Abstract
Prophylactic platelet transfusions are the standard of care for patients with hypoproliferative thrombocytopenia after receiving chemotherapy or radiation for the treatment of malignancy, for BM replacement by leukemia or solid tumor, or in preparation for a hematopoietic stem cell transplantation.1 During this time of thrombocytopenia, these patients may receive both prophylactic platelet transfusions, which are given to prevent potentially life-threatening bleeding when a patient's platelet count drops below a predetermined threshold, and therapeutic platelet transfusions, which are given to treat active or recurrent bleeding. In the 1950s, the invention of the plastic blood bag allowed for the production and storage of platelet concentrates,2 and in the 1960s, it was recognized that prophylactic platelet transfusions effectively reduced hemorrhagic death in patients with newly diagnosed leukemia.3,4 In 1962, Gaydos published the paper that is frequently credited with the inception of the 20 000/μL platelet transfusion threshold.5 Despite a half-century of experience with prophylactic platelet transfusions, there are still insufficient data to provide clinicians with evidence-based guidelines specific to pediatric oncology and hematopoietic stem cell transplantation (HSCT) patients.
Prophylactic platelet transfusions: current evidence in pediatric patients
Current guidelines for prophylactic platelet transfusions in pediatric patients are based on expert opinions and data from clinical trials and retrospective studies that have been performed primarily in adult patients with acute myelogenous leukemia (AML) or those undergoing HSCT. Many factors, such as underlying disease, treatment regimen, and comorbidities, may confer different bleeding risks to pediatric patients than adults. Therefore, the optimal platelet transfusion threshold for adults may not be appropriate for pediatric patients.
To date, there have been 4 large randomized, controlled trials (RCTs) designed to compare prophylactic platelet transfusion thresholds, either 10 000/μL versus 20 000/μL or 30 000/μL.6–9 Although 3 of these trials included patients < 18 years old, only Zumberg's paper stated the number of pediatric patients enrolled; none of the studies included a separate analysis of these patients.6,8,9 The primary outcome of 2 of these trials was the number of platelet transfusions, and they were powered accordingly, with bleeding incidence as a secondary outcome.6,8 The largest RCTs to evaluate the effect of platelet dose on bleeding are: the SToP (Strategies for Transfusions of Platelets) trial (patients ≥ 18 years of age)10 and the PLADO (Optimal Platelet Dose Strategy to Prevent Bleeding in Thrombocytopenic Patients) trial, which included a large cohort of pediatric patients (n = 198; age, 0-18 years).11 Of these 6 trials, only the PLADO trial had a separate analysis of pediatric patients, which was performed by Josephson et al.12
Before the PLADO trial and its subanalysis of pediatric patients, the only studies to report on the incidence of bleeding in thrombocytopenic pediatric oncology patients were published before modern chemotherapy regimens and pre-date the introduction of the World Health Organization (WHO) Bleeding Scale, which was designed to report bleeding toxicity in cancer treatment trials.13 These reports' current applicability is questionable for the following reasons: (1) the studies' inclusion of only acute leukemia patients; (2) changes in therapies, including HSCT and radiation treatment; (3) advancements in supportive care that have eliminated the use of aspirin; and (4) and improvements in the production of platelet components. Further, none of the pediatric reports referenced the controversy surrounding the use of nonsteroidal antiinflammatory drugs and their use or contraindication with thrombocytopenic patients. However, the available data do provide important insights into the relationship between thrombocytopenia and bleeding in pediatric oncology patients.
