Abstract
Central venous catheters (CVCs) are used extensively in cancer patients for the administration of therapy and phlebotomy. An important complication of CVCs is the development of catheter-related thrombosis (CRT), which becomes symptomatic in approximately 5% of the patients. Several factors, such as insertion location and position of the catheter tip, increase the risk of CRT. Prevention of CRT with systemic anticoagulant prophylaxis has largely been ineffective. In addition, the optimal diagnostic strategy and anticoagulant treatment are unclear due to the lack of well-designed studies. The most recent American College of Chest Physicians guidelines recommend (color) Doppler ultrasound more than venography as the initial diagnostic test in patients with suspected arm thrombosis. Only if the ultrasound is negative and clinical suspicion is high is further testing with D-dimer, serial ultrasound, or venography advocated. In case of CRT, removal of the catheter is not necessary if it is functional and needed for chemotherapy. Anticoagulant treatment of CRT consists of treatment with low-molecular-weight heparin (LMWH) followed by vitamin K antagonists for at least 3 months. Whether long-term treatment with LMWH is more effective than vitamin K antagonists in cancer patients with CRT is unknown, but LMWH may be advocated following the recommendations in lower limb thrombosis and cancer. In addition, the effect of new anticoagulants in CRT has not been studied.
Introduction
Central venous access devices or catheters are used extensively in cancer patients to secure vascular access to provide cancer treatment and supportive care therapies.1 These devices also improve patients' quality of life by reducing the need for venipunctures and allowing patients to receive chemotherapy, parenteral nutrition, and other IV therapies at home. All long-term central venous catheters (CVCs) are designed to have the distal catheter tip dwelling in the central venous system at the junction of the superior vena cava and the right atrium. CVCs can be grouped roughly into tunneled catheters with an anchoring cuff, nontunneled catheters, implanted ports, peripherally inserted central catheters (PICCs), and apheresis or dialysis catheters. They can have single, double, or triple lumens with valved or open ends. Valves prevent the reflux of blood into the lumen of the catheter and obviate the need to use heparinized solutions for flushing. In general, the smallest diameter catheter should be used to minimize venous obstruction in the cannulated veins, but catheters with multiple lumens are needed in patients who require infusion of drugs, blood products, or total parenteral nutrition.
Catheter-related thrombosis (CRT) is a relatively common complication in patients with long-term indwelling CVCs and may pose clinicians with difficult decisions on what anticoagulant treatment to choose and whether the CVC must be removed. In this review, we discuss the epidemiology of CRT, risk factors that predispose to CRT, and the diagnostic options to detect CRT in patients. We also summarize the current literature on the usefulness of anticoagulant prophylaxis to prevent CRT and provide current recommendations on the type and duration of anticoagulant treatment in patients with CRT. We focus largely on published data in adults, because CRT has a different natural history and carries a different prognosis in neonates and children.2 Thrombotic complications of hemodialysis and apheresis catheters or nonthrombotic occlusions are not discussed here.
Epidemiology of CRT
There are multiple types of thrombotic complications associated with CVCs. Although standard definitions are not available, most clinicians refer to venous thrombosis involving the vein(s) in which the catheter dwells as CRT. First, the fibrin sheath may be occluded, which may progress to an intraluminal occlusion that may progress to a mural thrombosis at the tip of the catheter. All of these complications may be managed with local thrombolytic treatment.3 When the thrombus extends into the vessel outside of the CVC and compresses the adjacent vein, a catheter-related venous thrombosis occurs. Most CRTs occur in the upper extremity, where most catheters are placed.
CRTs may be symptomatic or asymptomatic. Symptomatic cases occur in up to one-third of patients with a CVC.4 Symptoms of CRT include swelling, pain, redness, discoloration, and even cyanosis. Most patients with CRT are asymptomatic, even in the presence of an extensive, occlusive thrombus in the proximal veins. Some patients will complain of an ache in their shoulder or jaw without any other physical findings. Dilated superficial veins on the ipsilateral chest wall and neck can be observed. In patients who go on to develop superior vena cava syndrome, dyspnea, facial flushing and swelling, neck pain or swelling, headaches, or a sensation of head fullness or “head rush” are typical symptoms. It is not uncommon for patients to present with difficulty with infusion and/or aspiration of the catheter as the only sign of CRT. Therefore, the clinical picture of CRT is highly variable, varying from very mild symptoms to vena cava syndrome.
