Abstract
Although there have been recent advances with targeted therapies in chronic lymphocytic leukemia (CLL), chemoimmunotherapy remains the treatment of choice; however, this approach is not curative. A key feature of CLL is that it induces a state of immunosuppression, causing increased susceptibility to infections and failure of an antitumor immune response, often worsened by the immunosuppressive effect of treatment. Because of its improved specificity, immunotherapy potentially offers a way out of this dilemma. Allogeneic stem cell transplantation remains the only curative option, but is hampered by the toxicity of GVHD. After many years of promise but little reward, many other immunotherapeutic approaches are now in transition to the clinical setting. Clinical trials including CLL vaccines, CXCR4 antagonists, and adoptive cellular immunotherapies such as chimeric antigen receptor–modified T cells, CD40 ligand gene therapy, and the immunomodulatory drug lenalidomide are ongoing. Results to date suggest that immunotherapeutic approaches for the treatment of CLL might finally be fulfilling their promise.
Introduction
Over the past decade, there have been significant advances in our understanding of the underlying pathogenesis of chronic lymphocytic leukemia (CLL), and these have been accompanied by a dramatic increase in the number and range of treatment options.1 Combination immunochemotherapy with rituximab, fludarabine, and cyclophosphamide is currently established as the frontline therapy of choice, with overall response rates of 95% and complete remission (CR) rates of 44%.2 The current goal of therapy in CLL, as in other hematologic malignancies, is the eradication of minimal residual disease (MRD), with MRD eradication associated with improved outcome and improved progression-free and overall survival in CLL.3,4 There is, however, a need for alternative approaches to treat patients with “poor-risk” disease, such as those with 17p deletion or mutation and those who are refractory to, or relapse shortly after, immunochemotherapy.5 The results of ongoing and future trials with more targeted therapies (eg, ibrutinib) may show that novel agents can overcome these issues.
Role of hematopoietic stem cell transplantation in CLL
Currently, allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative option for CLL. The key to its activity is the GVL effect in which the transplanted hematopoietic stem cells differentiate into effector cells capable of mounting an antitumor immune response, which is likely directed at minor host antigenic variations. This effect is known to be primarily T-cell mediated, although it remains unclear whether it is due to improved T-cell function, the presence of allogeneic MHC molecules, or a combination of both.6 The major drawback of allogeneic transplantation is GVHD. HSCT is not a suitable treatment option for the majority of patients with CLL. The disease usually follows an indolent course; many patients never require any therapy and most patients are too elderly to undergo this procedure. However, high-risk patients can be identified using several clinical and biological features, and such younger patients are suitable candidates for enrolment in clinical trials evaluating the role of SCT in CLL. The biggest challenges remain the decisions regarding which patients are eligible for consideration of HSCT and when in their disease course HSCT should be offered.
The role of HSCT in CLL has been revolutionized by the introduction of reduced intensity conditioning (RIC),7 which can exploit GVL in CLL while avoiding the significant morbidity and mortality associated with myeloablative conditioning. RIC regimens allow transplantation in older patients, making this approach more applicable to increased numbers of CLL patients; results from the largest reported studies are shown in Table 1.8-11 RIC regimens appear to be associated with decreased mortality rates, but chronic GVHD remains a significant problem. All phase 2 studies have enrolled younger patients with “high-risk” disease, although this term is often rather loosely defined and it is difficult to determine precisely the risk factors used in each of the reported series. European Group for Blood and Marrow Transplantation guidelines have been established outlining indications for SCT in CLL.12 The guidelines conclude that there is an evidence base for the efficacy of allogeneic SCT in CLL and that this procedure is indicated in high-risk CLL patents. High-risk patients include those requiring treatment who have p53 abnormalities (who merit allogeneic SCT in first response), patients who fail to achieve CR or who progress within 12 months after purine