Abstract
Hairy cell leukemia (HCL) is a B-cell malignancy that in its classic form is exquisitely sensitive to single-agent purine analog therapy, but that is associated in many patients with late relapse and eventual purine analog resistance. Minimal residual disease, which is present in most patients achieving complete remission with purine analogs, retains Ags that are ideal for targeted therapy. Rituximab, which targets CD20, is active as a single agent, particularly if combined with purine analogs. Recombinant immunotoxins targeting either CD25 or CD22 and containing truncated Pseudomonas exotoxin have achieved major responses in relapsed/refractory HCL. Moxetumomab pasudotox in phase 1 testing achieved responses in 86% of such patients (complete in 46%) without dose limiting toxicity and often without MRD. Soluble CD22 has been used for improved detection and monitoring of HCL, particularly the poor-prognosis variant that lacks CD25. Ig rearrangements unique for each HCL patient have been cloned, sequenced, and followed by real-time quantitative PCR using sequence-specific reagents. Analysis of these rearrangements has identified an unmutated IGVH4-34–expressing poor-prognosis variant with immunophenotypic characteristics of either classic or variant HCL. The BRAF V600E mutation, reported in 50% of melanomas, is present in > 85% of HCL cases that are both classic and express rearrangements other than IGVH4-34, making HCL a potential target for specific inhibitors of BRAF V600E. Additional targets are being defined in both classic and variant HCL, which should improve both detection and therapy.
Introduction
Hairy cell leukemia (HCL) is an indolent B-cell leukemia first described by Bouroncle et al in 1958 as a distinct entity comprising 2% of all leukemias,1 which today would approximate 1000 of the 47 150 new cases of leukemia per year in the United States.2 Patients were noted to present with pancytopenia, splenomegaly, and malignant “reticulum” or “histiocyte” cells in the BM, blood, and spleen that contained hairy-like cytoplasmic projections.1 Originally called “leukemic reticuloendotheliosis,” the disease later became known as HCL. Malignant cells were characterized by flow cytometry because B cells strongly express the B-cell Ags FMC7, CD11c, CD20, CD22, and surface Ig. In the typical or classic form, CD103, CD25, and CD123 were also expressed.3 By 20 years after its original description, treatment for HCL was limited to cytotoxic chemotherapy and splenectomy, and the median survival of HCL patients was approximately 4 years.4 Splenectomy became the treatment of choice up until 1984, with retrospective studies suggesting modest survival benefits.5 The era of effective systemic treatment for HCL began in 1984 with the introduction of IFN. Overall response rates (ORRs) in several trials ranged from 69% to 87% and complete remission (CR) rates were up to 25%, with a large randomized trial showing a CR rate of 11%.5 Treatment of HCL was revolutionized with the advent of purine analog therapy in the late 1980s.