Considering the incidence of CRTs, 70%-80% of all upper extremity thromboses are associated with a CVC, and this involves approximately 10% of all cases of venous thromboembolism.5 The incidence of CRT depends heavily on the method of diagnosis. In earlier days, diagnosis was confirmed with venography, which shows more asymptomatic CRTs compared with ultrasonography. Conversely, catheter characteristics and maintenance care, patient-related risk factors for venous thromboembolism, and the type of infusions delivered by the catheter result in a lower incidence of CRT over time. Earlier studies published in the 1980s and 1990s reported rates as high as 66%, but more recent trials showed that only approximately 14%-18% of patients have evidence of CRT when screening venography or ultrasonography is performed.6–8 Symptomatic CRT occurs much less frequently, at approximately 5% or lower in prospective cohort studies.9,10 The majority of cases occur within the first 100 days after catheter insertion.11 Whether there are differences in patient outcomes between symptomatic and asymptomatic CRT is unknown. In children, it has been documented that asymptomatic CRT can have serious long-term consequences.12 With the evolution in insertion techniques (eg, increasing use of ultrasound guidance for insertion), catheter material and designs, maintenance care, and cancer-related treatments, it is expected that the risk of CRT will continue to change over time.
Complications of CRT
CRT may lead to pulmonary embolism, infection, and loss of catheter function. Delay in the diagnosis can lead to superior vena cava syndrome and formation of a right atrial thrombus. CRT also can delay the administration of chemotherapy and expose patients to the hazards of therapeutic anticoagulation. Pulmonary embolism has been detected in up to 15% of patients with symptomatic CRT, and fatal pulmonary embolism can occur rarely.13 In the RIETE registry, of the 512 patients with upper-extremity thrombosis, 9% had a pulmonary embolism compared with 29% in patients with venous thrombosis of the legs. In 45% of the patients with arm thrombosis, a CVC was present.14
Loss of catheter function or flow obstruction is probably the most frustrating complication of CRT because of the loss of venous access. Consequently, the use of heparin flushing has long been a part of standard practice in catheter maintenance to reduce catheter obstruction; however, the evidence for its efficacy is weak and the use of heparin flushes is now discouraged.4 In patients with catheter obstruction and CRT, therapeutic anticoagulation is necessary. Instilling small doses of a thrombolytic agent into the catheter lumen can sometimes open the blockage to allow infusion to continue.15 If this is not effective, because of the added morbidity and cost, consideration should be given to reassessing patency after a few days of therapeutic anticoagulation before replacing the catheter. If the catheter remains obstructed despite anticoagulation, it will be necessary to remove and replace it. Permanent loss of venous access of the cannulated veins can sometimes occur, which can be problematic in patients who need long-term access.
Another potential complication of CRT is the development of the postthrombotic syndrome. In a prospective study of 53 patients with upper-extremity deep vein thrombosis, 12 of 47 patients without catheters developed postthrombotic syndrome over 2 years of follow-up compared with 1 of 6 patients.16 In a retrospective study of 41 patients with axillosubclavian deep vein thrombosis, 28% developed postthrombotic syndrome but in the 8 patients who had CRT, none developed.17 Overall, it appears that postthrombotic syndrome, described in up to one-third of patients with lower extremity deep vein thrombosis, is less common after CRT.18 Obviously, the choice of CRT treatment may influence this outcome. In a retrospective series of 112 cancer patients with CRT who were treated with a variety of strategies (anticoagulation with or without line removal or line replacement, line removal alone, or line replacement alone), only 4 patients had persistent symptoms and were managed with line replacement without anticoagulation.19 Some patients with CRT are left with prominent superficial veins on the affected side of the chest wall because of collateral formation, especially if the thrombosis was extensive.
In conclusion, CRT is a serious disease with many clinical consequences. Adequate and prompt diagnosis upon clinical suspicion of CRT, followed by appropriate anticoagulant treatment, is necessary to prevent further complications.
Risk factors of CRT
Many observational studies have reported on potential risk factors of CRT. However, the reliability and accuracy of the data are limited by the retrospective design, small sample sizes, few CRT events, variable duration of follow-up, and heterogeneity in the baseline variables captured and outcome definitions. Risk factors may also vary in different patient populations. Furthermore, the use of prophylaxis is often unspecified and uncontrolled in these studies, leading to more uncertainty about the incidence and risk factors of CRT.