One such study, an RCT of 56 patients with newly diagnosed leukemia, found no difference in bleeding incidence between patients who received prophylactic transfusions versus those who only received therapeutic transfusions for gross bleeding.14 This study was unique even compared with modern studies, because it followed patients through the entire course of their therapy. Patients in the therapeutic cohort received fewer transfusions, but had a higher number of days with bleeding; there was no difference in overall survival.14 Another study, which compared historic controls with randomized cohorts receiving low- or high-dose platelets (defined in this study as 0.03 units/lb and 0.06-0.07 units/lb, respectively), found that the incidences of minor and severe bleeding per thrombocytopenic episode decreased from 48% and 11% down to 8.1% and 2.2%, respectively, once a prophylactic threshold of 25 000/μL was adopted.15 There was no statistical difference in bleeding incidence between the high- and low-dose cohorts.15
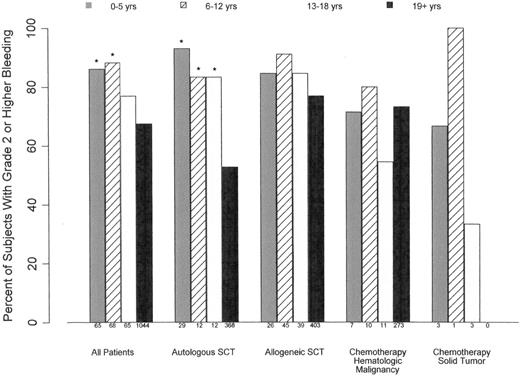
Like the pediatric subanalysis in the PLADO trial, Roy's study compared bleeding incidence between age groups.12,15 That study showed that bleeding incidence was highest in patients 0-4 years of age, a difference that was significant in both the transfused and untransfused cohorts.15 The pediatric subanalysis of the PLADO trial also found that the highest incidence of clinically significant bleeding was in patients 0-5 years of age undergoing autologous HSCT compared with older children and adults (Figure 1). As is the problem with many subanalyses, there was insufficient power to be able to determine differences in bleeding outcomes among all of the pediatric cohorts, and there may be additional covariables specific to pediatric patients that are not accounted for in this analysis.11,12
Relationship between age group and percentage of patients experiencing ≥ grade 2 bleeding in all patients and stratified by disease treatment category. For all treatment groups, there was significantly higher incidence of bleeding in patients 0-5 and 6-12 years of age compared with those ≥ 19 years (*P < .001). In HSCT patients, all pediatric age cohorts had a higher incidence of bleeding than adults (*P < .04).
Relationship between age group and percentage of patients experiencing ≥ grade 2 bleeding in all patients and stratified by disease treatment category. For all treatment groups, there was significantly higher incidence of bleeding in patients 0-5 and 6-12 years of age compared with those ≥ 19 years (*P < .001). In HSCT patients, all pediatric age cohorts had a higher incidence of bleeding than adults (*P < .04).
The PLADO trial was the largest trial to have a threshold of 10 000/μL in pediatric patients; however, because of its design, no conclusions can be drawn regarding the safety and efficacy of this regimen compared with other regimens. An important finding in this analysis was that the trigger of 10 000/μL was adhered to on approximately 90% of study days for all pediatric cohorts.12 This high adherence rate supports the feasibility of a clinical trial comparing transfusion thresholds, and recent surveys of pediatric HSCT physicians and oncologists suggest clinical equipoise between different threshold regimens.16,17 It is clear from these prior trials that there are likely factors that affect the relationship between thrombocytopenia and bleeding differently in pediatric and adult patients, highlighting the need for pediatric-specific trials.
