Abstract
The interplay between the complement and coagulation systems is just beginning to be explored and characterized. This interaction, however, is ancient. For example, if endotoxin is added to the hemolymph of the horseshoe crab, a protease is activated that triggers both the coagulation and complement systems. However, in extant mammals, these 2 cascades have diverged. These infamous “terrible C's” are the scourge of many a medical student (and possibly even a few hematologists). They also are intimately involved in the pathophysiology of thrombomicroangiopathies (TMAs). The complement system generates a procoagulant microenvironment and the coagulation system forms a clot in the renal microvasculature, and thus the 2 systems are partners in mediating multiple pathophysiological conditions.
Introduction
This review outlines how the complement system, in particular its primordial alternative pathway (AP), affects the endothelium after stress and injury.1,2 The focus is on patients with atypical hemolytic uremic syndrome (aHUS) or preeclampsia who carry genetic variants that lead to excessive AP activation. The consequences are fibrin- and platelet-rich clots in the renal microvasculature secondary to a disruption of hemostasis.
Complement function: the beneficial side
The complement system originated more than 1 billion years ago (Figure 1).3,4 In organisms such as Cnidaria (eg, anemones and coral) and protostomes (eg, crabs and nematodes), only a few complement components are present, but these are sufficient to constitute a primitive AP that provides an independent immune network.5 The human complement system differs mainly by having a more sophisticated and directed recognition system featuring antibodies (the classical pathway or CP) and lectins (the lectin pathway or LP). The name “complement” derived from the original discovery of the system as an effector arm of humoral immunity. A major goal of the complement system is to guard the intravascular (blood or hemolymph) space against bacterial invasion. To accomplish this, the AP activation cascade features a rapid and powerful amplification loop that proteolytically liberates small fragments to promote the inflammatory response, whereas the large fragments deposit on membranes to mediate opsonization and lysis (Figure 1).6
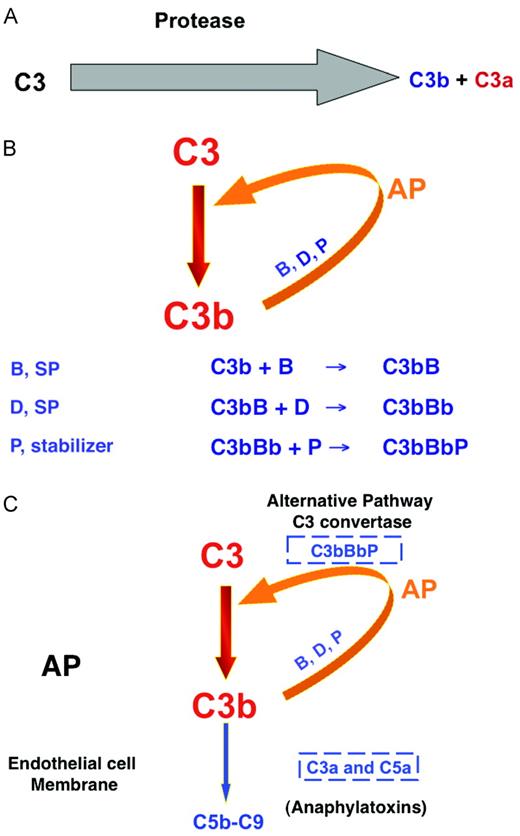
The AP of complement activation. (A) The AP of the complement system originally consisted of a serine protease that cleaved C3 to the opsonin C3b and the proinflammatory anaphylatoxin C3a. (B) An amplification loop was next evolved to more efficiently deposit C3b on a target and liberate C3a into the surrounding milieu. B indicates factor B, D indicates factor D, a serine protease; P, properdin, a stabilizer of the enzyme. (C) Development of a C5 convertase. The same enzyme that cleaves C3 (AP C3 convertase)can cleave C5 to C5a and C5b with the addition of a second C3b to the enzyme complex (AP C5 convertase).
The AP of complement activation. (A) The AP of the complement system originally consisted of a serine protease that cleaved C3 to the opsonin C3b and the proinflammatory anaphylatoxin C3a. (B) An amplification loop was next evolved to more efficiently deposit C3b on a target and liberate C3a into the surrounding milieu. B indicates factor B, D indicates factor D, a serine protease; P, properdin, a stabilizer of the enzyme. (C) Development of a C5 convertase. The same enzyme that cleaves C3 (AP C3 convertase)can cleave C5 to C5a and C5b with the addition of a second C3b to the enzyme complex (AP C5 convertase).