Treatment of HCL with purine analogs
Although systemic treatment of HCL was in its early stages, advances were made in identifying and inhibiting target pathways in leukemias. In the early 1970s, adenosine deaminase (ADA) was recognized as critical for the proliferation of lymphocytes. A specific inhibitor, 2-deocycoformycin (pentostatin), was developed as a potent transition state inhibitor to block the conversion by ADA of adenosine to inosine. HCL cells have unusually low levels of ADA, and by 1986, pentostatin was reported as highly effective in this disease, eventually documenting CR rates of > 75% and 10-year disease-free survivals of up to 68%.6 Soon after pentostatin became the treatment of choice for HCL, another purine analog, cladribine, took center stage. Originally called 2-chloro-2′-deoxyadenosine (CdA), cladribine was found to become phosphorylated to 2-Cl-dATP by deoxycytidine kinase, an enzyme found at high levels in HCL cells.7 Beginning in 1990, a single 5- to 7-day infusion of cladribine was found to be just as effective as pentostatin for the treatment of HCL, with CR rates of 87% and ORRs of 97% among 555 patients from 6 trials receiving frontline therapy.7 The advantage of treatment with a single cycle administered over 5-7 days made cladribine the most commonly used frontline treatment for HCL. However, the more gradual dosing of pentostatin, administered every other week for 3-12 months, was noted to be potentially advantageous in some clinical situations, such as in high-risk patients with severe cytopenias and/or infections who may require a lower dose at first.6
Long-term results of purine analog therapy for HCL
The excellent success of purine analogs seemingly left HCL a “beaten” disease, but long-term follow-up studies of cladribine and pentostatin failed to document a plateau in the relapse-free survival (RFS) curves.8–9 Counting nonresponders, the median RFS is 16, 11, and 6.5 years after first-, second-, and third-line purine analog treatment, respectively.8 Therefore, if a patient were retreated at relapse, the median time to failure of 3 courses of purine analog would be less than 35 years. Although patients responding poorly to the first course of purine analog can occasionally respond better to a subsequence course, the reverse is much more common. Purine analogs have significant toxicity to stem cells, particularly to CD4+ lymphocytes, with the median time to recovery of CD4+ cells being 54 and 40 months for pentostatin and cladribine, respectively, after a single course.10 Patients chronically immunosuppressed from purine analogs often encounter and occasionally succumb to opportunistic infections. There is an association, albeit controversial, between purine analogs and secondary malignancies. Purine analogs are associated with a 15% rate of neurotoxicity, and patients receiving multiple courses may have significant long-term complications.11 Purine analogs are clearly the treatment of choice for HCL, but the promise of life without relapse or eventual death from treatment-refractory disease cannot be made, particularly for younger patients. This imperfect outlook for the patient with HCL has stimulated the study of markers of response and molecular targets for biologic approaches.
Variant HCL
Approximately 10% of HCL patients have a variant called HCLv, which has a morphology intermediate between classic HCL and prolymphocytic leukemias.3,12 Patients typically present with worse splenomegaly and leukocytosis rather than leukopenia and often lack cytopenias.12–13 Although HCLv shares the B-cell Ags of classic HCL, the World Health Organization (WHO) defines HCLv as a separate disease lacking CD25, annexin 1a (ANXA1), or tartrate-resistant acid phosphatase (TRAP), and also lacking sensitivity to frontline purine analog therapy.3,12,14 In HCLv, purine analog therapy is associated with ORR < 50% and CR rates < 10%,12 with overall survival (OS) only 9 years from diagnosis.13 There have been case reports of major responses using rituximab or alemtuzumab for HCLv.12 Although somatic hypermutations in the IGHV domains of classic HCL cells resemble those of normal B cells exposed to Ag, the so-called “canonical” somatic hypermutation process is significantly less in HCLv, indicating that such cells (at least in some patients) have different origins from those with classic HCL.15
IGHV4-34 expression as a poor prognostic factor
HCL patients expressing unmutated rearrangements of the IGHV4-34 type, both those with immunophenotypic features of classic HCL and those with HCLv, are reported to have a particularly poor prognosis.13 IGHV4-34+ HCL is suggested clinically by lack of leukopenia at presentation and essentially universal failure of frontline purine analog therapy13 to achieve CR. Of 82 patients skewed toward those seeking salvage therapy, OS was 8.6 versus 26 years (P < .0001) for IGHV4-34 positive versus negative patients, respectively.13 IGHV4-34 remained important even when only classic HCL patients were considered, with OS of 4.7 versus 27 years (P < .0001) for 14 IGHV4-34+ versus 68 IGHV4-34− patients, respectively.13 Thus IGHV4-34 was independent from HCLv as a poor prognostic sign. OS was not significantly different comparing classic HCL and HCLv when IGHV4-34 cases were excluded (P = .2), suggesting that the poor OS believed to be due to HCLv may be due in part to the presence of previously unrecognized cases of HCLv that also express IGHV4-34.