In addition to standard prophylactic thresholds, there is the question of what is the appropriate pre-procedure platelet count to prevent bleeding during common procedures such as central venous catheter (CVC) placement, lumbar puncture (LP), BM aspirate or biopsy, or intestinal biopsy. Several clinical practice guidelines recommend the use of 50 000/μL as the minimum platelet count for most invasive procedures (eg, CVC placement, minor surgery, and procedures such as endoscopy and bronchoscopy) and 100 000/μL for procedures involving the CNS.1,18 In the absence of prospective RCTs, these recommendations are based on limited retrospective comparisons, case reports, and expert opinions. A recent retrospective review looked at bleeding incidence among 193 adult patients who underwent a total of 604 CVC insertions of nontunneled, 2- to 4-lumen catheters inserted either into the subclavian vein (85% of patients) or jugular vein (15%).19 Patients who received arterial catheters, port-a-caths, and inguinal catheters were excluded from analysis. This study found that 20 000/μL (not 50 000/μL) was the platelet count at which bleeding incidence increased. Although this study was limited to a specific type of CVC placement, and placement of other CVCs such as port-a-caths and tunneled lines may incur higher bleeding risk, the results show the importance of rethinking the current threshold paradigms for invasive procedures. The investigators estimated that by limiting pre-procedure platelet transfusions to patients with a platelet count < 20 000/μL (instead of 50 000/μL) would result in 40% fewer pre-procedure transfusions.19
Aside from CVC insertion, another common procedure in pediatric leukemia patients is the LP, whether performed at initial diagnosis or throughout the treatment course. Although the American Society of Clinical Oncology (ASCO) guidelines state that 20 000/μL is a safe pre-LP platelet count, there is a growing body of evidence showing that, in children with acute lymphoblastic leukemia (ALL) who have circulating leukemic blasts in the peripheral blood (ie, at time of diagnosis or before obtaining remission), a traumatic LP with blasts present (TLP+) in the cerebrospinal fluid (CSF) is associated with a lower event-free survival than patients who are CNS 1 (ie, no detectable leukemic blasts in the CSF).20,21 This difference in event-free survival is even greater in patients with 2 consecutive blast-positive traumatic LPs (TLP++); TLP++ patients were 4 times more likely to have an isolated CNS relapse (16% vs 4%) and 3 times as likely to have a hematologic relapse (32% vs 10%) than patients who were CNS 1 or TLP without blasts, even after controlling for other risk factors.20
In a large series of more than 5000 LPs performed in more than 950 children with ALL, serious complications such as subdural hematoma were rare at all platelet counts (including < 10 000/μL).22,23 The proportion of LPs that were traumatic (≥ 10 but < 500 RBCs/μL) or bloody (≥ 500 RBCs/μL) was 29% or 10%, respectively.22,23 Many factors affected the likelihood of traumatic or bloody LP: patient race and age, years of experience of the practitioner, time from most recent LP, and platelet count at the time of the procedure. Platelet counts < 100 000/μL were independently associated with an increased probability of traumatic or bloody LP, with an odds ratio of 1.5 (95% confidence interval, 1.2-1.8).23
Although there is no study that shows a direct correlation between platelet count at time of diagnostic LP and event-free survival, it appears that transfusing newly diagnosed ALL patients to a platelet count > 100 000/μL may be one strategy to minimize the risk of a traumatic or bloody LP and improve event-free survival. The data presented refer only to patients with ALL; however, a patient's leukemia type may not be known and clinicians can elect to take this approach with all newly diagnosed leukemia patients. Because multiple TLP+ increases a patient's risk for relapse, in patients with an initial TLP+, it may be prudent to continue to use a threshold of 100 000/μL for all LPs performed until the patient no longer has circulating blasts.
With the PLADO trial being the only RCT performed in pediatric patients who are receiving modern antineoplastic therapy, there is insufficient information in the published literature to determine the optimal prophylactic platelet transfusion strategy in these patients with respect to threshold or dose. There are even fewer data with respect to appropriate platelet count for invasive procedures. In the interest of reducing transfusion exposures and decreasing the use of a limited and costly resource, the direction in which the field of platelet transfusion research has been heading is toward the minimization of transfusions. However, there is evidence showing that, in some pediatric cohorts, the bleeding incidence is > 90%,12 and that a platelet count < 100 000/μL may place leukemia patients at higher risk for a traumatic LP at diagnosis, and therefore increase risk of both CNS and hematologic relapse.20 These data suggest that our current one-size-fits-all, less-is-more approach to platelet transfusions in pediatric oncology and HSCT patients needs to be reconsidered, and that well-designed RCTs are needed to address these issues.