The potent proinflammatory and membrane activities of the complement system require inhibitors to maintain homeostasis. These block inappropriate fluid-phase turnover (no target) and prevent activation on healthy self-tissue (wrong target). In essence, the regulators allow the complement system to distinguish “self” from “non-self.” The complement system also participates in the clearance of self-debris in injury states and promotes wound healing. In contrast to an immune response against an invading pathogen, an adaptive immune response and an exuberant inflammatory response are undesirable outcomes relative to the processing of damaged host tissue.
Pathways
Complement activation proceeds via 3 pathways: the CP, LP, and AP. Although triggered differently, a similar reaction sequence ensues with each, resulting in the cleavage of the third component of complement (C3). All 3 pathways generate enzymatic complexes called “convertases” that cleave C3 to generate C3a and C3b. C3a is an anaphylatoxin, whereas C3b binds covalently to a target, where it serves as an opsonin and as a site for further amplification of C3b deposition via the AP's feedback loop. The addition of a second C3b to the AP C3 convertase creates the C5 convertase. C5 is cleaved to C5b and the anaphylatoxin C5a by this convertase. The latter is a powerful chemoattractant and cell activator, whereas the former initiates the assembly of the membrane attack or lytic complex (MAC).
The CP is primarily triggered by IgM and IgG binding to antigen (ie, an immune complex). In an analogous process, mannose-binding lectin of the LP attaches to mannose groups displayed on the surface of microbes to trigger this pathway. In contrast, there is no initiating factor per se for the AP6 ; rather, the AP is in continuous (1%-2%/h) state of low-level activation as a result of the spontaneous hydrolysis of the thioester bond in native C3. This “tick-over” of C3 leads to its attachment to nonspecific acceptor molecules in plasma (such as water) or to hydroxyl groups on cell surfaces. Activated C3 molecules created in this manner or via activation of the CP or LP can form C3 convertases and begin the feedback loop. Control of the AP is therefore primarily aimed at regulating convertase assembly.
Regulation
Thrombomicroangiopathies (TMAs) are triggered by endothelial cell injury. A heterozygous mutation in one of 3 complement regulatory proteins (Figure 2) predisposes to aHUS. These regulators prevent activation on cells or in plasma via 2 mechanisms. One is called cofactor activity (CA; Figure 3). The plasma protein factor H or its cell-surface counterpart, membrane cofactor protein (MCP or CD46), binds to C3b. After this interaction, a plasma serine protease known as factor I proteolytically degrades C3b but in a precise and limited fashion. The initial cleavage reaction inactivates C3b (conversion to so-called iC3b), which no longer binds factor B and therefore cannot engage the AP's amplification loop. In the second type of regulatory mechanism, decay accelerating activity, the catalytic serine protease domain of the multiunit C3 convertase is disassociated (decayed); however, genetic variants in decay accelerating factor (DAF or CD55) have not been identified in aHUS.7
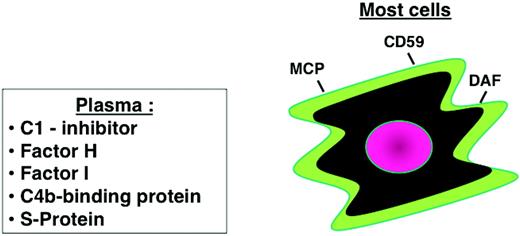
Cellular and plasma regulators of complement. Regulatory proteins on most host cells include MCP, DAF, and an inhibitor of the MAC (CD59). Plasma proteins also control activation in plasma and can assist on cell membranes. These include C1-inhibitor, factors H and I, C4b-binding protein, and S-protein (a MAC inhibitor).
Cellular and plasma regulators of complement. Regulatory proteins on most host cells include MCP, DAF, and an inhibitor of the MAC (CD59). Plasma proteins also control activation in plasma and can assist on cell membranes. These include C1-inhibitor, factors H and I, C4b-binding protein, and S-protein (a MAC inhibitor).