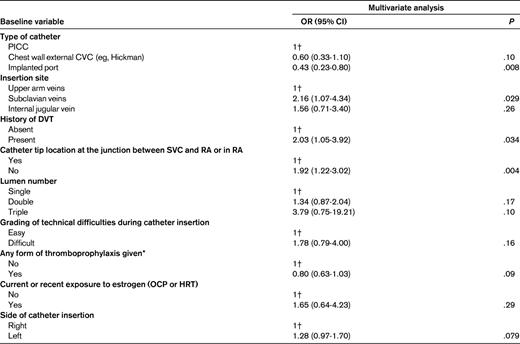
The largest study performed to date examined 17 baseline characteristics as potential risk factors for CRT.11 In a patient data-level meta-analysis that included data from 5636 subjects with or without cancer were enrolled in 5 randomized trials and 7 prospective studies. A total of 425 CRT events were observed and several variables were significantly associated with a higher risk of CRT (Table 1). The position of the catheter in the subclavian vein compared with the upper arm vein and improper positioning of the catheter tip doubled the risk of CRT. A previous history of deep vein thrombosis also resulted in a higher risk (overall risk [OR] = 2.03; 95% confidence interval [CI], 1.05-3.92). Furthermore, implanted ports were associated with a 57% relative risk reduction in CRT (OR = 0.43; 95% CI, 0.23-0.80) compared with PICC lines. Factors that showed a nonsignificant association with CRT included: (1) higher lumen number, (2) technically difficult procedures (3) left-sided insertions, (4) estrogen exposure, and (5) lack of thromboprophylaxis (Table 1). However, whether these latter factors increase the risk of CRT remains uncertain, because data on some of these baseline variables were missing or not captured in the original studies included in the meta-analysis. Given that it is biologically plausible for these factors to enhance thrombosis, it is reasonable to reduce exposure to these factors if possible. Because of the lack of data, the study was not able to account for competing events or determine the impact of other known risk factors for venous thromboembolism, such as heritable thrombophilia, underlying cancer type and status, and use of chemotherapeutic or antiangiogenic agents. A recent Cochrane review on the location of a CVC concluded that jugular and subclavian CVCs carry the same risk of CRT in long-term catheterization.20
The mechanisms for the various risk factors for CRT are poorly studied. A previous history of thrombosis is also a strong predictor of recurrent thrombosis in deep vein thrombosis and pulmonary embolism.21 Some of these cases may reflect the presence of an underlying thrombophilia or other undiagnosed genetic predisposition.22,23 In contrast, the entry/exit site of the catheter (eg, subclavian versus internal jugular vein) may be important because of anatomical or mechanical factors. Improper position of the catheter tip has been reported consistently to increase the risk of CRT. Having the tip at the junction of the superior vena cava and right atrium may be protective because of a greater dilutional effect when chemotherapeutic agents are infused or because there is a lower likelihood that the tip of the catheter will be in direct contact with the endothelium. Implanted port devices may be associated with a lower risk of CRT than PICCs because of a lower incidence of infection (which in turn can lead to thrombosis) or because there is less movement of the catheter with port devices, which thereby reduces endothelial trauma. Larger diameter catheters are likely to cause more venous stasis and turbulent flow, thus triggering activation of coagulation factors. Left-sided insertions and technically difficult insertions suggest that thrombus formation is initiated by endothelial injury secondary to the mechanics of the procedure. Given that the catheter is a foreign surface, the role of the contact pathway of coagulation and inflammatory responses require investigation. Better understanding the pathophysiologic mechanisms involved could help to reduce the risk of CRT.
Diagnostic strategy in patients with suspected CRT
In patients with suspected CRT, Doppler ultrasonography of venography of the arm veins is performed most often. However, in contrast to the numerous studies in suspected venous thrombosis of the legs, there is a paucity of diagnostic studies in upper-extremity thrombosis. It is unclear whether the results of leg thrombosis can be extrapolated to suspected arm thrombosis. The anatomy of the arm veins is different, and especially part of the subclavian vein that crosses the clavicle makes a proper diagnosis with ultrasound difficult. Venography is less often used, which threatens its place as the gold standard.