Measuring bleeding: the need for standardization in reporting bleeding severity and incidence
A primary outcome of interest in any platelet transfusion trial is bleeding frequency and severity, so it is critical to have a meaningful scale with which to measure bleeding. This scale needs to identify clinically significant bleeding in a consistent and reliable manner. The most commonly used scale is the one proposed by the WHO in 1979.13,24 The original scale has 5 grades: 0 = no bleeding, 1 = petechiae, 2 = mild blood loss, 3 = gross blood loss, and 4 = debilitating blood loss.13
These grades are broad and subjective, which leaves them open to individual interpretation. Despite its widespread use, the WHO scale has never been formally validated for use in research outcomes, and has also been shown to have poor inter-rater reliability.24 For research outcomes, clinically significant bleeding is defined as WHO grades 2-4. However, it has never been shown that WHO grade 2 bleeding predicts grade 3 or 4 bleeding, nor that it is associated with a worse outcome than grade 0-1 bleeding.24
A review of adult prophylactic platelet transfusion trials reveals a lack of consistency in the bleeding outcomes measured in the different studies. Bleeding scales used included an 8-point bleeding scale,6 a subjective “severe” versus “minor” bleeding assessment,7 a 4-point scale that defined severity based on the number of days of bleeding,25 and modified WHO scales.10,11 Even in trials using the WHO scale, the grade definitions vary. The PLADO trial defined an example of grade 2 bleeding as epistaxis for > 30 minutes,11 and the SToP trial used > 60 minutes as their definition.10 The inconsistent definitions of “severe” or “significant” bleeding make it difficult to compare studies or perform a meta-analysis. In the aforementioned studies, bleeding incidence ranged from 28%-77% in HSCT patients and 18%-73% in adult AML patients.6,10,11,25,26 With this broad range of bleeding incidence, it is impossible to calculate accurately the power for future trials.
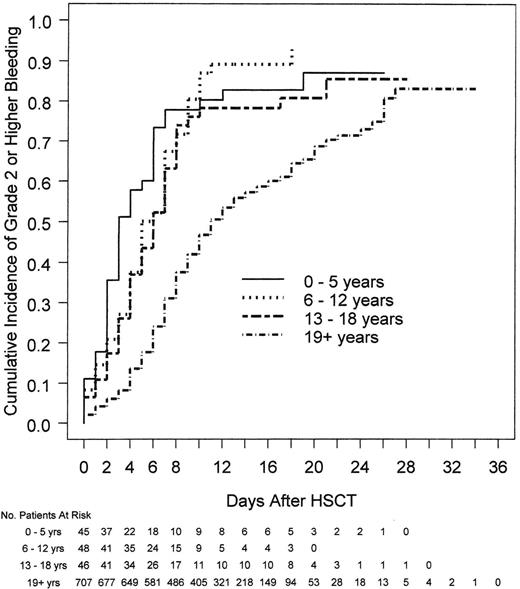
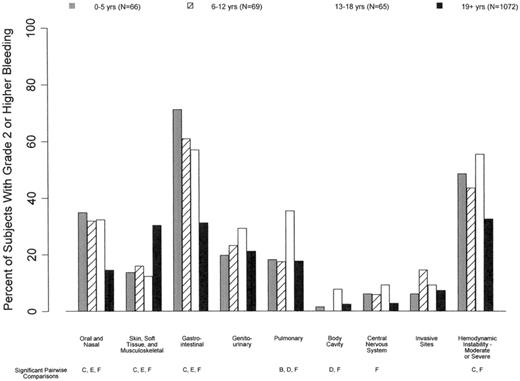
It is also important to take into consideration that patterns of bleeding in adult and pediatric patients differ. Among patients who received HSCT, all 3 of the pediatric cohorts (ages 0-5, 6-12, and 13-18 years) had significantly shorter times from HSCT to grade 2 or higher bleeding than adults (ages ≥ 19 years; median days 3.0, 5.5, 6.0, and 11.0 for the 4 age groups, respectively; P < .001; Figure 2).12 Pediatric patients were more likely to have oropharyngeal and gastrointestinal bleeding compared with adults, as well as a higher rate of hemodynamic instability associated with bleeding (Figure 3).12 Therefore, to be used in pediatric trials, tools that measure bleeding severity should be modified to take these bleeding differences into consideration and should undergo separate validation in pediatric patients of all ages.