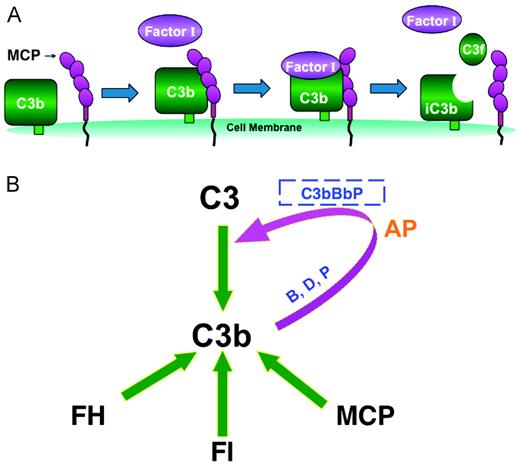
Cofactor activity controls complement activation. The most common functional defect in aHUS is reduced cofactor activity for C3b. (A) Cofactor activity. On the membrane of host cells, cofactors MCP and factor H bind C3b. This allows the serum protease factor I to cleave C3b and thereby prevent further C3 activation. (B) Complement regulators on cells and in plasma inhibit C3b. Mutations in one of the 3 inhibitors of the C3 and C5 convertases (factor H, factor I, and MCP) are seen in ∼ 50% of aHUS patients. MCP is widely expressed on almost all cells. Factor H, an abundant protein of plasma, is required for control in plasma and can bind to cell membranes and carry out CA. MCP and factor H both bind C3b, thereby enabling factor I to cleave C3b and thus prevent its participation in the AP's feedback loop.
Cofactor activity controls complement activation. The most common functional defect in aHUS is reduced cofactor activity for C3b. (A) Cofactor activity. On the membrane of host cells, cofactors MCP and factor H bind C3b. This allows the serum protease factor I to cleave C3b and thereby prevent further C3 activation. (B) Complement regulators on cells and in plasma inhibit C3b. Mutations in one of the 3 inhibitors of the C3 and C5 convertases (factor H, factor I, and MCP) are seen in ∼ 50% of aHUS patients. MCP is widely expressed on almost all cells. Factor H, an abundant protein of plasma, is required for control in plasma and can bind to cell membranes and carry out CA. MCP and factor H both bind C3b, thereby enabling factor I to cleave C3b and thus prevent its participation in the AP's feedback loop.
Complement dysfunction: the deleterious side
Typical or shiga toxin Esherichia coli (STEC) HUS has a good prognosis and usually resolves with no or minimal residual tissue damage. However, cases of lethal or “refractory” enteropathic HUS have now been reported in patients carrying mutations in complement regulators.8 Although it is rare, this scenario accounts for some of the “bad outcomes” in Stx-HUS. aHUS (or nonenteropathic HUS) is more severe, with an acute mortality up to 25% and often with recurrent episodes in which ∼ 50% of patients develop permanent kidney damage requiring long-term dialysis.9–18 The penetrance is ∼ 50%, suggesting that an environmental factor is required to trigger the disease process. A serious infection in early childhood was found to be the most common precipitating event in several studies.9–19 Whereas aHUS typically occurs in young children (mean age is less than 5 years), it may not manifest until later, particularly in association with pregnancy. The typical scenario for aHUS is that one parent, who has usually not developed aHUS, is a mutation carrier.
Much evidence implicates the AP in mediating undesirable tissue injury in aHUS (Figure 4).20 Heterozygous loss-of-function mutations in AP regulators, factor H, MCP, or factor I have been characterized over the past decade as predisposing to aHUS. Subsequently, rare variants in the activating components factor B17 and C316 were shown to represent a gain-of-function in activation of the AP. In addition, 5%-10% of patients have autoantibodies to factor H that disrupt its functional repertoire. Therefore, too much activation of the AP secondary to dysfunctional inhibitors predisposes to aHUS.