Overall, ultrasonography is used most often to diagnose upper-extremity thrombosis. However, prospective management studies are lacking to evaluate the efficacy of a single or serial ultrasound in suspected upper-extremity thrombosis. In a systematic review based on 793 patients from 17 studies, Di Nisio et al found that sensitivity was 97% (95% CI, 90%-100%) for compression ultrasonography, 84% (95% CI, 72%-97%) for Doppler ultrasonography, and 91% (95% CI, 85%-97%) for Doppler ultrasonography with compression, but noted that most studies were small and of poor quality.24
Clinical probability scores and D-dimer test are commonly used as safe diagnostic tests in the diagnostic workup for thrombosis of the lower limbs. Only limited data are available on the applicability of these diagnostic tools for upper-extremity thrombosis. A recent study attempted to develop a clinical score combining 4 items: presence of CVC or pacemaker, localized pain, unilateral pitting edema, and other diagnosis as plausible. Based on this score, 214 patients were divided into a low-, intermediate-, or a high-probability group.25 The prevalence of ultrasonography-confirmed upper-extremity deep vein thrombosis in the 3 groups was, respectively 12%, 20%, and 70%. The sensitivity of this score was 78% with a specificity of 64%. A prospective study in 52 patients evaluated the accuracy of the D-dimer test.26 The sensitivity, specificity, and positive and negative predictive values of the test were 100%, 14%, 32%, and 100%, respectively. Overall, the safety of withholding anticoagulant therapy based on a low clinical probability score and a normal D-dimer has not been evaluated in upper-extremity thrombosis. Moreover, no study has so far evaluated the safety and feasibility of (serial) ultrasonography within an algorithm in combination with a clinical decision rule and D-dimer. If proven safe, such a strategy could avoid unnecessary additional testing and confirm the value of ultrasonography for the diagnosis of upper-extremity thrombosis as for lower-extremity thrombosis. Currently, a large diagnostic study is ongoing assessing the efficacy and safety of this diagnostic strategy in more than 400 consecutive patients with suspected upper-extremity thrombosis (www.clinicaltrials.gov identifier NCT01324037). Results are expected later this year.
Another interesting technique is magnetic resonance venography, but management studies are currently lacking. The most recent ACCP guidelines suggest the use of a Doppler or color Doppler ultrasound over other initial tests such as venography (grade 2C).27 If the ultrasound is negative and clinical suspicion is high, further testing with D-dimer, serial ultrasound, or venography is advocated (grade 2C).27
Thromboprophylaxis to prevent CRT
Various strategies have been used to reduce thrombotic complications secondary to indwelling catheters. Using a heparinized saline solution to flush and/or lock catheters to reduce catheter occlusion has been the standard of care for many years, but recent reviews show that there is weak evidence for heparin flushing in reducing catheter occlusion.4 Heparin flushing can also increase the risk of heparin-induced thrombocytopenia and, possibly, bleeding. Medical errors associated with using the wrong concentration of heparin have also been reported. There is no evidence that heparin flushing reduces the incidence of CRT. In addition, the benefits of heparin-bonded catheters on reducing thrombosis are scarce, unpowered, and published more than 2 decades ago.4 The majority of trials in adults also focused on the rates of bacterial colonization or infection of the catheter rather than on thrombosis. In a small study of 49 patients, a reduction in thrombotic complications was not observed.28 Overall, this is no strong evidence that heparin-bonding reduces catheter thrombotic complications.
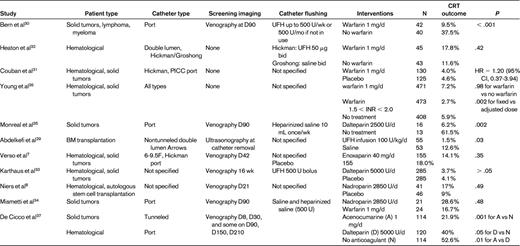
The efficacy and safety of using anticoagulant prophylaxis to prevent CRT has been investigated in several randomized trials (Table 2).7,8,29–37 The earliest studies suggested that low-dose warfarin (1 mg daily) or LMWH at a standard postoperative prophylaxis dose could reduce the incidence of CRT in cancer patients significantly.29,30 However, these were small, open-label studies that used screening venography to detect asymptomatic CRT. More contemporary randomized controlled trials evaluating warfarin and LMWH prophylaxis have failed to show any significant reduction in symptomatic or asymptomatic CRT (Table 2). In the open-label WARP study, the largest trial studying warfarin to date in 1590 patients, warfarin given in a fixed dose (1 mg daily) or adjusted dose (international normalized ratio, 1.5-2.0) did not lower the rate of symptomatic CRT compared with no prophylaxis (5.9% vs 5.9%; OR = 0.99; 95% CI, 0.57-1.72; P = .98).36 However, adjusted-dose warfarin was associated with a significant reduction in CRT compared with fixed-dose warfarin (2.7% vs 7.2%; OR = 0.38; 95% CI, 0.20-0.71; P = .002), with a trend toward more major bleeding (3.4% vs 1.5%; OR = 2.28; 95% CI, 0.95-5.48; P = .09). Similarly, randomized trials comparing LMWH with placebo or observation also have not been to demonstrate any efficacy in reducing symptomatic or any CRT using dalteparin, enoxaparin, or nadroparin.7,8,33,37 Increase in bleeding with LMWH prophylaxis in these trials was not reported.