analogs, those who relapse within 24 months after having achieved a response with purine-analog-based combination therapy, those who have relapsed after prior autologous SCT, or those patients who are fludarabine refractory. It should be noted that with the exception of cytogenetics for detection of p53 deletions, none of these categories requires assessment of biologic risk factors. Ongoing prospective clinical studies will determine the impact of biomarkers including IgVH mutational status and other cytogenetic abnormalities in the identification of patients at sufficiently high risk to merit the use of allogeneic SCT in first CR. Allogeneic SCT has the potential to induce long-term remission in patients with deletion 17p,13 and the mutational status of the TP53, SF3B1, and NOTCH1 genes had no significant effect on overall and event-free survival.8
Perhaps the most convincing proof of the principle for GVL comes from the ability of RIC plus allogeneic HSCT to induce durable eradication of MRD. In a report from the German CLL Study Group, of 52 patients who were available for longitudinal MRD monitoring, 54% were relapse free and MRD negative 12 months after allogeneic HSCT. Only 2 of these patients have subsequently relapsed, whereas 82% remain in MRD-negative clinical remission of CLL throughout long-term follow-up.8
There is currently no role for autologous HSCT in CLL except in the setting of a clinical trial. Although autologous SCT improved event-free survival, there was no difference in overall survival in phase 3 randomized trials.14 A retrospective matched-pair analysis suggested a survival advantage for autologous SCT over conventional therapy,15 but its clinical role in the era of improved frontline therapy with modern chemoimmunotherapy approaches remains uncertain.16
Autologous T-cell responses against CLL
Attempting to bolster an antitumor immune response against CLL is a particularly attractive treatment option (Table 2). The specificity of the adaptive immune response should make it possible to target leukemia cells. Immune-mediated recognition and destruction has the potential to circumvent established prognostic markers and provide improved treatments for high-risk patients. Finally, approaches aiming to enhance the immune response offer the capacity to improve immunity to infection in addition to repairing the antitumor response. However, attempts to use autologous T cells as therapy for CLL have been fraught with difficulties of overcoming the T-cell defects induced in this disease and require a fuller understanding of why patient T cells fail to recognize and attack the CLL cells.
Immune function in CLL
Immune dysfunction is a key feature of CLL, highlighted by hypogammaglobulinemia, increased susceptibility to infections, and an increased incidence of autoimmune anemia and thrombocytopenia that are commonly seen in this disease. The T-cell compartment is highly abnormal in CLL, with an increase in absolute numbers of peripheral blood T cells, primarily accounted for by an increase in CD8+ T cells, with a fall in the CD4:CD8 ratio. Despite their increased numbers, these T cells show profound defects in function and proliferative capacity, and abnormal cytokine secretion profiles. Although a shift toward a type 2 T-cell response was an attractive hypothesis, CLL T cells have increased production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), that can protect CLL cells from apoptosis and induce proliferation. CLL T cells show evidence of chronic activation, with up-regulation of activation markers such as CD69, HLA-DR and CD57, down-regulation of CD28 and CD62L and expansions of clonal and oligoclonal T cells. These oligoclonal expansions are primarily restricted to populations with an activated CD57+ phenotype, suggesting a role for chronic antigen stimulation in their development. T cells exhibit features of “exhaustion,” seen in chronic viral infections and it remained unclear whether this was directly related to CLL, or whether other factors such as cytomegalovirus (CMV) are involved. There is an expansion of CMV-specific CD4+ and CD8+ T cells in patients with CLL, with an effector phenotype.17 However CD8+ and CD4+ T cells from both CMV seropositive and seronegative CLL patients have increased expression of exhaustion markers CD244, CD160, and PD1, with expansion of a PD1+BLIMP1HI subset.18 These T cells have functional defects in proliferation and cytotoxicity, with impaired packaging of granzyme into vesicles and nonpolarized degranulation. In contrast to virally induced exhaustion, CLL T cells showed increased production of IFN-γ and TNFα and increased expression of TBET, and normal IL-2 production.