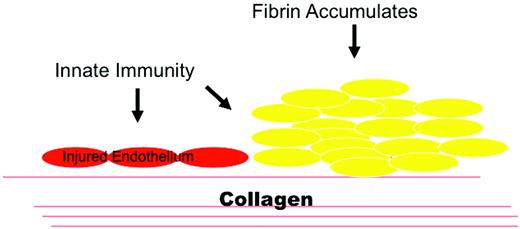
Innate immunity and hemostasis in aHUS. aHUS shows that regulatory proteins are vital for homeostasis. MCP and factor H protect cells from complement attack. Factor H can also transfer from plasma to cellular matrices such as an exposed basement membrane. Damage to an endothelial surface could result from a stressed, apoptotic, or necrotic cell that exposes the underlying basement membrane. The innate immune system responds to this injury state and facilitates wound repair. Excessive activation of the AP results in aHUS. (Used with permission from Tarr et al.20 )
Innate immunity and hemostasis in aHUS. aHUS shows that regulatory proteins are vital for homeostasis. MCP and factor H protect cells from complement attack. Factor H can also transfer from plasma to cellular matrices such as an exposed basement membrane. Damage to an endothelial surface could result from a stressed, apoptotic, or necrotic cell that exposes the underlying basement membrane. The innate immune system responds to this injury state and facilitates wound repair. Excessive activation of the AP results in aHUS. (Used with permission from Tarr et al.20 )
Mechanisms of cellular injury
An inherited or acquired deficiency in a complement inhibitor of the AP is commonly associated with renal disease.21 It is not clear, however, why this occurs. Part of the answer may relate to the presence in the kidney of a fenestrated glomerular endothelium and the absence of endogenous complement regulators on the glomerular basement membrane, especially in the setting of high shear stress. Recognized examples of endothelial injury leading to aHUS are infections such as HIV and Streptococcus pneumoniae, chemotherapeutic agents, and pregnancy.
Five mechanisms by which an abnormality of the complement system predisposes to aHUS have been demonstrated:
Haploinsufficiency resulting from a mutation preventing a protein's expression. Alternatively, the protein is expressed but has reduced function.9–19 The accumulated results in aHUS indicate that half normal levels of factor H or factor I predispose a child to a TMA. Interestingly, parents of aHUS patients, who are carriers, are usually healthy. In addition, the expression of 50% of normal levels of MCP/cell on endothelial cells predisposes to aHUS. Endothelial cells express the highest quantity of complement inhibitors of any cell type examined (MCP, ∼ 400 000; DAF, ∼ 200 000; CD59, the MAC inhibitor, ∼ 1 500 000 (A. Richards and J. Atkinson, unpublished results).
Autoantibody-mediated blocking of regulatory protein function is most commonly observed in association with deletion haplotypes of complement factor H–related protein 1 (CFHR1) and 3 (CFHR3).18,19,22
Convertase component mutations abnormally stabilize the AP C3 and C5 convertases.16,17
Polymorphisms in noncoding regions of complement regulators may associate with disease predisposition.14,19
Thrombomodulin23 or C4b-binding protein mutations14,23 influence CA.
The instructive value of cellular models
Common (> 5%) and uncommon (1%-5%) single nucleotide polymorphisms reflect mutations that occurred many generations ago, whereas rare variants (< 1%) are more likely to have arisen recently.24 On a population level, rare variants, by virtue of their low frequency alone, are more likely to be detrimental. This has turned out to be the case for mutations characterized in aHUS patients. For example, the initial 24 rare variants identified in MCP, in which a mutation was not found in 100-200 controls, were all defective.12 (Figure 5) In two-thirds of the cases, the mutation led to the protein not being expressed on the cell membrane. In the remaining one-third, albeit expressed, the variant protein was dysfunctional in carrying out cofactor activity.