MRD in HCL
CR in HCL is defined by complete resolution of hepatosplenomegaly, adenopathy, cytopenias, and HCL cells visible by nonimmunologic studies of the blood and BM, but patients may have low numbers of HCL cells assessable by immunologic staining. The lack of plateau on the RFS curve after purine analogs has paralleled the recognition of minimal residual disease (MRD) documented by immunohistochemistry of the BM biopsy, flow cytometry of the BM or blood, or PCR for the patient's Ig rearrangement. Early relapse may be predicted by MRD detected by immunohistochemistry,16 although studies have been small and largely retrospective. Although data are so far lacking, it is suspected that MRD assessed by flow cytometry or PCR will also be correlated with early relapse. We have used the Ig rearrangement (IGHV) sequence of each patient to create patient-specific probe and primer sets for real-time quantitative PCR capable of detecting 1 HCL cell in 106 normal cells. Time will be needed to determine whether such ultrasensitive tests can predict relapse or HCL eradication. Sigel et al have performed standard MRD studies in 19 of the 358 patients treated at the Scripps Clinic with cladribine who were in continuous hematologic CR for a median of 16 years, and found that 9 (47%) were MRD free. This suggested that cure is possible in a fraction of patients,17 although it is not clear that MRD negativity by standard tests at 16 years is equal to cure. The HCL cells composing MRD retain their bright positivity for HCL Ags, including CD20, CD25, and CD22, suggesting these markers as targets for biologic approaches such as mAbs and immunotoxins (Table 1).
Rituximab targeting CD20 in HCL
CD20 is one of the most densely expressed Ags in HCL. Rituximab is a human-murine chimeric mAb binding to CD20 with a Kd of approximately 8.0 nm,18 and kills cells by inducing apoptosis and mediating either complement-dependent cytoxicity (CDC) or Ab-dependent cytotoxicity (ADCC).19 CR rates among the 7 reported rituximab prospective and retrospective studies in HCL (6-25 patients each, total 103) varying from 10%-54%.19 However, there were 9 (19%) CRs and 10 (21%) partial remission (PRs) among 48 patients from 5 of these studies who demonstrated a need for treatment based on cytopenias and who had at least 1 prior course of purine analog. Rituximab was usually administered by 4-8 weekly doses of 375 mg/m2 and some patients required more than 4 doses to respond. Toxicities of rituximab are infusion related (eg, hypotension, bronchospasm, rhinitis, pruritus, rash, urticaria, and tumor pain) and decrease with repeated dosing. Rituximab appears effective and well tolerated in HCL, but the number of reported patients is limited. The lower response rate of rituximab relative to the purine analogs may be related to cellular resistance to the apoptotic pathway induced by rituximab, insufficient immunity to mediate CDC or ADCC, and/or limited tumor penetration of densely packed clumps of cells.
Retrospective data for rituximab combined with cladribine or pentostatin
It is known that rituximab synergistically increases the apoptotic effect of cladribine or bendamustine toward human lymphoma cell lines and chronic lymphocytic leukemia (CLL) samples20 and improves the outcome of several B-cell leukemias and lymphomas when combined with chemotherapy. In retrospective analyses, 18 HCL patients received purine analog plus rituximab,8,21 including 11 with rituximab concurrent with pentostatin, 1 with rituximab following pentostatin by 1 month, 3 with rituximab concurrent with cladribine, and 3 with rituximab following cladribine by 1-2 months. All (100%) 18 responded, with 16 (89%) CRs, and all 6 had CR after cladribine and rituximab. Response in these patients was improved compared with previous purine analogs given without rituximab. In this analysis, combination purine analog-rituximab therapy was used as the second- to sixth-line therapy, highlighting its efficacy.8,21 There are no reported prospective trials of pentostatin plus rituximab for HCL.