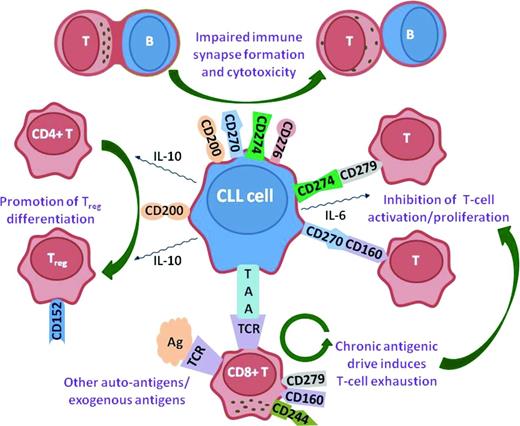
T cells in CLL exhibit profound changes in their global gene expression profiles, with alterations in the expression of genes involved in cytoskeletal formation.19 Similar defects in gene expression are induced in healthy T cells after coculture with CLL cells, demonstrating that it is the leukemia cells that are inducing these changes. The altered expression of cytoskeletal genes translated into a functional defect in filamentous actin polymerization, with CLL T cells exhibiting defective immunological synapse formation with APCs.20 Functional screening assays were developed to elucidate the molecular mechanisms whereby CLL cells induce these T-cell changes and found that the proteins CD200, CD270 (Herpes virus entry mediator; HVEM), CD274 (Programmed death ligand-1; PD-L1), and CD276 (B7-H3) induced impaired immunological synapse formation in both allogeneic and autologous T cells.21 Decreased actin polymerization also contributes to impaired integrin-mediated migration, with altered Rho-GTPase activation.22 In addition to increased expression of inhibitory receptors in CLL, there is also an increase in CD4+CD25hi regulatory T cells (Tregs) in CLL, which increases in advanced-stage disease.23 This increase may be mediated by increased expression on CLL cells of CD27 and CD200.24 Tregs express the inhibitory receptor cytotoxic T-lymphocyte associated antigen-4 (CTLA-4; CD152), and T cells from CLL patients have increased CTLA-4 expression. Therefore, it is likely that CTLA-4 signaling is yet another inhibitory pathway mediating T-cell dysfunction in CLL, in addition to the PD-1:PD-L1, CD160:HVEM, and CD200:CD200R axes (Figure 1).
Inhibitory signaling axes in CLL. Up-regulation of CD200, CD270, CD274, and CD276 induces impaired actin polymerization and immunological synapse formation in CLL T cells. This results in nonpolarized degranulation and impaired cytotoxicity. CD200 also promotes the differentiation of CD4+ T cells into Tregs, which express CD152(CTLA-4). CD270(HVEM) and CD274(PD-L1) interact with their ligands, CD160 and CD279(PD-1), respectively, to inhibit T-cell activation and proliferation. Chronic antigenic stimulation of T cells by either TAAs expressed by the CLL cells or other auto-antigens/exogenous antigens drives T-cell exhaustion and increased expression of inhibitory ligands such as CD279(PD-1), CD160, and CD244.
Inhibitory signaling axes in CLL. Up-regulation of CD200, CD270, CD274, and CD276 induces impaired actin polymerization and immunological synapse formation in CLL T cells. This results in nonpolarized degranulation and impaired cytotoxicity. CD200 also promotes the differentiation of CD4+ T cells into Tregs, which express CD152(CTLA-4). CD270(HVEM) and CD274(PD-L1) interact with their ligands, CD160 and CD279(PD-1), respectively, to inhibit T-cell activation and proliferation. Chronic antigenic stimulation of T cells by either TAAs expressed by the CLL cells or other auto-antigens/exogenous antigens drives T-cell exhaustion and increased expression of inhibitory ligands such as CD279(PD-1), CD160, and CD244.
T cells appear to play a central role in the development and maintenance of CLL by providing a protective niche.25 Nurselike cells (NLCs) are another key constituent of the immune microenvironment implicated in providing such a protective niche for CLL cells. NLCs appear to promote CLL cell survival via CXCL12 and increased expression of B-cell-activating factor of the tumor necrosis factor family (BAFF) and a proliferation inducing ligand (APRIL), perhaps explaining the increased numbers of T cells in CLL pseudofollicles.26 Increased CCL3 levels are correlated with other adverse prognostic markers such as advanced clinical stage and poor-risk cytogenetics and are predictive of shorter time to first treatment.27 This mixture of CXCL12, CCL3, and CCL4 may result in colocalization of stromal cells, NLCs, and activated CD4+ T cells, which provide “sanctuary sites” for the tumor cells, protecting them from immunochemotherapy.