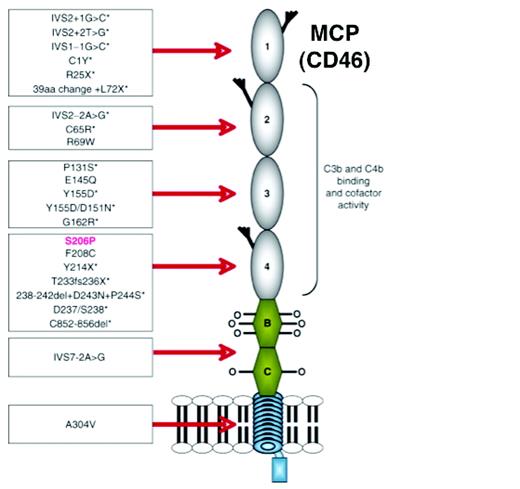
Schematic diagram of the structure of MCP and depiction of the initial 24 mutations associated with aHUS. MCP is an ∼ 65-kDa type 1 transmembrane glycoprotein. Beginning at the N-terminus, it consists of 4 ∼ 60 amino acid complement control repeats (also called short consensus repeats and occasionally referred to as “Sushi domains”). Modules 1, 2, and 4 each contain one N-glycosylation site. Next is an alternatively spliced region, rich in serines, threonines, and prolines that are sites for O-glycosylation. This is followed by a juxtamembraneous group of 12 amino acids (encoded by a separate exon) of unknown function, a transmembrane hydrophobic domain, a charged intracellular anchor, and the alternatively spliced cytoplasmic tail (tail 1 or 2). The MCP-BC isoform is shown. Mutations associated with aHUS are primarily clustered in the 4 extracellular modules. Mutations associated with reduced expression levels are marked by an asterisk. The mutation in red is S206P, which is discussed in the text. (Used with permission from Richards et al.15 )
Schematic diagram of the structure of MCP and depiction of the initial 24 mutations associated with aHUS. MCP is an ∼ 65-kDa type 1 transmembrane glycoprotein. Beginning at the N-terminus, it consists of 4 ∼ 60 amino acid complement control repeats (also called short consensus repeats and occasionally referred to as “Sushi domains”). Modules 1, 2, and 4 each contain one N-glycosylation site. Next is an alternatively spliced region, rich in serines, threonines, and prolines that are sites for O-glycosylation. This is followed by a juxtamembraneous group of 12 amino acids (encoded by a separate exon) of unknown function, a transmembrane hydrophobic domain, a charged intracellular anchor, and the alternatively spliced cytoplasmic tail (tail 1 or 2). The MCP-BC isoform is shown. Mutations associated with aHUS are primarily clustered in the 4 extracellular modules. Mutations associated with reduced expression levels are marked by an asterisk. The mutation in red is S206P, which is discussed in the text. (Used with permission from Richards et al.15 )
An example that illustrates these points is the initial rare variant described in MCP associated with aHUS.12 Whereas the putative MCP variant S206P was expressed normally on patients' and family members' leukocytes, it tracked with the disease in 3 Belgian brothers afflicted with aHUS. This variant was not found in > 200 controls (400 alleles). The mutation was in a region that previously had been mapped by alanine scanning mutagenesis to a C3b/C4b interactive site, being in a hypervariable loop of MCP.25 Recombinant protein carrying the mutation was expressed and analyzed. In vitro assays demonstrated that S206A had modestly reduced C3b binding, but only ∼ 10% of wild-type cofactor activity. The patient's mutation (S206P) had an even more severely reduced function.12 It bound C3b poorly (7% of normal) and also had minimally detectable CA.
This mutant was next analyzed in situ in a model system designed to assess complement regulators on the surface of cells.12,26 Chinese hamster ovary (CHO) cell line clones bearing equivalent copy numbers of wild-type or mutant protein were prepared by transfecting recombinant DNA. CHO cells were chosen because they do not spontaneously activate human complement and because their membrane regulators do not possess activity against human C3b or C4b.26 The derived cell lines were sensitized with a polyclonal rabbit anti–CHO IgG and exposed to a human serum source in which only the AP can be activated. The magnitude of complement challenge can be varied by altering the concentration of the sensitizing Ab and of the serum. Flow cytometry and Western blotting were used to monitor C3 fragment deposition and degradation. Cytotoxicity was assessed using colorimetric indicators of viability and growth. Data generated using this model system indicated that the S206P mutant also had impaired (∼ 10% of wild-type) ability to prevent C3b deposition on the surface of the cell in response to the AP challenge. These results point out the advantages of having both in vitro and cellular models to provide a more comprehensive and quantitative measure of the functional activity of a variant.
Preeclampsia as a TMA
Preeclampsia occurs in 4%-6% of all first pregnancies worldwide, leading to substantial maternal and neonatal mortality.27 It has defied reliable prediction and eluded satisfactory medical intervention and is often diagnosed at ∼ 20 week's gestation due to the development of hypertension. However, the syndrome begins earlier with abnormal placental development.27,28 The specific “anatomical” defect, the failure of uterine spiral arteries to remodel into dilated, flaccid vessels, leads to underperfusion of the intervillous space and placental hypoxia.28–32
Preeclampsia has been classified as a TMA based in part on the renal pathologic findings of endothelial cell swelling, expansion of the subendothelial zone, duplication of the glomerular basement membrane, mesangial cell interposition, and glomerular or microvascular thrombi.13–15,18,19,27,29 Clinically, preeclampsia syndromes may be characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal involvement with refractory hypertension. Preeclampsia is a multifactorial syndrome that produces clinical symptoms with gradations of vasospasm, endothelial damage, and an inflammatory response. Undelivered mothers risk life-threatening disease secondary to progression to the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), seizures (eclampsia), or stroke.8,28–32
Recent evidence indicates that mutations in the same AP genes involved in aHUS are present in 5%-15% of preeclampsia cases33 and in a higher percentage of HELLP syndrome cases.8,29,30 Mutations in MCP, factor H, and factor I have been associated with the HELLP syndrome.8,29,30 The HELLP syndrome may be a placenta instigated and liver targeted acute inflammatory condition that is comparable to the kidney and CNS involvement in preeclampsia and eclampsia.