Prospective trials of rituximab and cladribine for early HCL
To determine whether rituximab could improve the outcome of early HCL by removal of MRD left over after cladribine, Ravandi et al enrolled 31 HCL and 5 HCLv patients, all without prior therapy, in a phase 2 trial in which cladribine in 5 daily doses was followed 1 month later by 8 weekly doses of rituximab.22 Patients with classic HCL had relatively mild disease at baseline, with WBC counts of 0.9-8.2 × 109/L (median, 2.7 × 109/L) and platelet counts of 36-206 × 109/L (median, 77 × 109/L) cells; spleens were usually nonpalpable. At the 1-month time point before the rituximab, 12 (44%) of 27 adequately restaged patients had achieved CR. However, by the end of the rituximab, all 36 patients eventually achieved CR. None of the HCL and 1 of 5 HCLv patients relapsed at 2-68 months (median, 25) of follow-up. The relapsed HCLv patient died, as well as 2 additional HCLv patients who developed pancreatic and metastatic lung secondary malignancies, but the 2 remaining HCLv patients remained in CR at 12 and 35 months. MRD in the BM was assessed by flow cytometry and PCR using IGHV consensus primers before and at 1 and 3 months after cladribine. Flow cytometry was positive in all 31 HCL patients before cladribine, 22 (85%) of 26 at 1 month, and 6 (21%) of 28 at 3 months, whereas PCR was positive in 17 (81%) of 21 before cladribine, 13 (54%) of 24 at 1 month, and 8 (30%) of 27 at 3 months.22 MRD was harder to detect in the peripheral blood, which was followed for MRD at 6 months and beyond. No patients were positive by PCR of blood, and at least 3 patients were positive by flow cytometry of blood at 6, 12, 15, or 42 months. IgG but not IgM or IgA decreased after cladribine and then returned to baseline. It was concluded that rituximab may improve the CR rate of cladribine in previously untreated classic HCL patients. However, the investigators suggested that the cost of therapy may not justify this approach for all patients with HCL, particularly those with low risk, and suggested larger randomized trials with longer follow-up times to resolve the issue.22 To objectively determine the outcome of adding rituximab to cladribine, classic HCL patients with 0-1 prior courses of cladribine have been randomized at the National Institutes of Health (NIH) to 8 weekly doses of rituximab beginning either on day 1 or at least 6 months after cladribine. A second course of rituximab is given to either arm if and when any MRD is detected. So far, of 55 patients no longer under treatment to improve their response, all (100%) have achieved CR. MRD is being compared in these arms to determine whether rituximab should be added upfront to the standard treatment of early HCL. Early use of rituximab, with its reversible effects on normal cells, may be justified if it can prevent or even delay relapse and the eventual use of repeated courses of purine analogs with cumulative and often irreversible toxicities. Alternatively, the use of purine analogs alone, with rituximab reserved until MRD is detected, may provide equal or even longer MRD-free survival and may avoid the use of rituximab in the fraction of patients who might never relapse after purine analogs alone. It should be emphasized that, regardless of treatment, these studies must follow MRD in patients for very long periods of time.
Studies of rituximab combined with other purine analogs
Fludarabine has shown limited activity in HCL in small trials. Gerrie et al recently reported a retrospective review of 15 HCL patients treated over a 7-year period in British Colombia with combination fludarabine and rituximab, all of whom were previously treated and most of whom were multiply relapsed.23 Of 13 evaluable patients, 1 had PR and progressed at 31 months, 3 had MRD− CRs, and 9 met the criteria for CR without having BM studies. Patients with CR or CR without BM studies had not relapsed at 10-80 months (median, 37) of follow-up.23 The efficacy of bendamustine, which is part purine analog and part alkylating agent, was recently described in HCL.24 To more objectively determine the efficacy of combinations of pentostatin-rituximab and bendamustine-rituximab in patients with HCL, a prospective randomized phase 2 trial is underway at NIH testing each of these 2 combinations. Patients on this trial are multiply relapsed and have received at least 2 prior courses of purine analog unless refractory to the first course (defined as responding in < 1 year). Before beginning the trial, an initial cohort received bendamustine at 70 mg/m2 on days 1 and 2 in combination with rituximab (375 mg/m2) on days 1 and 15. Based on its acceptable tolerability, bendamustine is currently being administered to randomized patients at a dose level of 90 mg/m2 on days 1 and 2.