Induction of autologous T-cell responses against CLL
Immune checkpoint blockade
Suppression of antitumor immunity by inhibitory pathways such as PDL1:PD1 appears to be an important mechanism underlying the failure of immune responses in CLL.28 The initial studies highlighting the importance of PD1 in suppressing T-cell function also showed that blocking PD1:PDL1 interactions could restore T-cell effector responses.29,30 These findings have led to considerable interest in the potential for blockade of these “immune checkpoints” to enhance antiviral and antitumor immunity in chronic viral infections and cancers.31 Initial work highlighted the potential for blockade of CTLA4 to induce clinical responses in patients with advanced melanoma.32 Subsequent studies have also investigated the effect of blocking the PD1:PDL1 axis. A recent phase 1 study of an anti-PD1 monoclonal antibody (Nivolumab; BMS-936558) in patients with non-small-cell lung cancer, melanoma, or renal cell cancer showed overall response rates of 18% to 28% depending on the cancer type.33 These response rates are very encouraging given the 10% to 15% “ceiling” of durable tumor response rates seen with trials of other immunotherapeutic approaches in solid cancers over the last 30 years.34 A further phase 1 trial of an anti-PDL1 antibody (BMS-936559) in a variety of advanced solid cancers also showed efficacy, with response rates of 6% to 17%, again depending on the cancer type.35 Clinical trials of these agents in hematological malignancies have been notably absent, although these cancers are generally more “immunosensitive,” with the potential for higher response rates. A single published phase 1 dose-escalation study of another anti-PD1 antibody (CT-011; Pidilizumab) showed evidence of response in 6 of 18 patients. Of the 3 CLL patients who were enrolled, 2 showed evidence of stable disease on the second and third dose levels.36 Given the preclinical data highlighting the significance of the PD1:PDL1 axis in suppressing T-cell function in CLL, there is a strong rationale for clinical assessment of immune checkpoint blockade in this disease.
CLL vaccines
Autologous (and allogeneic) T-cell responses can be generated after stimulation with dendritic cells pulsed with HLA-restricted immunodominant peptides or dendritic cells can be genetically modified by an adenoviral vector encoding full antigens from viruses such as CMV.37 A vital component of this approach is the identification of a suitable tumor-associated antigen (TAA) with which to induce an immune response. An ideal TAA should be only expressed on the tumor cells to avoid induction of autoimmunity, should be expressed on all of the tumor cells, and should be essential for tumor cell survival to prevent the emergence of antigen-negative variants and immune escape. The obvious major challenge to cancer vaccines is that the tumor cells are derived from “self,” with the consequence that few TAAs have these characteristics. In light of this, the search for possible TAAs has focused on proteins that are either commonly mutated in the malignant cells or are aberrantly expressed. Recent attention has focused on the oncofetal surface antigen receptor tyrosine kinase-like orphan receptor 1 (ROR1), which has the advantage that it is selected expressed by malignant B cells, although it is also expressed by undifferentiated embryonic stem cells and at low levels in adipose tissue.38 After infusion of autologous CLL cells that had undergone adenoviral transfection with CD154, 50% of patients subsequently generated antibodies against ROR1.39
Modification of T cells
Although a large number of tumor-associated antigens have been identified in CLL, it has proven difficult to generate autologous tumor-antigen-specific T cells in CLL and other approaches are required. The first involves the gene transfer of TCRs with known specificity into autologous or allogeneic T cells, which are then expanded in vitro and infused into patients.40 A potential risk with this approach is that the α- or β-subunit of the transgenic TCR could misassociate with the α- or β-subunits of the endogenous TCR, resulting in an autoreactive T cell. A second strategy involves the use of the single chain variable fragment from an antibody molecule fused with an internal signaling domain such as CD3ζ to form a chimeric antigen receptor (CAR).41 A major advantage of this approach is that it eliminates MHC restriction, enabling the same CAR to be used for several different patients. Furthermore, the use of an antibody receptor means that potential targets can be increased to include a wide range of surface proteins, sugars, and lipids.42 Several phase 1/2 clinical trials are under way using anti-CD19 CAR T cells for the treatment of B-cell malignancies, and impressive results have been observed in CLL43-47 and in acute lymphoblastic leukemia.48
A key observation in preclinical studies was that the addition of costimulatory domains such as CD28 significantly improved the efficacy of CAR T cells, overcoming the reduced expression of CD80 and CD86 seen in B-cell malignancies such as CLL. A clinical trial with anti-CD19 CAR T cells in a patient with advanced follicular lymphoma resulted in regression of lymphadenopathy associated with B-lymphopenia and hypogammaglobulinemia. Unfortunately, the CAR T cells did not persist long term, with the anti-CD19 CAR becoming undetectable at 27 weeks, followed by the development of progressive disease at 32 weeks.46 In a series of 8 patients with relapsed CLL in 2 cohorts, the first cohort of 3 patients did not receive any conditioning and did not show any objective responses. The next patient received lymphodepleting chemotherapy with cyclophosphamide as part of the trial design. Unfortunately, this patient rapidly developed hypotension, respiratory distress, and renal failure secondary to a combination of sepsis and tumor lysis syndrome and died within 48 hours of infusion of the T cells.49 This highlights the risks associated with this approach, but also emphasizes the potential potency of CAR T-cell therapy. In 4 further patients treated with cyclophosphamide conditioning and a reduced dose of T cells, 3 had disease stabilization or lymph node responses.43 Although this work is still in the early stages, it has underscored the importance of the conditioning regimen in promoting T-cell engraftment and activation, analogous to the impact of conditioning in allogeneic transplantation. In particular, it may be vital to eliminate Tregs, which are known to be expanded in CLL and can be suppressed with fludarabine treatment and potentially other subpopulations such as immature dendritic cells, as well as cell populations that act as “cytokine sinks” by competing for the same survival and stimulatory factors. It may also be important to debulk patients' disease before CAR T-cell infusion, because their use in patients with a lower tumor burden may avoid massive tumor lysis syndrome and cytokine storm.