The clinical manifestations of preeclampsia represent the maternal response to an excess of antiangiogenic factors released by the hypoperfused placenta. These include vasculopathic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1), a potent vascular endothelial growth factor (VEGF) antagonist, and soluble endoglin, an inhibitor of TGF-β signaling.31–34 A genetic contribution is suggested by the higher rates of preeclampsia in sisters, daughters, and mothers of affected women. Further, evidence for a paternal component to the predisposition supports the hypothesis that the genotype of the fetus contributes to the overall risk of preeclampsia.34
Complement activation products have been identified in deciduas, chorionic villi, and vessel walls in the placentas of patients with preeclampsia.35 In normal pregnancies, excessive complement activation is prevented by complement regulatory proteins that are highly expressed on trophoblast membranes. Elevated levels of the AP-derived complement activation fragments C3a and Bb in the first 20 weeks of pregnancy were recently found to be independently associated with preeclampsia later in pregnancy.36–38 These data support an important role for complement, in particular the AP, in this disorder (and its newly acquired inclusion as a TMA; Figure 6).
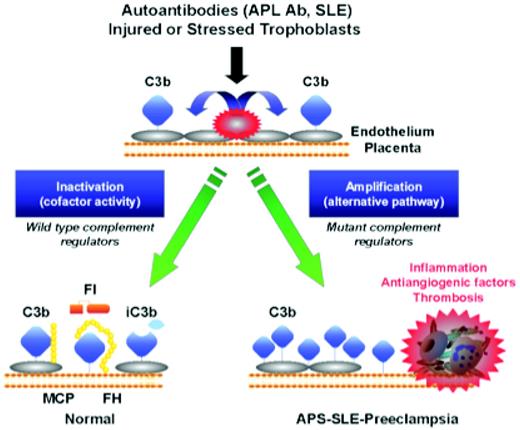
Activation and regulation of the complement system on injured or stressed endothelium or trophoblasts. Regulation of the feedback loop of the AP on placental trophoblasts occurs through limited proteolytic cleavage of C3b to generate iC3b. This reaction is carried out by a serine protease factor I and the cofactor protein MCP or factor H. Cofactor activity terminates the feedback loop because iC3b does not bind factor B, thereby shutting down the amplification loop. Because SLE and APL syndrome are characterized by autoantibodies that trigger the CP, defective regulation of C4b (a component of the CP C3 convertase) by MCP is also likely to influence the severity of tissue injury and risk for preeclampsia. When tissues are damaged, a delicate balance must be established to allow for repair and recovery. If regulators such as MCP, factor I, or factor H are dysfunctional, excessive complement activation occurs. This may result in placental damage, thrombophilia, and the release of antiangiogenic factors, culminating in preeclampsia. Endothelial cells could be substituted for trophoblasts in this schematic. (Figure provided by Jane Salmon, Hospital for Special Surgery, New York.)
Activation and regulation of the complement system on injured or stressed endothelium or trophoblasts. Regulation of the feedback loop of the AP on placental trophoblasts occurs through limited proteolytic cleavage of C3b to generate iC3b. This reaction is carried out by a serine protease factor I and the cofactor protein MCP or factor H. Cofactor activity terminates the feedback loop because iC3b does not bind factor B, thereby shutting down the amplification loop. Because SLE and APL syndrome are characterized by autoantibodies that trigger the CP, defective regulation of C4b (a component of the CP C3 convertase) by MCP is also likely to influence the severity of tissue injury and risk for preeclampsia. When tissues are damaged, a delicate balance must be established to allow for repair and recovery. If regulators such as MCP, factor I, or factor H are dysfunctional, excessive complement activation occurs. This may result in placental damage, thrombophilia, and the release of antiangiogenic factors, culminating in preeclampsia. Endothelial cells could be substituted for trophoblasts in this schematic. (Figure provided by Jane Salmon, Hospital for Special Surgery, New York.)