Relationship between age and time to first bleed in HSCT patients. Pediatric patients (≤18 years of age) had a shorter time from day of HSCT to day of first bleed than adult patients (P < .001).
Relationship between age and time to first bleed in HSCT patients. Pediatric patients (≤18 years of age) had a shorter time from day of HSCT to day of first bleed than adult patients (P < .001).
Relationship between age and bleeding site. Pediatric patients were more likely than adults to have oral/nasal and gastrointestinal bleeding and less likely to have cutaneous/soft tissue bleeding (P < .01). In addition, patients 0-5 and 13-18 years of age were more likely to have hemodynamic instability associated with bleeding than patients ≥ 19 years of age (P < .01).
Relationship between age and bleeding site. Pediatric patients were more likely than adults to have oral/nasal and gastrointestinal bleeding and less likely to have cutaneous/soft tissue bleeding (P < .01). In addition, patients 0-5 and 13-18 years of age were more likely to have hemodynamic instability associated with bleeding than patients ≥ 19 years of age (P < .01).
In addition to having a reliable, objective measurement of bleeding severity, there should also be agreement in the field with respect to the best way to report bleeding incidence. There are several ways to do so, including the percentage of patients with one or more episodes of bleeding,8–11 time to first bleeding event,10,11 number of days with bleeding,6,10,11 number of bleeding episodes per patient,6,7 and highest bleeding grade per patient.10,11 The method used to measure bleeding incidence can alter the study's results.27 Cook et al reanalyzed the primary data from Rebulla's trial in 19976 using the following measurements of bleeding incidence: proportion of patients with bleeding, time to first bleed, proportion of days with bleeding, and recurrent days of bleeding.27 Although none of the methods found a statistically significant difference in bleeding between the 2 patient groups (P = 0.1-0.7), each method found a trend toward increased bleeding in the lower threshold cohort of various degrees.
There are broader questions that the clinician needs to ask. What is the goal of giving patients prophylactic platelet transfusions? Is it to prevent all episodes of bleeding, or only those that expose a patient to additional medical interventions or put them at risk for short- or long-term morbidity? Given the risks associated with platelet transfusions (ie, alloimmunization, fever, transfusion-transmitted infection, transfusion-related acute lung injury, and allergic reactions), coupled with platelets being a limited and costly resource, clinicians and researchers need to determine the incidence and severity of bleeding that is considered tolerable. Much of the bleeding recorded in platelet transfusion trials has been equivalent to WHO grade 2, mild bleeding that did not expose the patient to an invasive medical intervention (ie, surgery or endoscopy) or additional blood transfusions.6,9–11
There are many reasons that researchers include WHO grade 2 bleeding in the primary outcome. It is the most common type of bleeding in thrombocytopenic patients receiving prophylactic platelet transfusions.9–11 Grade 3 and 4 bleeding only make up approximately 15%-20%8–11 of total bleeding in patients, with an overall incidence of 7%-11%.24 With this relatively low incidence of severe bleeding, the number of patients needed to have an adequately powered noninferiority trial would be prohibitively high. However, because grade 2 bleeding is so common, it can have a significant effect on a patient's quality of life. Therefore, a consideration for future platelet trials using a composite outcome of grade 2-4 bleeding would be to include quality-of-life measurements as secondary outcomes.