Recombinant immunotoxins for targeting HCL
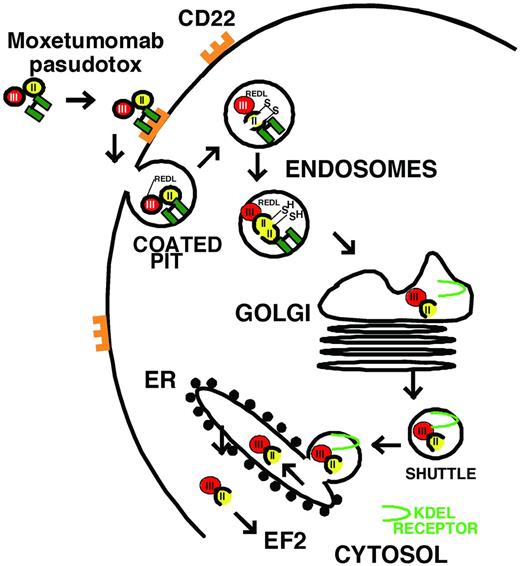
Unfortunately, mAbs targeting Ags other than CD20, including CD52,12 have not been very effective in HCL. Many HCL patients experience failure of rituximab, probably due to resistance to apoptotic signaling and inadequate immunity to mediate CDC and ADCC. To improve the cytotoxicity of targeted therapy, protein toxins may be connected to mAbs to create chimeric immunotoxins, which kill cells by catalytic inhibition of protein synthesis and induction of apoptosis.25 Compared with chemical conjugates of whole Abs and toxins, recombinant immunotoxins contain only the Fv fragment of the Ab fused to a truncated toxin. This allows a smaller targeting molecule with less exposure to endothelial cells, a more defined toxin-ligand junction, and a recombinant construction that can be produced in bacteria with low endotoxin content. Recombinant immunotoxins previously made to target HCL in patients include LMB-2, which targets CD25, and both BL22 and moxetumomab pasudotox, which target CD22.26 As shown in Figure 1 for moxetumomab pasudotox, recombinant immunotoxins containing truncated Pseudomonas exotoxin (PE) bind to the targeted surface Ag, internalize via a coated pit, become proteolyzed within an acidic endosome, traffic to the endoplasmic reticulum using the KDEL receptor, translocate to the cytosol, and ADP-ribosylate elongation factor-2 (EF2), leading to protein synthesis inhibition and apoptotic cell death. Protein toxins are extremely toxic due to their enzymatic mechanisms of action. Once in the cytosol, 1 molecule has been shown sufficient for cell death.27 Due to poor efficiency of the steps needed for intoxication, several hundred toxin molecules likely need to bind for 1 to make it to the cytosol, and this protects normal cells, which might express < 100 sites/cells. Although targeted bacterial toxins have disadvantages with respect to immunogenicity and unintended targeting of normal tissues, their advantage lies in their extreme potency. A less toxic targeted drug requiring thousands of molecules to kill a cell may be effective in killing a cell line densely expressing Ag, but may have little activity on primary malignant cells expressing a few hundred Ag sites/cell.
Intoxication of CD22+ B cells by moxetumomab pasudotox. The recombinant immunotoxin is composed of VH and VL (green), which are disulfide bonded together by cysteines replacing Arg44 of VH and Gly100 of VL. The carboxy terminus of VH is fused to the toxin. VH contains a mutation of SSY to THW at positions 100, 100a, and 100b, respectively. The toxin PE38 is composed of domain 2 (amino acids 253-364, yellow), 1a (amino acids 381-399, not shown), and the catalytic domain 3 (amino acids 400-613, red). After the variable domains bind to CD22, the immunotoxin internalizes into a coated pit by endocytosis, domain 2 is proteolytically cleaved between Arg279 and Gly280 by Furin, the carboxy terminus of the toxin traffics to the endoplasmic reticulum using the KDEL receptor (light green), and then, once in the cytosol, the enzymatic domain within domain III ADP ribosylates EF2, leading to protein synthesis inhibition and apoptotic cell death.