The most recent Cochrane systematic review published in 2011 included 12 studies enrolling 36 111 patients with cancer.38 It found that prophylactic doses of unfractionated or LMWH were not associated with a statistically significant effect on symptomatic CRT (relative risk [RR] = 0.54; 95% CI, 0.28-1.05), asymptomatic CRT (RR = 0.81; 95% CI, 0.64-1.02), major bleeding (RR = 0.68; 95% CI, 0.10-4.78), or infection (RR = 0.91; 95% CI, 0.49-1.68). Similarly, low-dose vitamin K antagonists were not associated with a statistically significant reductions in symptomatic CRT (RR = 0.63; 95% CI, 0.35-1.11) or major bleeding (RR = 6.93; 95% CI, 0.86-56.08), but were associated with a significant reduction in asymptomatic CRT (RR = 0.42; 95% CI, 0.28-0.61). Differences in major bleeding and mortality were not observed, but the low event rates preclude any conclusion about the safety of anticoagulant prophylaxis regimens.
Overall, the available evidence does not support the use of anticoagulant prophylaxis to prevent CRT in cancer patients. Based on the WARP study, bleeding may also be a concern with higher intensity regimens. Therefore, major consensus guidelines do not recommend anticoagulant prophylaxis routinely in this setting.39
Treatment of CRT
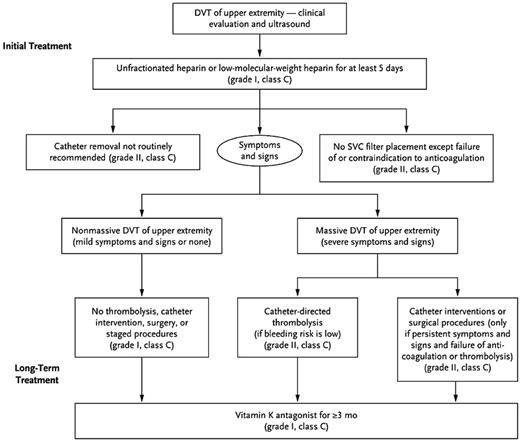
Treatment of CRT aims at preventing recurrent events, including potentially fatal pulmonary embolism, which in turn could reduce the morbidity, use of health care resources, and, above all, mortality. As with the diagnosis of CRT, prospective studies of the treatment of CRT are scarce, so recommendations are generally based on results from venous thrombosis of the legs. There are no randomized controlled trials comparing different treatment strategies. The ACCP guidelines do not recommend removing the catheter in case of a CRT if the catheter is functional and needed.40 If removal of the catheter is necessary, this may be performed after 3-5 days of anticoagulant treatment, although the optimal timing is unclear. Anticoagulant treatment of CRT follows the recommendations of venous thrombosis of the legs: initial treatment with LMWH, unfractionated heparin, or fondaparinux, followed by vitamin K antagonists for at least 3 months (Figure 1). The ACCP recommends anticoagulant treatment alone over thrombolysis for the treatment of acute upper-extremity thrombosis based on the absence of a better efficacy-risk benefit of the latter treatment.40 However, the very recent the CaVenT study suggested that thrombolysis resulted in a better patency and lower rate of postthrombotic syndrome in acute iliofemoral thrombosis.41 This study involved only patients with lower leg thrombosis, so no conclusions can be drawn for catheter-related thrombosis of the arm.
Management of upper-extremity thrombosis. Reprinted with permission from Kucher.5
Management of upper-extremity thrombosis. Reprinted with permission from Kucher.5
Although treatment with LMWHs is more effective than vitamin K antagonists in cancer patients with acute VTE, there are no data in cancer patients with CRT available. Nevertheless, LMWH as long-term treatment in CRT and cancer may be advocated following the recommendations in VTE and cancer. In addition, the effect of new anticoagulants in CRT has not been studied.
Conclusions
CRT is associated with significant morbidity to individual patients and represents a significant economic burden on the population as a whole because of the total number of patients affected. Anticoagulant prophylaxis to prevent CRT is not recommended, but future research in high-risk patients should give more definitive answers. The absence of large-scale studies in this area is worrisome. The optimal diagnostic strategy, adequate initial and long-term anticoagulant treatment, and potential role of new oral anticoagulants in CRT are largely unknown and should be studied.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Pieter W. Kamphuisen, Department of Vascular Medicine, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; Phone: 31-50-3612943; Fax: 31-50-3619069; e-mail: p.w.kamphuisen@umcg.nl.