In an experimental model of the antiphospholipid syndrome, a C5a-C5a receptor interaction triggered the release of sFlt-1.39 This antiangiogenic factor is associated with hypertension, proteinuria, and glomerular endotheliosis in rodents and is elevated in the circulation of pregnant women destined for preeclampsia.31,32,40 In a mouse model developed at our institution, uncontrolled complement activation due to deficiency of a membrane complement regulator analogous to human MCP is embryonically lethal due to maternal complement–triggered damage. Pregnancies are rescued in mice with reduced maternal AP function.41
Due to histopathologic similarities in the kidney between aHUS and preeclampsia, the hypothesis that impaired capacity to limit the AP of complement activation underlies preeclampsia was tested in a prospective study of 250 pregnant patients with systemic lupus erythematosus (SLE) and/or antiphospholipid (APL) Abs.33 Pregnancies in women with autoimmune conditions characterized by complement-mediated injury are associated with increased risk of preeclampsia and miscarriage.42 Genes that encode 3 complement inhibitors were sequenced in 40 patients with preeclampsia, and heterozygous mutations in 7 (18%) were found.33 Five patients had risk variants in MCP or factor I that were previously identified in aHUS. One patient had a novel mutation in MCP that impaired regulation of C4b but not C3b, a finding consistent with defective control of CP activation via immune complexes leading to preeclampsia. These variants constituted the first genetic defects associated with preeclampsia in the context of SLE and/or APL Abs. The association of hypomorphic variants of MCP and factor I were confirmed in a cohort of nonautoimmune preeclampsia patients, in which 5 of 59 were heterozygous for mutations.33 The presence of these risk variants in patients who develop preeclampsia links complement activation to disease pathogenesis.
Therapeutic implications
The treatment implications of these observations in aHUS and preeclampsia are that the AP contributes to tissue damage, so inhibiting it could ameliorate the 2 syndromes. Excessive activation of the AP leads to the release of 2 mediators of inflammation, the anaphylatoxins C3a and C5a. It also leads to 2 membrane-altering events: opsonization by C3b and sublytic membrane perturbation or lysis by C5b-9 (MAC). C3a and C5a interact with their respective G-coupled protein receptors to alter endothelial cells, a process that includes enhancing the expression of adhesins and attracting and activating inflammatory cells to a site of injury. C3b is the major opsonin of the complement system, and its limited degradation products (iC3b and C3d) are ligands for additional complement receptors. Sublytic injury and, of course, lysis are long-appreciated effector activities of the MAC. As described in the accompanying article by Tim Goodship (see pages 15-20), early clinical trial results implicate C5a and/or C5b-9 because a mAb to C5 that prevents its cleavage to C5a and C5b appears to be highly efficacious in the treatment of aHUS.43
Conclusion
The role of the complement system in health and disease continues to be elucidated. Dysfunction of its regulatory proteins teaches us the importance of maintaining homeostasis with the given expression levels nature uses as a “set point.” In the example of aHUS, disruption of that delicate balance leads to an assault on the integrity of the endothelium that is moderated by both the complement and coagulation systems. The incomplete penetrance of disease also points out that other genetic and environmental factors play an important role in pathological development. Continuing evolution of structural insights into complement activation and its proper regulation are likely to suggest therapeutic interventions.44
Disclosures
Conflict-of-interest disclosures: M.K.L. declares no competing financial interests. J.P.A. is on the board of directors or an advisory committee for Genentech and Compliment Corporation; has consulted for Genentech, Idera Pharmaceuticals, Compliment Corporation, and KEREOS; and has received honoraria from Idera Pharmaceuticals and KEREOS. Off-label drug use: Soliris (eculizumab) is a complement inhibitor—specifically, it is a monoclonal antibody that blocks the activation of C5, is currently in the marketplace to treat paroxysmal nocturnal hemoglobinuria, and is in clinical trials for aHUS.
Correspondence
John P. Atkinson, Washington University School of Medicine, Department of Medicine/Rheumatology Division, 660 South Euclid Ave, Campus Box 8045, St Louis, MO 63110; Phone: (314) 362-8391; Fax: (314) 362-1366; e-mail: jatkinso@dom.wustl.edu.