Intoxication of CD22+ B cells by moxetumomab pasudotox. The recombinant immunotoxin is composed of VH and VL (green), which are disulfide bonded together by cysteines replacing Arg44 of VH and Gly100 of VL. The carboxy terminus of VH is fused to the toxin. VH contains a mutation of SSY to THW at positions 100, 100a, and 100b, respectively. The toxin PE38 is composed of domain 2 (amino acids 253-364, yellow), 1a (amino acids 381-399, not shown), and the catalytic domain 3 (amino acids 400-613, red). After the variable domains bind to CD22, the immunotoxin internalizes into a coated pit by endocytosis, domain 2 is proteolytically cleaved between Arg279 and Gly280 by Furin, the carboxy terminus of the toxin traffics to the endoplasmic reticulum using the KDEL receptor (light green), and then, once in the cytosol, the enzymatic domain within domain III ADP ribosylates EF2, leading to protein synthesis inhibition and apoptotic cell death.
Recombinant immunotoxin targeting CD25 on HCL
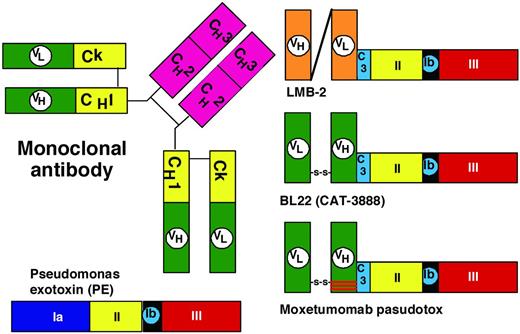
The first recombinant immunotoxin created contained a 38-kDa truncated form of PE called PE38 fused to a single-chain Ab containing the variable domains of the anti-Tac mAb, and was called anti-Tac(Fv)-PE40. A slightly shortened form of this molecule called anti-Tac(Fv)-PE38 or LMB-2 (Figure 2) was developed for the targeting of CD25+ hematologic malignancies.27 A phase 1 trial was associated with major response in hematologic malignancies, including adult T-cell leukemia, Hodgkin lymphoma, chronic lymphocytic leukemia, and cutaneous T-cell lymphoma, but response was best in HCL, with 1 CR and 3 PRs of 4 evaluable patients. Subsequent patients with HCL are receiving LMB-2 in a phase 2 trial, particularly those unable to receive anti-CD22 immunotoxin. One of the 4 patients responding to LMB-2 had selection for CD25− HCL cells not observed pretreatment, but all HCL cells remained bright CD22+. Additional development of recombinant immunotoxins for HCL targeted CD22.
Domains of PE. The domains of PE include the binding domain (1a); amino acids 1-252; and domains II, Ib, and III, as noted in Figure 1. C3 is a connector (ASGGPE) between VH and the toxin. To allow recombinant immunotoxins to bind selectively to target cells, the VH and VL domains of the mAb are connected to a truncated form of the toxin devoid of its binding domain.
Domains of PE. The domains of PE include the binding domain (1a); amino acids 1-252; and domains II, Ib, and III, as noted in Figure 1. C3 is a connector (ASGGPE) between VH and the toxin. To allow recombinant immunotoxins to bind selectively to target cells, the VH and VL domains of the mAb are connected to a truncated form of the toxin devoid of its binding domain.