Most approaches for genetic manipulation of engineered T cells have used retrovirus and lentivirus for stable expression of CARs. Transfer of nonviral plasmids is an appealing alternative to transduction because it can be produced to clinical grade much cheaper than the cost of recombinant GMP-grade virus. The Sleeping Beauty (SB) transposon and transposase has been developed for human application using DNA plasmids that consist of a transposon encoding for the gene of interest and a transposase to insert the transgene. To generate clinically sufficient numbers of genetically modified T cells, artificial APCs are modified to express a TAA such as CD19 and T-cell costimulatory molecules and cytokines. Manufactured T cells originally derived from peripheral or umbilical cord blood can then be stimulated by these APCs to enable the expansion of clinical grade CD19-specific T cells.50
The effect of enhanced costimulation on T-cell-mediated antitumor responses is important in the context of CAR T cells. In preclinical studies, human anti-CD19 CAR T cells containing the costimulatory domain CD137 (4-1BB) were significantly more effective, showed longer survival times than cells expressing CARs containing the CD28-signaling domain and were less likely to trigger the induction of a “cytokine storm” and differentiation of Tregs. The use of anti-CD19 CAR T cells incorporating a CD137-costimulatory domain resulted in the achievement of CR in a case of a heavily pretreated patient with refractory CLL.44 A significant feature of this case was that these cells persisted and were still detectable 6 months after infusion and the CAR T cells had started to express molecules associated with a “central memory” phenotype, which is important in maintaining robust and persistent antitumor immune responses. One other heavy pretreated patient also achieved CR, and a third patient, who also had deletion of p53, only showed a partial response to anti-CD19 CAR T cells, but had required corticosteroids for persistent fevers, constitutional, and cardiac symptoms at day 18.45 Although this is encouraging, many unresolved questions remain. As described above, T cells from CLL patients have profound defects in proliferative capacity and cytotoxicity. However, they can be successfully transfected and induced to proliferate in vitro and proceed to rapidly expand and cause extensive tumor lysis after infusion back into patients. There are several potential explanations for this apparent paradox. First, simply removing T cells from the inhibitory effect of the tumor cells may contribute to improved function, which may be maintained after reinfusion. Second, the strong pro-proliferative signals provided by anti-CD3/anti-CD28 beads in vitro and binding of the CAR to CD19 in vivo may overcome more subtle functional defects. Third, proliferative stimuli applied in vitro could “select out” cells that have retained the ability to proliferate, leading to restoration of global proliferative capacity. A final potential explanation is the effect of the CAR construct itself. A reduction in proliferative capacity is associated with defects in costimulatory pathways, with down-regulation of molecules such as CD28 and decreased production of IL-2. The nature of any interactions between the costimulatory domain introduced as part of second- and third-generation CAR constructs and endogenous costimulatory elements remain unclear. Understanding any such interactions may be important for enhancing the durability of CAR T-cell activity by promoting the formation of memory T-cell populations with high proliferative capacity. A correlative study that accompanied a clinical report of CAR T cells in CLL noted high expression of CD45RA, PD-1, and CD57 at day 169 after infusion, which may reflect the emergence of T-cell exhaustion and incipient loss of function.45 Other aspects of this therapy remain unquantified, such as the risk associated with profound long-term B-cell lymphopenia and hypogammaglobulinemia, which could result in increased susceptibility to infections analogous to Bruton's X-linked agammaglobulemia. However, the potential of targeting alterative tumor antigens, in particular those not expressed in normal tissues such as the oncofetal antigen ROR1,38,51 other costimulatory approaches, and the engineering of other cell types with CARs, such as NK and NKT cells, means that this remains an extremely exciting area of research.
