the role of homeostatic T-cell proliferation in tumor eradication in humans comes from a clinical trial at the National Institutes of Health (NIH) involving 35 patients with advanced metastatic melanoma refractory to conventional treatments. Patients received in vitro expanded autologous tumor-infiltrating lymphocytes directed against overexpressed melanoma self-antigens in combination with IL-2 after conditioning with total body irradiation, fludarabine, and cyclophosphamide, resulting in huge expansions of the adoptively transferred clones with sustained regression of melanoma in 50% of cases.40 A drawback of this approach in the clinical setting is the technical difficulty of producing sufficient quantities of antigen-specific T cells for adoptive transfer. To overcome this limitation, in a subsequent study, the investigators genetically engineered peripheral blood lymphocytes carrying TCR chains specific for a melanoma antigen. The TCR-engineered T cells were transferred into 13 patients, with objective regression of metastatic melanoma lesions in 2 subjects.41
Transient lymphopenia induced by sublethal total body irradiation or other chemotherapeutic regimens is thought to enhance the efficiency of adoptive immunotherapy by altering homeostatic mechanisms that promote the expansion and stimulation of tumor-reactive effector T cells and minimize tumor-induced immune suppression.42 In the allogeneic HSCT setting, the incoming donor T cells are not tolerized to the leukemia, and after transplantation, the lymphopenic environment allows strong expansion of respective antitumor T cells in the presence of cytokines responsible for thymic-independent homeostatic T-cell proliferation, such as IL-7, IL-15, and IL-21. In addition to eradicating cells that may suppress antitumor responses, such as regulatory T cells, lymphoid reconstitution of either donor or host origin may overcome inherent defects in T-cell signaling, processing, or presentation and may strengthen the costimulatory functions of APCs.42
Because reconstitution of the T-cell compartment in lymphopenic hosts is regulated by peptides occupying MHC class I and II molecules,43 at the time of T-cell recovery, there may be an opportunity to skew the T-cell repertoire by engaging the available MHC class I and class II molecules with peptides of particular interest. Therefore, if tumor-associated peptides are presented to the proliferating lymphocytes during a lymphopenic episode, the host may be repopulated with tumor-reactive T cells that could lead to better tumor control. These observations imply that the first few months after transplantation offer a unique environment for delivering GVL directed against both leukemia-associated antigens and mHags expressed by the leukemia.
Vaccination in the context of allogeneic HSCT
Combining vaccination with transplantation strategies for the prevention of relapse
There are several reasons for considering vaccines after allogeneic HSCT. First, with a global decrease in transplantation-related mortality, relapse remains the major unresolved obstacle to overcome in allogeneic HSCT for malignant diseases. Second, given the lower tumor burden, the early posttransplantation phase should be an ideal setting for antitumor immune responses to operate. Third, the unique immune milieu around the time of the transplantation is permissive to the generation of antileukemia immune responses. Ideally, an effective immunotherapeutic strategy should result in the in vivo generation of large numbers of high avidity, antileukemia lymphocytes without the induction of immune tolerance. As described in the previous section, the reconstituting immune system after allogeneic HSCT provides a unique approximation to this setting.
Several studies in patients with multiple myeloma and lymphoma incorporated vaccination in the posttransplantation setting.44,45 Whereas immunological responses could be detected, convincing clinical responses were absent. Notably, these studies administered the vaccines months after transplantation, after exponential T-cell proliferation had likely occurred.
Limitations to vaccine therapies after ASCT
One potential limitation of vaccinating early posttransplantation is that antigen-specific CD8+ T cells during this time may be at risk for rapid induction of senescence. Indeed, a recent study showed that while circulating LAA-specific CD8+ T cells are prominent in patients with myeloid leukemia after allogeneic HSCT, cells are functionally unresponsive and display features of replicative senescence.46 Another concern is the potential impact of immunosuppression on the vaccine-induced immune response. However, even in the face of immunosuppression to prevent GVHD, GVL responses can be observed. We showed that even in unvaccinated patients, levels of WT1- and PR1-specific T cells increase during the first 3 months after transplantation despite the use of low-dose cyclosporin,10,11 suggesting that the administration of vaccines in patients treated with low-dose calcineurin inhibitors may not interfere with the vaccine-induced T-cell response. In a recent phase 1 trial, Ho et al vaccinated patients with high-risk MDS and AML with lethally irradiated, autologous, GM-CSF–secreting leukemia cells early after allogeneic HSCT. They reported that despite the use of the calcineurin inhibitor tacrolimus as GVHD prophylaxis, immunization in this setting was safe, immunogenic, and associated with biological activity.19
Combining vaccines with adoptive transfer of vaccine-primed lymphocytes after transplantation