Development of recombinant immunotoxins targeting CD22
To target CD22, the anti-CD22 mAb RFB4, which had previously been chemically conjugated to ricin derivatives for targeting B-cell malignancies, was cloned to obtain the variable domains. Originally a single-chain Fv and then a disulfide-stabilized Fv was produced and genetically fused to PE38 (Figure 2), resulting in BL22, also called CAT-3888.26–27 BL22 was cytotoxic to cells with as few as 350 CD22 sites/cell,28 induced regressions in CD22+ tumor xenografts, and began clinical testing at the NIH in 1999. The phase 1 trial included 31 HCL patients with relapsed or refractory HCL after purine analog, treated with 3-50 μg/kg every other day for 3 doses (QOD × 3). A hemolytic uremic syndrome (HUS), composed of thrombocytopenia, hemolytic anemia and renal insufficiency, was observed in 4 HCL patients during retreatment cycles, and completely resolved after 6-10 days of plasmapheresis. Dose-limiting capillary leak syndrome appeared in 1 HCL patient, and the MTD was established at 40 μg/kg × 3.29–30 BL22 was clinically active in HCL, with 19 (61%) CRs and 6 (19%) PRs and an ORR of 81%. BL22 pharmacokinetics documented a strong CD22 sink effect mainly during the first cycle. During this trial, a new marker of HCL, soluble CD22, was detected as an approximately 100-kDa protein with an associated approximately 75-kDa product, and an ELISA was developed to use this marker to assess tumor burden and response.31 Because most (65%) CRs but no HUS occurred after just 1 cycle of BL22, a phase 2 trial was performed in 36 patients limiting BL22 to 1 cycle and retreating only patients not achieving resolution of cytopenias.32 Of these 36 phase 2 patients, 9 (25%) CRs and 9 (25%) PRs were achieved with 1 cycle (ORR 50%) and, after retreating 56% of the patients needing it, best response improved to 47% CRs and 25% PRs (ORR 72%). An additional 3 cases of HUS on study were observed, although 1 of these was grade I. These data supported further clinical development, but because BL22 was less effective in more common diseases with lower CD22 density, including CLL and acute lymphoblastic leukemia,30,33 an effort was made to improve the immunotoxin.
Development of an affinity-matured version of BL22
To improve the targeting of BL22, which binds to CD22 with a Kd of approximately 10nM, the approach was to decrease the off-rate and thereby increase the percentage of bound molecules that become internalized. Molecules containing mutations in the “hot spots” of the CDR3 region of the variable heavy (VH) domain were screened by phage display and a mutant was found with VH residues 100, 100a, and 100b mutated from SSY to THW. This mutant had 14-fold improved affinity due to lower off-rate and was more cytotoxic toward HCL and CLL than BL22,34 but had similar animal toxicity and preclinical antitumor activity.35 As shown in Figure 2, moxetumomab pasudotox contains only 3 mutations compared with BL22, and was prepared for further clinical development.
Dose-escalation phase 1 testing of moxetumomab pasudotox
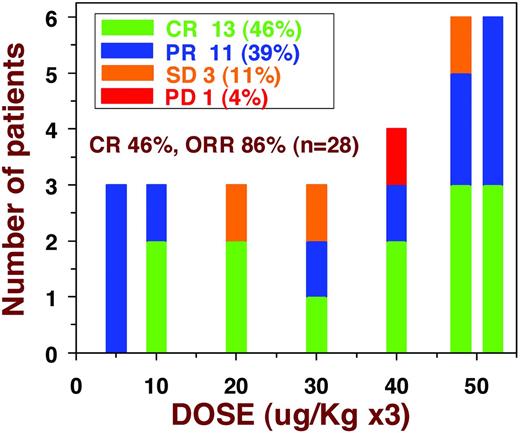
A dose-escalation phase 1 trial of moxetumomab pasudotox in 28 patients with multiply relapsed/refractory HCL was recently reported.36 Patients received a total of 114 cycles, 1-16 (median 4) per patient. No dose-limiting toxicity was observed, and in particular no cases of grade III-IV HUS or capillary leak syndrome were observed.36 However, 2 patients had grade II HUS after cycle 3 of 30 and after 5 of 50 μg/kg QOD × 3, but in these 2 patients, the platelet nadirs were 106 000-120 000/μL, creatinine peaks were 1.53-1.66, and both resolved without specific therapy. As shown in Figure 3, 16 patients received lower doses, 5-40 μg/kg QOD × 3, and 12 patients received the highest dose level, 50 μg/kg QOD × 3. Responses were observed at all doses levels, and CRs