There is more to bleeding than platelets and more to platelets than bleeding
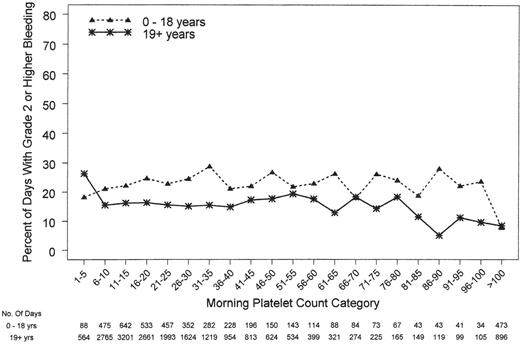
Platelet count is not the only factor that determines a patient's propensity to bleed, as illustrated by several studies showing that major bleedings events can occur at any platelet count.11,12,25,26 The pediatric subjects in the PLADO subanalysis underscored this concept, because the children were at a much higher risk of bleeding over a wide range of platelet counts compared with adults, indicating that their excess bleeding risk may be due to factors other than platelet counts (Figure 4).12 Further, the fact that platelet dose did not predict bleeding for any age group and that children had a significantly higher risk of grade 2 or higher bleeding than adults (86%, 88%, 77%, and 67% of patients 0-5 years of age, 6-12 years of age, 13-18 years of age, and adults, respectively) suggests that dose does not play a role in these patients' proclivity to bleed.11 Finally, to provide more insight into other factors influencing bleeding tendency in this population, the effect of age differed by disease treatment category and was most pronounced among autologous transplantation recipients.
Relationship between morning platelet count category and the occurrence of ≥ grade 2 bleeding on that day. Both pediatric and adult patients had bleeding at all platelet counts. Pediatric patients had a higher incidence of bleeding at the same platelet count as their adult counterparts (P < .001).
Relationship between morning platelet count category and the occurrence of ≥ grade 2 bleeding on that day. Both pediatric and adult patients had bleeding at all platelet counts. Pediatric patients had a higher incidence of bleeding at the same platelet count as their adult counterparts (P < .001).
Hemostasis is controlled by a variety of systems, including platelets and clotting factors, and there is increasing recognition of the role that the vascular endothelium plays in hemostasis. Damage to the vascular endothelium is common in HSCT patients and is a major component of many of the posttransplantation complications: venoocclusive disease, GVHD, idiopathic pulmonary syndrome, and thrombotic angiopathy.28,29 This damage can occur as a result of the preparative regimen, either chemotherapy or total body irradiation; posttransplantation GVHD prophylactic medications such as cyclosporine; or systemic inflammation.30–32 The degree of endothelial damage has been shown to be associated with increased platelet utilization in the posttransplantation period, likely due to platelet consumption during the reparative process.33 The degree of damage to the vascular endothelium may affect the incidence and severity of bleeding in a manner independent of platelet count, and may explain the differences in bleeding outcomes between pediatric and adult patients.
It is therefore vital to evaluate other factors that may put patients at risk for bleeding independent of platelet count and platelet transfusions. Studies have shown that endothelial-specific markers are elevated after transplantation, being both above the pretransplantation baseline and above healthy controls.29 To get an accurate idea of a patient's vascular endothelium integrity, it is important to evaluate the building blocks, circulating endothelial cells, as well as the cytokines that serve as indicators of endothelial damage and repair.
Angiopoietin-2 (Ang2) and VEGF are both involved in vascular repair.34 Ang2 levels on day 15 after HSCT was correlated directly with number of platelet transfusions.33 Both ICAM and VCAM play a role in maintaining the integrity of the endothelial barrier. ICAM-1 is expressed primarily by the pulmonary endothelium and VCAM-1 by the hepatic endothelium. In both adult and pediatric patients undergoing cardiac surgery with cardiopulmonary bypass (CPB), soluble ICAM-1 increased in relation to the length of time the patient was on CPB.35,36 E-selectin is expressed by endothelial cells, and its expression mediates leukocyte adhesion and rolling along the endothelium in response to inflammation.37 As yet, these markers have only been used in a research setting; however, clinical correlation may allow for a more precise understanding as to whether they are indicative of global or local endothelial damage; they may ultimately be used as clinical markers to follow the progression of diseases such as venoocclusive disease, GVHD, and interstitial pulmonary syndrome.