CD40 ligand gene therapy
A further area of intense investigation was stimulated by the observation that patient T cells show reduced expression of CD154 (CD40L). The CD40/CD40L axis is critical for B-cell maturation, expansion, and survival. CLL B cells have reduced expression of CD80 and CD86 and are functionally poor at antigen presentation. CD40 ligation of CLL cells up-regulates expression of costimulatory molecules and significantly improves their antigen-presenting function. Several strategies have been developed to capitalize on the activating effect of CD40L. One such strategy was to use adenoviral vectors to transduce CLL cells to express CD40L. In addition to inducing expression of costimulatory and adhesion molecules on the transduced cell, increased expression of CD40L in the CLL microenvironment can activate other B cells even if they were not transfected. An early clinical trial investigated the effects of infusions of autologous tumor cells that had been transduced ex vivo with murine CD40L, which was found to be more efficiently expressed than human CD40L. This treatment was well tolerated and the patients did show some peripheral blood and lymph node responses. However, some of the patients developed antibodies against the murine CD40L.52 A recombinant humanized CD40-binding protein, ISF35, was developed and tested in a phase 1 study. The infusions of transduced autologous tumor cells were again well tolerated and were consistently followed by reductions in circulating lymphocyte counts and lymphadenopathy. The increased levels of this humanized CD40L was associated with induction of a pro-apoptotic state in the circulating CLL B cells, with increased expression of the pro-apoptotic molecules CD95, DR5, p73, and BCL-2 interacting domain (BID) and reduced levels of the antiapoptotic molecule MCL-1. Significantly, these findings were also observed in patients with deletion of chromosome 17p53 and intranodal injection was safe and induced clinical responses.54
Lenalidomide
Lenalidomide is approved for the treatment of multiple myeloma and 5q− myelodysplastic syndrome. It is not licensed for use in CLL, but is being evaluated in clinical trials in CLL, where it has shown clinical activity alone,55,56 in combination with rituximab,57 and as consolidation after immunochemotherapy.58 Its mechanism of action in CLL appears to be primarily by enhancing antitumor immunity.59 Lenalidomide treatment of both autologous T cells and CLL cells results in repair of T-cell defects, suggesting that this may be a key component of this agent's activity in CLL.20 Lenalidomide down-regulates the expression of T-cell-inhibitory molecules in CLL21 and enhances T-cell motility.22 Enhancing immune cell function with immunomodulatory agents may therefore be useful to enhance T-cell-mediated responses such as vaccines or adoptive T-cell transfer.
Conclusions
New treatments are resulting in improved survival for younger CLL patients, but older patients remain a particular challenge. There has been a rapid expansion of new treatments for CLL, including drugs targeting BCR signaling and novel monoclonal antibodies, many of which are significantly less toxic than traditional chemotherapeutic agents. Allogeneic HSCT remains potentially curative, but is associated with significant morbidity and mortality. Our increased understanding of the immunobiology of CLL is now starting to translate into a wide variety of therapies that target the immune microenvironment and have the potential to use patients' own activated or modified T cells to induce antitumor control. Furthermore, a particularly interesting aspect of agents such as ibrutinib and idelalisib is their ability to mobilize CLL cells from the tissues to the peripheral blood. This “eviction from the niche” may allow for increased T-cell accessibility in addition to enhancing the susceptibility of the tumor cells to immunotherapeutic approaches, thereby providing a rationale for novel treatment combinations. These treatments have the potential to be highly effective, and their more favorable side effect profile should allow for their eventual application to older and more vulnerable patient subgroups.
Disclosures
Conflict-of-interest disclosure: J.G.G. is on the board of directors or an advisory committee for Pharmacyclics and Celgene, has received research funding from Celgene, and has received honoraria from Celgene and Roche/Genentech. J.C.R. declares no competing financial interests. Off-label drug use: Drugs in clinical development that are not yet approved and their potential future use.