were observed at as low as 10 μg/kg QOD × 3 (Figure 3). In the 28 evaluable patients, there were 13 (46%) CRs and 11 (39%) PRs for an ORR of 86%. Tumor burden was associated with lower peak plasma levels, which increased significantly between days 1 and 5, because tumor burden decreased significantly during this time. Immunogenicity by ELISA assay was positive in 17 (65%) of 26 evaluable patients after a median of 2 cycles. Neutralizing Abs, detected by the interference by serum of the killing of CD22+ cells by moxetumomab pasudotox, were high in 10 (38%) of the 26 evaluable patients.36 A total of 2-5 cycles of moxetumomab pasudotox were required to achieve CR, and up to 2 consolidation cycles could be administered in increase the durability of CR. A total of 10 consolidation cycles were given to 5 patients achieving CR. The other 8 patients who achieved CR could not get consolidation cycles because of immunogenicity in 6 patients and grade II HUS in 2 patients. Of the 13 CRs, 10 (83%) patients were still in CR at a median of 29 months and only 1 patient relapsed before 1 year. It was determined that response was not related to prior purine analog. However, CR rate was related to prior splenectomy in that 0 of 7 with prior splenectomy achieved CR versus 13 of 21 with spleens (P = .007). Nevertheless, of the 7 with prior splenectomy, 6 (86%) patients had PRs. The absence of CRs in those with prior splenectomy is probably due to more advanced disease in these patients, and it is possible that in the absence of a spleen, there is increased BM infiltration with HCL, requiring more cycles for resolution to the level needed for CR. Therefore, moxetumomab pasudotox showed activity in HCL and its safety profile supported further clinical development toward the goal of an approved and effective nonchemotherapy option for HCL.
Development of additional targets for the treatment of HCL
Hematologic improvement was recently reported with the cyclin-dependent kinase inhibitor flavopiridol in a patient with multiply relapsed HCL refractory to both purine analog and rituximab.37 Treatment was complicated by tumor lysis syndrome, which was successfully handled with successive dose reductions. Both hemoglobin and platelet counts improved significantly, and at 28 months after initiation of flavopiridol, the patient met the criteria for CR.37 The discovery by the Italian group that the BRAF V600E mutation is present in 100% of classic HCL patients opened the door for exploration of BRAF V600E inhibitors such as Vemurafenib for HCL treatment.38 Approximately half of melanomas have this mutation, and in V600E+ melanoma, Vemurafenib was associated with an improvement in OS with a hazard ratio for death of 0.37 compared with dacarbazine.39 Recent HCL BRAF data indicate that both HCLv and IGHV4-34+ HCL lack the V600E+ mutation.40 In fact, of 42 classic HCL patients with known IGHV rearrangements other than IGHV4-34, most of whom had relapsed disease, 5 (12%) were wild-type at V600, indicating that other genetic defects must be important in the origin and pathogenesis of HCL. In the coming years, it is hoped that other genetic events in HCL and its variants will be discovered, and that new agents will assist and perhaps replace standard therapy for this disease, particularly for those who now have a less than optimal long-term outlook.
Disclosure
Conflict-of-interest disclosure: The author is employed by and holds patents with or receives royalties from the National Institutes of Health. Off-label drug use: moxetumomab pasudotox and LMB-2, recombinant immunotoxins targeting CD22 and CD25, respectively.
Correspondence
Robert J. Kreitman, MD, Laboratory of Molecular Biology, National Cancer Institute, National Institutes of Health, 37/5124b, 9000 Rockville Pike, Bethesda, MD 20892; Phone: 301-496-6947; Fax: 800-864-7554; e-mail: kreitmar@mail.nih.gov.