In HSCT and oncology patients, the risk of bleeding associated with thrombocytopenia versus the risk of platelet transfusion must also be considered in the context of a transfusion's effect on hematopoiesis. The recovery of hematopoietic function is an important milestone in the posttransplantation period because it decreases the patient's risk of serious bacterial infection and allows for independence from RBC and platelet transfusions. Myriad factors affect time to engraftment, including indication for transplantation, source of donor cells, infectious complications, and intrinsic individual patient factors.38 There are 3 primary hematopoietic growth factors: G-CSF, erythropoietin, and thrombopoietin. Rather than only being effective in their respective cell lines, there is growing evidence that these growth factors are synergistic in their effects on trilineage hematopoiesis.39,40
The liver constitutively produces thrombopoietin, an essential growth factor in megakaryocyte development and platelet production. Its serum concentration is inversely related to platelet count.41,42 Platelets have a receptor that avidly binds to thrombopoietin, effectively clearing it from the circulation.41 Studies in both adults and infants have demonstrated that transfused platelets have the same ability to clear thrombopoietin, resulting in significantly decreased levels after transfusion.43,44 In animal models, thrombopoietin has been shown to increase pluripotent hematopoietic stem cell proliferation and differentiation.45 In fact, thrombopoietin has demonstrated efficacy in the ex vivo expansion of CD34+ cells in umbilical cord blood units.46
There is clinical evidence to suggest that platelet recovery time is directly correlated with neutrophil engraftment time.9,47 A recently presented abstract evaluated adults with AML receiving intensive chemotherapy with regard to platelet transfusion strategy and time to neutrophil and platelet recovery.47 The study compared a therapeutic transfusion strategy (patients were only transfused if bleeding complications occurred) versus a prophylactic transfusion strategy (with a platelet threshold of 10 000/μL). The patients in the prophylactic group received twice the number of transfusions than the therapeutic group (8.5 ± 5.1 vs 4.2 ± 2.5, respectively) and had a longer time to neutrophil and platelet recovery (26 ± 12 vs 32 ± 7 days and 21 ± 11 vs 32 ± 12 days, respectively, P < .01).47 The therapeutic cohort had an increased incidence of bleeding, especially gastrointestinal bleeding, but this study was not powered to detect a difference in mortality. These data suggest that a reduction in platelet transfusions may lead to a decreased number of transfusions and faster recovery of hematopoiesis, resulting in fewer in-hospital days. Larger randomized studies in adult and pediatric patients are needed to further evaluate this possibility in both oncology patients and HSCT patients and to determine its effect on posttransplantation mortality.
Summary and future directions
The question of the optimal platelet transfusion strategy in pediatric oncology and HSCT patients is a complicated conundrum to address. There are several issues to consider before RCTs can be designed to explore this issue. The development of a validated and reliable pediatric-specific scale to measure the severity and incidence of bleeding is a necessary first step in this process. Once such a scale is established, it will be important to define and then determine the incidence of clinically significant bleeding (ie, bleeding that prophylactic platelet transfusions are used to prevent) among pediatric oncology and HSCT patients. Future RCTs that plan to compare platelet transfusion regimens should also be designed to address the other issues that contribute to bleeding risk and to determine the effect of platelet transfusions on outcomes not related to bleeding (eg, time to engraftment and event-free and overall survival).
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Cassandra D. Josephson, MD, Children's Healthcare of Atlanta, Egleston Campus-Pathology, 1405 Clifton Rd NE, Atlanta, GA 30332; Phone: 404-785-4553; Fax: 404-785-1370; e-mail: cjoseph@emory.edu.