The efficiency of vaccination can be increased further by combining adoptive T-cell transfer with vaccination. This involves vaccinating the patient, collecting vaccine-primed lymphocytes by apheresis before chemotherapy, and reinfusing them with further vaccination after lymphoreductive chemotherapy. Work published by June et al supports the feasibility and efficacy of this approach in stimulating specific immunity to influenza and pneumococcal antigens in the autologous setting.47,48 In randomized phase 1/2 trials, the investigators demonstrated that patients who received a single infusion of in vivo vaccine-primed, ex vivo–costimulated autologous T cells early posttransplantation, followed by booster immunizations posttransplantation, had accelerated immune reconstitution and enhanced antigen-specific CD4+ and CD8+ T-cell function in vivo. They subsequently extended the applicability of this approach to vaccination against tumor antigens in a phase 1/2 trial in 54 patients with myeloma. All patients received an autologous stem cell transplantation followed by vaccine-primed, ex vivo–costimulated autologous T cells at day 2 after transplantation. The patients were genetically randomized based on their HLA-A0201 genotype to receive an HLA-A0201–restricted multipeptide tumor antigen vaccine derived from the human telomerase reverse transcriptase and the antiapoptotic protein survivin and the pneumococcal conjugate vaccine before and after transplantation. HLA-A0201–negative patients received the pneumococcal conjugate vaccine only. The investigators reported augmented and accelerated cellular and humoral immune reconstitution, including antitumor immunity in 36% of HLA-A0201–positive patients, although no impact on event-free survival was noted.49
This approach was also explored in a phase 2 study by investigators at Johns Hopkins University using autologous leukemia cells admixed with GM-CSF–secreting K562 cells (the GVAX platform). After a single pretransplantation dose of the vaccine, the primed lymphocytes were collected and reinfused with the stem cell graft as postremission therapy after autologous stem cell transplantation for AML.39 Fifty-four subjects were enrolled, 46 of whom achieved CR with chemotherapy and 28 of whom received a total of 9 vaccinations at 3-weekly intervals posttransplantation. For all patients who achieved CR, the 3-year relapse-free survival rate was 47.4% and the overall survival rate was 57.4% compared with 61.8% and 73.4%, respectively, in the 28 immunotherapy-treated patients. After posttransplantation immunotherapy, immune responses to the vaccine were detected in 100% of patients, including delayed-type hypersensitivity reactions in 7 of 18 (39%), T-cell responses (assessed by 7-day ELISpot assay) in 15 of 17, and antibody responses to GVAX in 17 of 17 (100%) of patients.
In the setting of allogeneic HSCT, the opportunity exists to vaccinate the donor before lymphocyte collection. This approach has the advantage that vaccine-primed lymphocytes are collected from a healthy donor with a healthy immune system, rather than from patients tolerant to their own tumor antigens with reduced immunity from prior chemotherapy. The first study to show that tumor-specific T cells can be safely induced in a healthy donor and transferred to the recipient after allogeneic HSCT was published by Kwak et al in 1995.50 These investigators immunized an HLA-matched donor with a patient-derived idiotype vaccine before stem cell collection, and demonstrated the successful transfer of idiotype-specific T cells posttransplantation, which was associated with a significant reduction in the serum paraprotein levels.
The next logical step will be to enhance the GVL effect of adoptively transferred vaccine-primed lymphocytes further by booster vaccinations early after allogeneic HSCT. Investigators at Stanford University recently demonstrated the feasibility of this approach, which they termed “immunotransplantation,” in a preclinical lymphoma model.51 They showed that the efficiency of intratumoral vaccination with an immunostimulatory CG-enriched oligodeoxynucleotide can be augmented significantly by the adoptive transfer of vaccine-primed, tumor-specific T cells into syngeneic HSCT recipients, followed by posttransplantation booster vaccinations.51 Clinical trials to assess the efficacy of this approach after allogeneic HSCT are under development.
Future directions
Allogeneic HSCT continues to play a unique role in achieving cure of hematological malignancies that are otherwise resistant to treatment. However, as alternative treatment approaches continue to improve and increasing numbers of older patients present with leukemia, the challenge to cure more resistant malignancies with allogeneic HSCT will increase. Allogeneic HSCT is least effective against high-risk leukemias and current manipulations of conditioning regimens, posttransplantation immunosuppression, and donor lymphocyte infusions have probably reached their capacity to deliver GVL. To improve the natural GVL reactivity of allogeneic HSCT, it will be necessary to adopt new targeted treatments to boost GVL further; such approaches include the use of leukemia vaccines and the adoptive transfer of leukemia-specific T cells. Several improvements can be made to optimize the vaccine-transplantation strategy. Most vaccines currently used in clinical trials are small peptides presented to CD8+ T cells with no recruitment of CD4+ help. Whereas CD4+ T cells are dispensable for primary expansion of CD8+ T cells and their differentiation into cytotoxic effectors, secondary CTL expansion is wholly dependent on the presence of CD4+ T cells. Inclusion of MHC class II binding peptides in a vaccine or immunization using whole-tumor protein or lysate to elicit both CD4+ and CD8+ T cells may enhance the persistence of vaccine-induced responses. Ways to overcome tolerance are also being explored. Experimental murine models and human vaccine studies have shown that the preferential depletion of CD4+CD25+ regulatory T cells before vaccination enhances the vaccine-induced T-cell response.52,53
In summary, vaccines targeting leukemia antigens make a logical adjuvant to the allogeneic GVL effect. In the next few years, we should be able to define the optimum way to use these 2 powerful immune modalities to reduce relapse after allogeneic HSCT for otherwise incurable malignancies. It is possible that a multifaceted approach to immunotherapy involving allogeneic HSCT, adoptive T-cell transfer, and vaccination could prove to be a highly effective strategy for the control of refractory leukemia.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Published results of phase 1/2 studies of vaccination against leukemia-associated antigens.
Correspondence
Katayoun Rezvani, Hammersmith Hospital, Imperial College, Du Cane Road, London W12 0HS United Kingdom; Phone: +44(0)2083832175; Fax: +44(0)2033138223; e-mail: k.rezvani@imperial.ac.uk.